Abstract
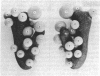
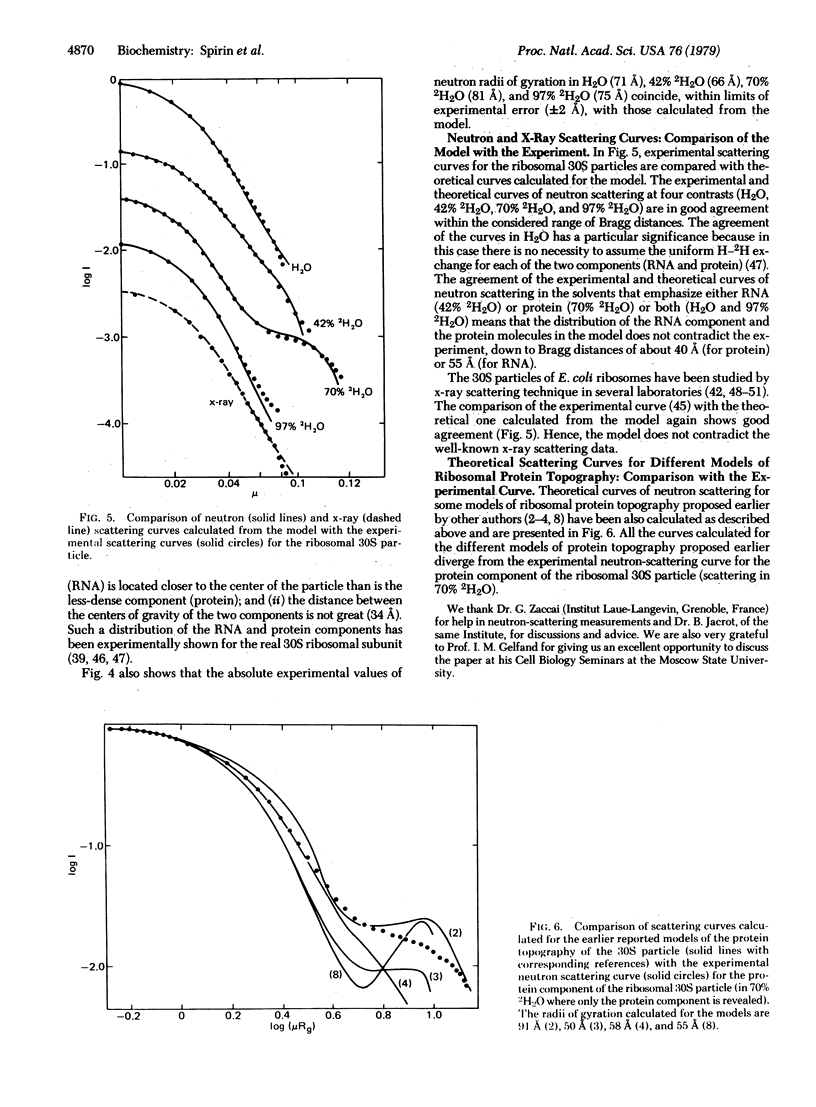
In considering the structure of the 30S subunit of the Escherichia coli ribosome, we have assumed that: (i) all or almost all the proteins within the 30S particle are compact and globular, as recently shown for the isolated proteins S4, S7, S8, S15, and S16 in solution [Serdyuk, I.N., Zaccai, G. & Spirin, A.S. (1978) FEBS Lett. 94, 349-352]; (ii) the RNA within the 30S particle has approximately the same specific V-like or Y-like shape that was demonstrated for the isolated 16S RNA in a compact conformation [Vasiliev, V.D., Selivanova, O.M. & Koteliansky, V.E. (1978) FEBS Lett. 95, 273-276]. From these assumptions and using the numerous data reported on neighboring ribosomal proteins, we have constructed a model of the quaternary structure of the ribosomal 30S subunit. The model has been tested by calculation of the theoretical curves of neutron scattering at different contrasts, as well as those of x-ray scattering, and their comparison with the experimental scattering curves for E. coli 30S particles. It has been found that the calculated scattering curves for the model practically coincide with the experimental scattering curves for the 30S particles in the range of Bragg distances down to 40-55 A. The scattering curves calculated for several three-dimensional patterns of arrangement of the 30S subunit proteins proposed earlier have been shown to be inconsistent with the experiments.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaudry P., Petersen H. U., Grunberg-Manago M., Jacrot B. A neutron study of the 30 S-ribosome subunit and of the 30 S-IF3 complex. Biochem Biophys Res Commun. 1976 Sep 20;72(2):391–397. doi: 10.1016/s0006-291x(76)80055-1. [DOI] [PubMed] [Google Scholar]
- Bollen A., Cedergren R. J., Sankoff D., Lapalme G. Spatial configuration of ribosomal proteins: a computer-generated model of the 30S subunit. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1069–1078. doi: 10.1016/s0006-291x(74)80088-4. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Nierhaus K. H., Garrett R. A., Wittmann H. G. The ribosome of Escherichia coli. Prog Nucleic Acid Res Mol Biol. 1976;18:1-44, 323-5. doi: 10.1016/s0079-6603(08)60585-1. [DOI] [PubMed] [Google Scholar]
- Bäumert H. G., Sköld S. E., Kurland C. G. RNA-protein neighbourhoods of the ribosome obtained by crosslinking. Eur J Biochem. 1978 Sep 1;89(2):353–359. doi: 10.1111/j.1432-1033.1978.tb12536.x. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. Proximity relationships among the 30 S ribosomal proteins during assembly in vitro. J Mol Biol. 1977 Jun 15;113(1):103–122. doi: 10.1016/0022-2836(77)90043-2. [DOI] [PubMed] [Google Scholar]
