Abstract
Background
T-cell lymphomas (TCL) are uncommon diseases in the US. Accurate diagnosis is challenging and requires morphologic interpretation, immunophenotyping, and molecular techniques. We compared pathologic diagnoses at referring centers against expert hematopathology review to determine concordance rates and characterize the usefulness of second opinion pathology review for TCL.
Methods
Patients in the National Comprehensive Cancer Network non-Hodgkin's lymphoma database with peripheral T-cell lymphoma, NOS (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and ALK-positive and ALK-negative anaplastic large cell lymphomas (ALCL) were eligible if they had prior tissue specimens examined at a referring institution. Pathologic concordance was evaluated using available pathology and diagnostic testing reports, and provider progress notes. The etiology of discordance and the potential impact on treatment was examined.
Results
Among 131 eligible cases, 57 (44%) were concordant, totaling 64% of the 89 cases referred with a final diagnosis. 32 (24%) cases were discordant, representing 36% of cases with a final referring diagnosis. The rates of discordance among cases of PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL were 19%, 33%, 34%, and 6%, respectively. In 14 (44% of discordant cases) cases, pathologic reclassification could have resulted in a different therapeutic strategy. 42 (32%) cases were referred for classification with a provisional diagnosis.
Conclusion
In our large cohort of patients with TCL referred to NCCN centers, the likelihood of a concordant final diagnosis at a referring institution was low. As current and future therapies target subsets of TCL, our data suggest that suspected TCLs would benefit from evaluation by an expert hematopathologist.
Keywords: Lymphoma, T-cell Lymphoma, Diagnosis, Outcomes Research, Pathology
Introduction
Peripheral T-cell lymphomas (TCL) are an uncommon group of diseases that were recently updated in the World Health Organization (WHO) classification of non-Hodgkin lymphomas (NHL). The accurate diagnosis of TCL is challenging, requiring morphologic interpretation, immunophenotyping, and molecular techniques. Establishing a precise diagnosis in TCLs is critical for determining prognosis and has the potential to impact both therapeutic decisions and clinical trial enrollment.
Although TCLs are generally associated with poor outcomes, prognosis varies with disease subtype. ALK-positive ALCL has the most favorable prognosis1, though some studies suggest outcomes in ALCL are dependent on age rather than ALK status.2 Most patients with TCL receive anthracycline-based induction combination chemotherapy; however, with the exception of ALK-positive anaplastic large cell lymphoma (ALCL), relapse rates are high and a subset of patients may benefit from consolidation with autologous hematopoietic stem cell transplantation (HSCT).3-7 In recent years, a number of novel therapies, including histone deacetlyase inhibitors8, 9, pralatrexate10, and the novel CD30 antibody-drug conjugate, brentuximab vedotin11, 12, have shown significant promise in treating TCLs. As new targeted therapies become available, accurate classification of TCL will be crucial for determining appropriate candidates for clinical trial enrollment and treatment.
Despite the use of advanced techniques, prior studies evaluating the diagnostic accuracy of expert hematopathologists using both older classification systems and the newer WHO classification for TCLs have shown suboptimal rates of agreement with consensus diagnoses. Historical studies evaluating expert hematopathologist agreement rates with consensus panel diagnoses for TCLs have shown similar diagnostic accuracy, ranging from 72% for angioimmunoblastic T-cell lymphoma (AITL) and peripheral TCL, not otherwise specified (PTCL-NOS) to 85% for ALCLs.13-15 In a series of recent studies of 1314 patients with peripheral TCL and NK/T-cell lymphoma (NKTCL) by the International T-cell Lymphoma Project, the agreement rates between the diagnoses assigned by individual expert hematopathologists and the consensus diagnoses assigned by panels of expert hematopathologists were in the 66 to 97% range for various TCL subtypes. The agreement rates for the more common TCL histologies—PTCL-NOS, AITL, and ALK-negative and ALK-positive ALCL—were 75%, 81%, 74%, and 97%, respectively.1, 16, 17 Additionally, in a recent study of upfront autologous HSCT for TCL by the Nordic Lymphoma Group, referral pathology was reanalyzed by national reference center pathologists with an agreement rate of 87%.6 In another study from the UK, all lymphomas diagnosed within a hospital network underwent central review by an expert hematopathologist, and the agreement rate was also 87% for TCLs.18
Though the above studies suggest consensus expert panel hematopathology review of TCLs is beneficial, convening an expert panel for each case of suspected TCL is not feasible. Instead, when a community pathologist is unsure of a diagnosis of TCL, the biopsy specimen is referred for second opinion review, often to a tertiary center. In real-world practice, expert hematopathology review (often with departmental consensus review) is the standard of care for TCL diagnosis. Although concordance between community and expert hematopathology review has been evaluated in B-cell NHLs19, little data exists regarding the rates of agreement between referring diagnoses and expert review for TCLs in the US, and the potential impact of pathologic reclassification on treatment recommendations. We evaluated the rate of diagnostic concordance between referring center diagnoses and expert hematopathology review for 4 subtypes of T-cell lymphoma at 7 tertiary centers in the National Comprehensive Cancer Network (NCCN).
Patients and Methods
The NCCN NHL Outcomes Project is a multicenter prospective registry of comprehensive clinical, treatment, and outcome data for patients with NHL established on July 1, 2000. Data collection for patients with TCLs was initiated on April 1, 2007. Seven institutions contributed patients to this analysis: City of Hope Cancer Center, Duarte, CA; Dana-Farber Cancer Institute, Boston, MA; Fox Chase Cancer Center, Philadelphia PA; Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, IL; University of Michigan Cancer Center, Ann Arbor, MI; The University of Texas M.D. Anderson Cancer Center, Houston, TX; and Roswell Park Cancer Institute, Buffalo, NY. The institutional review boards at all participating centers approved the data collection protocol. When required, we obtained written informed consent for medical record review.
All patients with TCL presenting to participating NCCN centers between April 1, 2007 and June 15, 2012 were eligible for inclusion. Additional inclusion criteria included a documented pathologic review at a referring center prior to expert hematopathology review, and a final diagnosis of one of the following 4 TCL WHO subtypes: PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL.20
The pathologic diagnosis from the referring center was compared to the final WHO diagnosis at the NCCN centers to establish pathologic concordance rates. Pathologic concordance was defined as the same pathologic diagnosis at both the referring and NCCN center, considering all supporting documentation, including: pathology reports, immunohistochemistry (IHC), flow cytometry, fluorescence in situ hybridization (FISH) and cytogenetics, T-cell gene rearrangement studies, and physician progress notes. Review of the records of all cases was performed by three of the authors (A.F.H, A.C., A.S.L.) to determine pathologic concordance.
Cases were separated into the following categories:
Concordant, same referral and NCCN diagnoses;
Provisional diagnosis before second opinion referral with further work-up suggested;
Discordant, different referral and NCCN diagnoses.
Cases referred with a provisional diagnosis to a non-NCCN tertiary academic referral center or commercial hematopathology service prior to the NCCN presentation were placed in the same provisional diagnosis category as cases with a provisional referral diagnosis referred directly to an NCCN center for diagnosis. Cases with a provisional diagnosis before second opinion referral in which additional biopsy was necessary at the NCCN center to make a final diagnosis were included in the provisional diagnosis category.
To characterize the etiology of discordance, reviewers assigned each pathologically discordant case to one of the following categories:
Discordant final referral diagnosis, based on NCCN interpretation of existing data;
Discordant final referral diagnosis, based on additional studies performed at NCCN center.