- Cole M. D., Beer M., Koller T., Strycharz W. A., Nomura M. Electron microscopic determination of the binding sites of ribosomal proteins S4 and S8 on 16S RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):270–274. doi: 10.1073/pnas.75.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick G. G., Kretsinger R. H. The 30 S subunit of the Escherichia coli ribosome. Topographical model of its component proteins. Biochim Biophys Acta. 1977 Feb 3;474(3):398–410. doi: 10.1016/0005-2787(77)90269-6. [DOI] [PubMed] [Google Scholar]
- Ehresmann B., Backendorf C., Ehresmann C., Ebel J. P. Characterization of the regions from E. coli 16 S RNA covalently linked to ribosomal proteins S4 and S20 after ultraviolet irradiation. FEBS Lett. 1977 Jun 15;78(2):261–266. doi: 10.1016/0014-5793(77)80319-0. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Moore P. B., Schoenborn B. P. Neutron scattering measurements of separation and shape of proteins in 30S ribosomal subunit of Escherichia coli: S2-S5, S5-S8, S3-S7. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3888–3892. doi: 10.1073/pnas.72.10.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert-Bezançon A., Barritault D., Milet M., Guérin M. F., Hayes D. H. Identification of neighbouring proteins in Escherichia coli 30 S ribosome subunits. J Mol Biol. 1977 Jun 5;112(4):603–629. doi: 10.1016/s0022-2836(77)80166-6. [DOI] [PubMed] [Google Scholar]
- Gaffney P. T., Craven G. Use of computerized multidimensional scaling to compare immunoelectron microscopy data with protein near-neighbor information: application to the 30S ribosome from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3128–3132. doi: 10.1073/pnas.75.7.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri L., Franz A. The shape of proteins S15 and S18 from the small subunit of the Escherichia coli ribosome. FEBS Lett. 1978 Mar 1;87(1):31–36. doi: 10.1016/0014-5793(78)80126-4. [DOI] [PubMed] [Google Scholar]
- Gulik A., Freund A. M., Vachette P. Small-angle X-ray scattering study of ribosomal proteins S3, S4 S7 and s20. J Mol Biol. 1978 Mar 5;119(3):391–397. doi: 10.1016/0022-2836(78)90221-8. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Fessenden R. J. Structural studies on the 30 S ribosomal subunit from Escherichia coli. J Mol Biol. 1974 Dec 25;90(4):719–726. doi: 10.1016/0022-2836(74)90535-x. [DOI] [PubMed] [Google Scholar]
- Hill W. E., Thompson J. D., Anderegg J. W. X-ray scattering study of ribosomes from Escherichia coli. J Mol Biol. 1969 Aug 28;44(1):89–102. doi: 10.1016/0022-2836(69)90406-9. [DOI] [PubMed] [Google Scholar]
- Huang K. H., Fairclough R. H., Cantor C. R. Singlet energy transfer studies of the arrangement of proteins in the 30 S Escherichia coli ribosome. J Mol Biol. 1975 Oct 5;97(4):443–470. doi: 10.1016/s0022-2836(75)80053-2. [DOI] [PubMed] [Google Scholar]