Finally, five situations were identified when pathologic reclassification might influence a patient's treatment: (1) benign diagnosis changed to TCL; (2) malignancy other than NHL changed to TCL; (3) B-cell NHL or classical Hodgkin lymphoma changed to TCL; (4) NKTCL changed to TCL; and (5) incorrect or undefined ALK status in patients with ALCL. Patients with pathologic discordance who met one of these criteria were considered as having potentially experienced a change in treatment based on pathologic reclassification.
When reported in the materials reviewed, information regarding the type of biopsy performed (core needle or excisional biopsy), materials received for review by the NCCN center (number of paraffin-embedded tissue blocks and/or slides), number and type of studies performed at the referring and NCCN centers (e.g. number of immunostains, TCR gene rearrangement), duration of the pathology review at the referring and NCCN centers, and number of pathologists involved with the case review was collected. Descriptive statistics were used to estimate concordance rates, rates of potential treatment difference, and rates of additional testing among groups and subgroups. Analyses were performed to evaluate whether the type of biopsy or number or type of ancillary testing performed at the referring center was associated with pathologic concordance or the referring center arriving at a final diagnosis using Fisher's exact test or the student's t-test, as appropriate.
Results
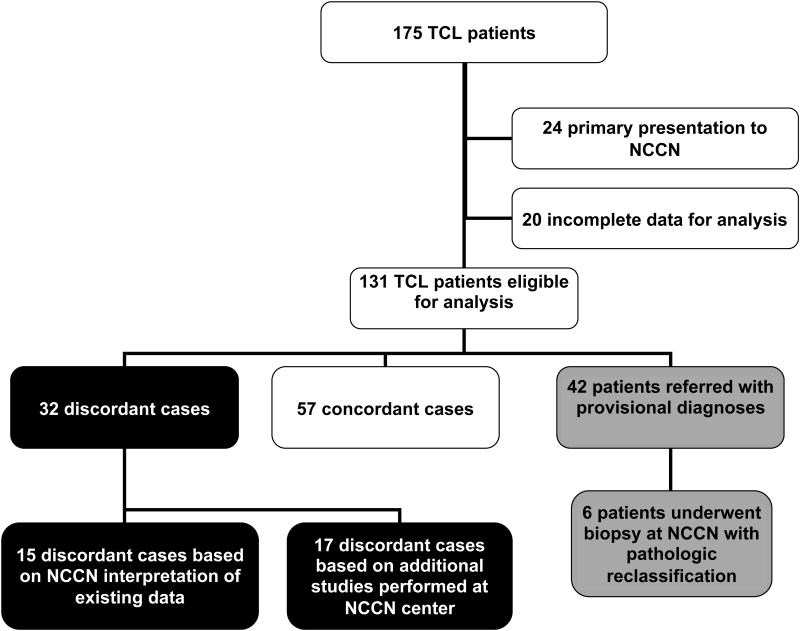
There were 175 patients with TCL enrolled into the NCCN NHL database between April 1, 2007 and June 15, 2012. Twenty-four patients had a primary presentation to a NCCN center and, therefore, had no referring pathology and were ineligible for the study. Twenty patients had incomplete or insufficient data for analysis, usually unavailable referral pathology reports for comparison, and were excluded.
Of 131 eligible cases, 89 were assigned a final diagnosis at the referring center. 57 (44%) cases were concordant with the NCCN center diagnosis, and 32 (24%) were discordant. 42 (32%) were referred for second opinion with a provisional diagnosis and further work-up or additional biopsy suggested. The rates of pathologic discordance among cases of PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL were 19%, 33%, 34%, and 6%, respectively (Table 1). Among cases referred for second opinion with a final diagnosis, the overall discordance rate was 36%, and the discordance rates among cases of PTCL-NOS, AITL, ALK-negative ALCL, and ALK-positive ALCL were 38%, 50%, 38%, and 7%, respectively. Table 1 lists the various referring and final diagnoses assigned to cases diagnosed as TCL at the NCCN centers.