- Khechinashvili N. N., Koteliansky V. E., Gogia Z. V., Littlechild J., Dijk J. A heat denaturation study of several ribosomal proteins from Escherichia coli by scanning microcalorimetry. FEBS Lett. 1978 Nov 15;95(2):270–272. doi: 10.1016/0014-5793(78)81008-4. [DOI] [PubMed] [Google Scholar]
- Lake J. A., Kahan L. Ribosomal proteins S5, S11, S13 and S19 localized by electron microscopy of antibody-labeled subunits. J Mol Biol. 1975 Dec 25;99(4):631–644. doi: 10.1016/s0022-2836(75)80177-x. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Ribosome structure determined by electron microscopy of Escherichia coli small subunits, large subunits and monomeric ribosomes. J Mol Biol. 1976 Jul 25;105(1):131–139. doi: 10.1016/0022-2836(76)90200-x. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Engelman D. M., Moore P. B. Neutron-scattering studies of the ribosome of Escherichia coli: a provisional map of the locations of proteins S3, S4, S5, S7, S8 and S9 in the 30 S subunit. J Mol Biol. 1978 Mar 15;119(4):463–485. doi: 10.1016/0022-2836(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Laughrea M., Engelman D. M., Moore P. B. X-ray and neutron small-angle scattering studies of the complex between protein S1 and the 30-S ribosomal subunit. Eur J Biochem. 1978 Apr 17;85(2):529–534. doi: 10.1111/j.1432-1033.1978.tb12268.x. [DOI] [PubMed] [Google Scholar]
- Laughrea M., Moore P. B. Ribosomal components required for binding protein S1 to the 30 S subunit of Escherichia coli. J Mol Biol. 1978 Jun 15;122(1):109–112. doi: 10.1016/0022-2836(78)90111-0. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Langer J. A., Schoenborn B. P., Engelman D. M. Triangulation of proteins in the 30 S ribosomal subunit of Exherichia coli. J Mol Biol. 1977 May 15;112(2):199–227. doi: 10.1016/s0022-2836(77)80139-3. [DOI] [PubMed] [Google Scholar]
- Morgan J., Brimacombe R. A preliminary three-dimensional arrangement of the proteins in the Escherichia coli 30-S ribosomal sub-particle. Eur J Biochem. 1973 Sep 3;37(3):472–480. doi: 10.1111/j.1432-1033.1973.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Morrison C. A., Garrett R. A., Zeichhardt H., Stöffler G. Proteins occurring at, or near, the subunit interface of E. coli ribosomes. Mol Gen Genet. 1973 Dec 31;127(4):359–368. doi: 10.1007/BF00267106. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B., Garrett R. A., Littlechild J. Small-angle x-ray scattering study of the 16S RNA binding protein S4 from Escherichia coli ribosomes. FEBS Lett. 1977 Jan 15;73(1):25–28. doi: 10.1016/0014-5793(77)80007-0. [DOI] [PubMed] [Google Scholar]
- Osterberg R., Sjöberg B. Small-angle X-ray scattering study of the proteins S1, S8, S15, S16, S20 from Escherichia coli ribosomes. FEBS Lett. 1978 Sep 1;93(1):115–119. doi: 10.1016/0014-5793(78)80817-5. [DOI] [PubMed] [Google Scholar]
- Paradies H. H., Franz A. Geometry of the protein S4 from Escherichia coli ribosomes. Eur J Biochem. 1976 Aug 1;67(1):23–29. doi: 10.1111/j.1432-1033.1976.tb10627.x. [DOI] [PubMed] [Google Scholar]