Table 1. Referral and Final Pathologic Diagnoses for Patients with T-cell Lymphoma (n = 131).
| NCCN Diagnosis | |||||
|---|---|---|---|---|---|
| Referral Diagnosis | PTCL NOS | AITL | ALK+ ALCL | ALK-ALCL | Total |
| PTCL NOS | 15 | 4 | 0 | 3 | 22 |
| AITL | 0 | 11 | 0 | 0 | 11 |
| ALK+ ALCL | 0 | 0 | 13 | 0 | 13 |
| ALK- ALCL | 1 | 0 | 0 | 18 | 19 |
| ALCL, no ALK status | 0 | 0 | 0 | 2 | 2 |
| Anaplastic T-cell/NK-cell lymphoproliferative neoplasm | 0 | 0 | 0 | 1 | 1 |
| DLBCL | 1 | 1 | 0 | 0 | 2 |
| EMZL | 0 | 1 | 0 | 0 | 1 |
| Classical Hodgkin lymphoma | 1 | 1 | 0 | 3 | 5 |
| EATL | 1 | 0 | 0 | 0 | 1 |
| T-cell lymphoma without WHO designation | 4 | 1 | 0 | 1 | 6 |
| Atypical lymphoid proliferation | 1 | 1 | 0 | 0 | 2 |
| Benign/Reactive | 0 | 2 | 1 | 0 | 3 |
| No definitive diagnosis rendered | 0 | 0 | 0 | 1 | 1 |
| Final diagnosis | 24 | 22 | 14 | 29 | 89 |
| Provisional diagnosis | 24 | 11 | 4 | 3 | 42 |
| Total cases evaluated | 48 | 33 | 18 | 32 | 131 |
| Discordant cases | 9 (19%) | 11 (33%) | 1 (6%) | 11 (34%) | 32 (24%) |
PTCL NOS indicates peripheral T-cell lymphoma, not otherwise specified, AITL indicates angioimmunoblastic T-cell lymphoma, ALK+ ALCL indicates ALK-positive anaplastic large cell lymphoma, ALK-ALCL indicates ALK-negative anaplastic large cell lymphoma, DLBCL indicates diffuse large B-cell lymphoma, EMZL indicates extranodal marginal zone lymphoma, EATL indicates enteropathy-associated T-cell lymphoma, TCL indicates T-cell lymphoma
Of the 32 discordant cases referred to an NCCN center with a final diagnosis, 15 (47%) were reclassified based on a different interpretation of the same data or non-contributory additional studies. Non-contributory additional studies represented studies performed at the NCCN center that were not originally performed at the referring center and were negative, or studies that were repeated at the NCCN center that had been originally performed and merely confirmed positivity. In the remaining 17 (53%) discordant cases, additional studies were performed at the NCCN center that led to a different diagnosis. Additional IHC led to a reclassification in 14 cases, a positive TCR result led to one reclassification, an additional biopsy with repeat TCR testing led to one reclassification, and a negative FISH test for a 9q34 abnormality supported a reclassification based on morphology from enteropathy-associated TCL to PTCL-NOS. Of the cases reclassified because of additional IHC: 2 cases were ALCLs in which ALK staining had not been performed at the referring center; in 5 cases, CXCL13 and/or PD-1 and/or CD21 stains were performed that led to a reclassification to AITL; in 3 cases, BSAP/PAX5 stains, usually in concert with repeat CD15, CD30, and on one occasion OCT2 and BOB1 stains, led to a reclassification from classical Hodgkin lymphoma to ALCL. The remaining 4 cases were reclassified based on IHC for standard T-cell markers or CD30.
In 14 cases (11% overall, 16% of cases with a final referring diagnosis, and 44% of discordant cases), pathologic reclassification may have resulted in a change in treatment. Three patients referred with benign diagnoses were diagnosed with TCL at an NCCN center and required treatment. Eight patients were referred with a diagnosis of B-cell NHL or classical Hodgkin lymphoma and were reclassified as having TCL. One patient referred with a diagnosis of NKTCL was reclassified as a TCL. Two patients were diagnosed with ALCL without evaluation of ALK status.
In 112 cases (86%), an excisional biopsy was performed at the referring center and was subsequently submitted for NCCN hematopathology review. In 19 cases (14%), a core needle biopsy or other type of sample represented the primary tissue sample referred for NCCN hematopathology review. Among 42 cases referred to the NCCN center with a preliminary diagnosis, 9 had a core biopsy or other type of sample referred for review and 33 had an excisional biopsy. In the 89 cases referred with a final diagnosis, 10 were referred with core biopsy or other type of sample and 79 had an excisional biopsy. Of the 10 finalized cases referred with a core biopsy or other type of sample, there was 1 discordant case and 9 concordant cases. There were 48 concordant cases and 31 discordant cases among finalized cases referred with an excisional biopsy. There was no association between biopsy type and the pathologic concordance among cases referred with final diagnoses (p=0.18) or between biopsy type and whether a final diagnosis was rendered at the referring center (p=0.09).
Additional testing beyond histologic evaluation of biopsy material was performed at the referring institution prior to the second opinion referral in 95% of all eligible cases. Immunohistochemical stains, flow cytometry, T-cell receptor gene rearrangement testing, and FISH testing (usually for ALK rearrangement) were performed in 84%, 52%, 36%, and 6% of cases, respectively. Table 2 describes the studies performed at the referring center in cases where a final diagnosis was conferred, separated into cases that were concordant or discordant. There was no association between pathologic concordance or discordance and the number of immunohistochemical stains performed (p = 0.23), or the type of study performed – immunohistochemistry (p = 0.66), flow cytometry (p = 0.83), TCR gene rearrangement testing (p = 0.5), the combination of immunohistochemistry and flow (p = 0.825), the combination of immunohistochemistry and flow and TCR testing (p = 0.6).
Table 2. Number and Type of Studies Performed at Referring Centers in Cases Assigned a Final Diagnosis.
| Type of Study Performed at Referring Institution | Concordant cases n = 57 | Discordant cases n = 32 | ||
|---|---|---|---|---|
|
|
|
|||
| Number | Percent | Number | Percent | |
| IHC | ||||
| Yes | 54 | (95) | 29 | (91) |
| No/not mentioned | 3 | (5) | 3 | (9) |
| Flow cytometry | ||||
| Yes | 32 | (56) | 17 | (53) |
| No/not mentioned | 25 | (44) | 15 | (47) |
| TCR gene rearrangement | ||||
| Yes | 19 | (33) | 13 | (41) |
| No/not mentioned | 38 | (67) | 19 | (59) |
| FISH | ||||
| Yes | 5 | (9) | 0 | (0) |
| No/not mentioned | 52 | (91) | 32 | (100) |
| IHC+Flow | 31 | (54) | 16 | (50) |
| IHC+Flow+TCR | 15 | (26) | 6 | (19) |
| # of referring IHCs (median) | 14 (range, 0-35) | 11 (range, 0-31) | ||
IHC indicates immunohistochemical stain, TCR indicates T-cell receptor
The median number of paraffin-embedded tissue blocks and slides received for review at the NCCN centers was 0 (range, 0-10 blocks) and 19 (range, 0-65 slides), respectively. The median duration of time spent reviewing a case at the NCCN center was 5 days (range, 1-34 days). From the available documentation, in 72% of the NCCN pathology reviews, a single NCCN hematopathologist was reported to have reviewed the case. In 28% of cases, it was reported that cases were referred for intradepartmental consultation by at least one hematopathologist or were reviewed at an intradepartmental conference. Comparatively, in 76% of referring center pathology reviews, it was reported that cases were reviewed by one pathologist, and in 24% of cases, it was reported that cases were referred for intradepartmental consultation by at least one pathologist or were reviewed at an intradepartmental conference. At the NCCN center, additional immunohistochemical stains, flow cytometry, T-cell receptor gene rearrangement testing, and FISH testing were performed in 53%, 18%, 18%, and 6% of cases, respectively. The median number of immunohistochemical stains performed at the NCCN centers was 2 (range, 0-29 stains) compared to 11 (range, 0-35 stains) performed at the referring centers. In 31 cases, a median 10 (range, 1-40) immunohistochemical stains were performed at an institution other than the referring institution prior to NCCN referral.