- Rohde M. F., O'Brien S., Cooper S., Aune K. C. Physical properties of some ribosomal proteins in solution and evidence for molecular interactions between isolated ribosomal proteins. Biochemistry. 1975 Mar 11;14(5):1079–1087. doi: 10.1021/bi00676a031. [DOI] [PubMed] [Google Scholar]
- Serdyuk I. N. A method of joint use of electromagnetic and neutron scattering: a study of internal ribosomal structure. Methods Enzymol. 1979;59:750–775. doi: 10.1016/0076-6879(79)59123-x. [DOI] [PubMed] [Google Scholar]
- Serdyuk I. N., Grenader A. K. On the distribution and packing of RNA and protein ribosomes. Eur J Biochem. 1977 Oct 3;79(2):495–504. doi: 10.1111/j.1432-1033.1977.tb11833.x. [DOI] [PubMed] [Google Scholar]
- Serdyuk I. N., Zaccai G., Spirin A. S. Globular conformation of some ribosomal proteins in solution. FEBS Lett. 1978 Oct 15;94(2):349–352. doi: 10.1016/0014-5793(78)80974-0. [DOI] [PubMed] [Google Scholar]
- Sommer A., Traut R. R. Identification by diagonal gel electrophoresis of nine neighboring protein pairs in the Escherichia coli 30 S ribosome crosslinked with methyl-4-mercaptobutyrimidate. J Mol Biol. 1975 Oct 5;97(4):471–481. doi: 10.1016/s0022-2836(75)80054-4. [DOI] [PubMed] [Google Scholar]
- Stuhrmann H. B., Koch M. H., Parfait R., Haas J., Ibel K., Crichton R. R. Determination of the distribution of protein and nucleic acid in the 70 S ribosomes of Escherichia coli and their 30 S subunits by neutron scattering. J Mol Biol. 1978 Feb 25;119(2):203–212. doi: 10.1016/0022-2836(78)90434-5. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Heimark R. L., Traut R R. The protein topography of the E. coli 30S ribosomal subunit: a preliminary model E. coli/30S subunit/ribosome/spatial arrangement of proteins. Mol Cell Biochem. 1975 Jan 31;6(1):33–41. doi: 10.1007/BF01731864. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Traut R. R., Kahan L. Protein-protein proximity in the association of ribosomal subunits of Escherichia coli: crosslinking of 30 S protein S16 to 50 S proteins by glutaraldehyde or formaldehyde. J Mol Biol. 1974 Aug 15;87(3):509–522. doi: 10.1016/0022-2836(74)90101-6. [DOI] [PubMed] [Google Scholar]
- Tischendorf G. W., Zeichhardt H., Stöffler G. Architecture of the Escherichia coli ribosome as determined by immune electron microscopy. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4820–4824. doi: 10.1073/pnas.72.12.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliev V. D. Morphology of the ribosomal 30S subparticle according to electron microscopic data. Acta Biol Med Ger. 1974;33(5-6):779–793. [PubMed] [Google Scholar]
- Vasiliev V. D., Selivanova O. M., Koteliansky V. E. Specific selfpacking of the ribosomal 16 S RNA. FEBS Lett. 1978 Nov 15;95(2):273–276. doi: 10.1016/0014-5793(78)81009-6. [DOI] [PubMed] [Google Scholar]