Discussion
Our review of second opinion pathology in the NCCN showed a high rate of pathologic discordance for most TCL subtypes included in our study with the exception of ALK-positive ALCL. The discordance rates were particularly high for cases assigned a “final” diagnosis at the referring center. Compared to other studies reporting central review of clinical trial subject specimens or central review of all lymphoma cases in a geographic region, the discordance rates in our study are high.6, 18 We also found that in about half of the discordant cases, the pathologic reclassification may have impacted treatment.
Unlike a prior study by our group demonstrating a high rate of pathologic concordance between referring and NCCN centers for B-cell NHLs19, the lower rate of agreement between referring and NCCN centers suggests that community pathology review is not equivalent to expert hematopathology review of TCLs. Often, referring pathologists did not assign a specific diagnosis according to the WHO classification. There was no association between the type of biopsy performed, or the number or type of study performed at the referring center and pathologic concordance or discordance. In fact, in about half of discordant cases, NCCN hematopathology review of already available diagnostic testing resulted in a pathologic reclassification. The other half of cases were reclassified based on additional testing performed at the NCCN center. The most common additional testing performed was IHC, including novel stains like CXCL13 and PD-1 that may not be available in the community. Common reclassifications included a referral diagnosis of PTCL-NOS reclassified as AITL or ALK-negative ALCL, and a referral diagnosis of classical Hodgkin lymphoma reclassified as ALK-negative ALCL. Notably, 3 patients originally diagnosed with benign conditions were reclassified as TCL at NCCN centers, which would have resulted in a major difference in treatment. Two of these cases were ultimately diagnosed as AITL, an aggressive TCL that is notoriously difficult to accurately diagnose and distinguish from non-malignant lymphoid proliferations. As new advanced diagnostic tools, including molecular profiling of TCLs21, become available to enhance diagnostic accuracy of TCLs, cases will require review at centers capable of performing and interpreting these analyses.
Importantly, a number of cases were referred for second opinion without a final diagnosis from the referring institution and were referred immediately for second opinion with only a provisional diagnosis. Referring pathologists frequently recognized atypical lymphoid populations, and sometimes, lymphoid proliferations suggestive of T-cell lymphoma, but often referred cases for expert hematopathology review for final diagnosis and classification. The considerable proportion of patients referred with a provisional diagnosis likely reflects how infrequently TCL is encountered in the community and the inherently challenging nature of accurately diagnosing TCLs. The high early referral rate suggests that it may already be common practice for community pathologists to refer these complicated cases to a tertiary center.
Our study has limitations. First, the numbers of cases within each subtype of TCL is small. Second, we compared community pathology review to expert hematopathology review at a tertiary center. For the purposes of this analysis, we assumed that the diagnosis rendered by the NCCN hematopathologist was the “correct diagnosis”. Prior studies evaluating expert hematopathology review against consensus expert panels have demonstrated diagnostic accuracy rates in the 72-97% range for the lymphoma subtypes included in this study.1, 14, 16, 17 Therefore, there might be an inherent discordance rate in the expert review that should be considered when interpreting the data.
Next, since our study population was entirely composed of patients referred to tertiary centers for further management and pathologic review, our population may have been enriched for complex cases that were more challenging to accurately diagnose. This may explain the higher discordance rate in our study relative to studies evaluating all cases in a geographical region or all subjects enrolled on a specific clinical trial. Additionally, in estimating the impact of pathologic reclassification, we did not examine the actual therapy received by patients in the study. Finally, the hematopathologists at NCCN centers were not blinded to the referring pathology, which may have influenced their decisions regarding a final pathologic diagnosis. Nevertheless, awareness of a previously assigned diagnosis should have biased the hematopathologists towards a concordant diagnosis and should not have altered the high discordance rates demonstrated in this study.
Establishing a precise diagnosis by differentiating between different TCL subtypes is important for determining prognosis and impacts both therapeutic decisions and clinical trial eligibility. Prognosis and response to standard chemotherapy differs between TCL subtypes, with ALK-positive ALCL associated with higher remission rates and improved survival following induction chemotherapy.1, 6 Because of the poor prognosis associated with non-ALK-positive ALCL TCLs, many of these patients are considered for up-front consolidation with autologous HSCT. Recent data suggests that there may be differences in outcomes following autologous HSCT according to TCL subtype, with ALK-negative ALCL patients having increased progression-free and overall survival when compared to other TCL subtypes.6 Additionally, a number of currently available therapies for relapsed or refractory TCLs have differential activity across different TCLs, with AITL patients less likely to respond to pralatrexate and exhibiting longer duration of responses to romidepsin.8-10 Thus, accurate TCL histologic classification is critical for making treatment decisions in these patients and will become increasingly important as we continue to learn about the differences in outcomes according to TCL subtype following HSCT and various therapies. Furthermore, with the evolution of clinical trials examining the activity of novel agents among TCL subtypes, such as brentuximab vedotin, the anti-CD30 antibody drug conjugate, proper classification will be important for understanding and identifying these differential responses. The low rate of pathologic concordance seen in our study stresses the importance of centralized expert hematopathology review in these trials.
In summary, over one-third of TCL cases referred to NCCN centers for second opinion with a final diagnosis were reclassified at the NCCN center. About one in ten patients with TCL in the NCCN NHL Outcomes Project database had a pathologic reclassification at the NCCN center that may have impacted their treatment. The NCCN NHL Outcomes Project database is one of the largest reported T-cell lymphoma series to date using the WHO classification. Given the frequency of pathologic reclassification for TCLs we observed, our data supports obtaining an expert hematopathology review for any patient suspected of having TCL as well as centralized hematopathology review for TCL clinical trials. Indeed, TCLs are uncommon and difficult to diagnose accurately. As current and future therapeutic approaches target subsets of TCLs, accurate diagnosis and distinguishing between TCL subtypes promises to become even more important.
Figure 1.
Comparison of Referral and Final Pathology for TCL
Acknowledgments
There was no specific funding for this project.
We would like to acknowledge the hematopathologists at each NCCN institution who reviewed the cases included in this analysis. We thank the Clinical Research Associates at each site for their assistance with assembling all of the review materials.
Footnotes
Precis: A high discordance rate was observed between referring diagnoses and expert hematopathology review of T-cell lymphomas. In 1 in 10 cases, expert review resulted in a pathologic reclassification that may have impacted treatment.
Authorship Contributions: Conception and Design: A.F.H., A.C., J.W.F, G.A.A., M.S.C, L.I.G., M.S.K., M.M.M., A.P.N., J.C.N., S.J.R., M.A.R., A.D.Z., A.S.L.
Collection and Assembly of Data: A.F.H., A.C., A.S.L.
Data Analysis: A.F.H., G.A.A., J.W.F., A.S.L.
Manuscript Preparation: A.F.H., G.A.A., J.W.F., S.J.R., A.S.L.
Final Approval of Manuscript: A.F.H., A.C., J.W.F, G.A.A., M.S.C, L.I.G., M.S.K., M.M.M., A.P.N., J.C.N., S.J.R., M.A.R., A.D.Z., A.S.L.
Conflict of Interest Disclosures: The authors have no financial interests to disclose.
Presented in part at the Annual Meeting of the American Society of Hematology, 2012, abstract 1610: “Comparison of Referring and Final Pathology for T-Cell Lymphomas in the NCCN”
References
- 1.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. doi: 10.1200/JCO.2008.16.4558. [DOI] [PubMed] [Google Scholar]
- 2.Sibon D, Fournier M, Briere J, et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d'Etude des Lymphomes de l'Adulte trials. J Clin Oncol. 2012;30:3939–3946. doi: 10.1200/JCO.2012.42.2345. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez J, Conde E, Gutierrez A, et al. Prolonged survival of patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation: the GELTAMO experience. Eur J Haematol. 2007;78:290–296. doi: 10.1111/j.1600-0609.2007.00815.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez J, Conde E, Gutierrez A, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group. Eur J Haematol. 2007;79:32–38. doi: 10.1111/j.1600-0609.2007.00856.x. [DOI] [PubMed] [Google Scholar]
- 5.Schetelig J, Fetscher S, Reichle A, et al. Long-term disease-free survival in patients with angioimmunoblastic T-cell lymphoma after high-dose chemotherapy and autologous stem cell transplantation. Haematologica. 2003;88:1272–1278. [PubMed] [Google Scholar]
- 6.d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30:3093–3099. doi: 10.1200/JCO.2011.40.2719. [DOI] [PubMed] [Google Scholar]
- 7.Smith SM, Burns LJ, van Besien K, et al. Hematopoietic cell transplantation for systemic mature T-cell non-hodgkin lymphoma. J Clin Oncol. 2013;31:3100–3109. doi: 10.1200/JCO.2012.46.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30:631–636. doi: 10.1200/JCO.2011.37.4223. [DOI] [PubMed] [Google Scholar]
- 9.Piekarz RL, Frye R, Prince HM, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood. 2011;117:5827–5834. doi: 10.1182/blood-2010-10-312603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182–1189. doi: 10.1200/JCO.2010.29.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 12.Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 13.Rudiger T, Weisenburger DD, Anderson JR, et al. Peripheral T-cell lymphoma (excluding anaplastic large-cell lymphoma): results from the Non-Hodgkin's Lymphoma Classification Project. Ann Oncol. 2002;13:140–149. doi: 10.1093/annonc/mdf033. [DOI] [PubMed] [Google Scholar]
- 14.Weisenburger DD, Anderson JR, Diebold J, et al. Systemic anaplastic large-cell lymphoma: results from the non-Hodgkin's lymphoma classification project. Am J Hematol. 2001;67:172–178. doi: 10.1002/ajh.1102. [DOI] [PubMed] [Google Scholar]
- 15.A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997;89:3909–3918. [PubMed] [Google Scholar]
- 16.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood. 2011;117:3402–3408. doi: 10.1182/blood-2010-09-310342. [DOI] [PubMed] [Google Scholar]
- 17.Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31:240–246. doi: 10.1200/JCO.2011.37.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Proctor IE, McNamara C, Rodriguez-Justo M, Isaacson PG, Ramsay A. Importance of expert central review in the diagnosis of lymphoid malignancies in a regional cancer network. J Clin Oncol. 2011;29:1431–1435. doi: 10.1200/JCO.2010.31.2223. [DOI] [PubMed] [Google Scholar]
- 19.LaCasce AS, Kho ME, Friedberg JW, et al. Comparison of referring and final pathology for patients with non-Hodgkin's lymphoma in the National Comprehensive Cancer Network. J Clin Oncol. 2008;26:5107–5112. doi: 10.1200/JCO.2008.16.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 21.Piccaluga PP, Fuligni F, De Leo A, et al. Molecular profiling improves classification and prognostication of nodal peripheral T-cell lymphomas: results of a phase III diagnostic accuracy study. J Clin Oncol. 2013;31:3019–3025. doi: 10.1200/JCO.2012.42.5611. [DOI] [PubMed] [Google Scholar]