Abstract
Objectives
This study evaluated the relationship between intracellular tenofovir diphosphate concentrations in peripheral blood mononuclear cells and prophylactic efficacy in a macaque model for HIV pre-exposure prophylaxis (PrEP).
Methods
Macaques were challenged with simian HIV (SHIV) via rectal inoculation once weekly for up to 14 weeks. A control group (n = 34) received no drug, a second group (n = 6) received oral tenofovir disoproxil fumarate/emtricitabine 3 days before each virus challenge and a third group (n = 6) received the same dosing plus another dose 2 h after virus challenge. PBMCs were collected just before each weekly virus challenge. The relationship between tenofovir diphosphate in PBMCs and prophylactic efficacy was assessed with a Cox proportional hazards model.
Results
The percentages of animals infected in the control, one-dose and two-dose groups were 97, 83 and 17, respectively. The mean (SD) steady-state tenofovir diphosphate concentration (fmol/106 cells) was 15.8 (7.6) in the one-dose group and 30.7 (10.1) in the two-dose group. Each 5 fmol tenofovir diphosphate/106 cells was associated with a 40% (95% CI 17%–56%) reduction in risk of SHIV acquisition, P = 0.002. The tenofovir diphosphate concentration associated with a 90% reduction in risk (EC90) was 22.6 fmol/106 cells (95% CI 13.8–60.8).
Conclusions
The prophylactic EC90 for tenofovir diphosphate identified in macaques exposed rectally compares well with the EC90 previously identified in men who have sex with men (MSM; 16 fmol/106 cells, 95% CI 3–28). These results highlight the relevance of this model to inform human PrEP studies of oral tenofovir disoproxil fumarate/emtricitabine for MSM.
Keywords: HIV pre-exposure prophylaxis, non-human primate model, intracellular pharmacology, pharmacodynamics, nucleoside analogues
Introduction
HIV pre-exposure prophylaxis (PrEP) involves the administration of antiretroviral therapy prior to potential HIV exposures to prevent HIV acquisition. It is intended for individuals at high risk of HIV infection, including injection drug users, men who have sex with men (MSM), sero-discordant couples and heterosexual adults. Traditional Phase 1–2 drug development for PrEP is challenging because efficacy is measured as the rate of HIV acquisition.1 The generally low rates of HIV infection (<1%–9%), even in high-risk populations, mean that large sample sizes are required for proof of concept and dose-ranging studies.1–7 For these reasons, non-human primate (NHP) and humanized mouse models are especially important in the development and evaluation of PrEP therapies.8,9 Animal models enable investigators to control the virus challenges and drug dosing, thereby providing a cost-effective way to advance the most promising PrEP regimens towards Phase 3 clinical trials.
NHP models were pivotal in the development, evaluation and eventual approval of oral tenofovir disoproxil fumarate/emtricitabine for PrEP.9 In particular, the repeat low virus dose macaque model was used extensively to measure the efficacy of different oral tenofovir disoproxil fumarate/emtricitabine regimens against mucosal transmission. These models demonstrated that daily and intermittent oral tenofovir disoproxil fumarate/emtricitabine provided high efficacy during weekly rectal virus challenges.10–12 This pre-clinical work provided proof of concept of efficacy for clinical trials in humans, including the iPrEx study in MSM and transgendered women.13 The iPrEx study randomized MSM at high risk of HIV infection to daily placebo versus tenofovir disoproxil fumarate/emtricitabine.4 The study demonstrated 44% efficacy for tenofovir disoproxil fumarate/emtricitabine versus placebo, but variable adherence appeared to play a major role in efficacy in this PrEP trial, as well as others.2–7 In iPrEx, a post hoc analysis was performed evaluating the relationship between intracellular tenofovir diphosphate, the pharmacologically active moiety of tenofovir, and HIV-1 risk reduction. The analysis identified a 90% effective concentration (EC90) value of 16 fmol/106 cells (95% CI 3–28) in viably cryopreserved peripheral blood mononuclear cells (PBMCs); this was the concentration associated with 90% reduction in risk of HIV acquisition compared with placebo.14 This type of information is important because it can help set target concentrations for studying alternative dosing regimens.
The availability of human efficacy and concentration–effect data such as that described for iPrEx provides an opportunity to evaluate how well the repeat low-dose macaque model correlates with the human results. Strong correlation would support using the NHP model and corresponding target concentrations for studying alternative dosing regimens. The aim of the present study was to estimate the concentration–effect relationship between intracellular tenofovir diphosphate and reduced risk of virus acquisition in the macaque rectal challenge model.
Methods
Study design
The methods and procedures for the repeat low-dose rectal challenge model in macaques were described previously.10–12 Briefly, anaesthetized adult rhesus macaques were inoculated once weekly for up to 14 weeks with SHIVSF162P3, a chimeric virus with tat, rev and env coding regions from HIV-1SF162 on the background of SIVmac239. Each weekly inoculation consisted of 10 TCID50 or 7.6 × 105 RNA copies. The virus was introduced atraumatically into the rectal vault with a gastric feeding tube. The animals remained anaesthetized and recumbent for 15 min after virus challenges. Blood for drug concentrations (described below) was collected at the time of each weekly virus challenge.
Two different tenofovir disoproxil fumarate/emtricitabine dosing strategies were evaluated in the macaques. One group of six macaques were given one tenofovir disoproxil fumarate/emtricitabine dose 3 days prior to each weekly virus challenge (−3 days). A second group of six macaques were given two tenofovir disoproxil fumarate/emtricitabine doses: one 3 days prior to each weekly virus exposure and another dose 2 h after each weekly virus exposure (−3 days/+2 h). All doses consisted of 20 mg/kg of emtricitabine and 22 mg/kg of tenofovir disoproxil fumarate, prepared in PBS buffer and delivered orally via a gastric tube to anaesthetized macaques. These doses, although ∼6-fold higher on an mg/kg basis, were shown previously to approximate human plasma concentrations obtained with 200 mg of emtricitabine and 300 mg of tenofovir disoproxil fumarate.11 A total of 34 control animals underwent the same once-weekly virus challenges using the same virus with the same procedures, but did not receive drug. The virus challenges and infection of the 34 control animals occurred between 2004 and 2010. Of the 34 controls, 20 were historical controls exposed and infected between 2004 and 2007, 11 were real-time controls for the −3 day/+2 h and −3 day studies performed in 2008/2009 and 3 were controls exposed and infected in 2010.11 All SHIV inoculations were prepared from the same NIH virus stock maintained in liquid nitrogen until use. The median time for infection in the real-time controls was 1 inoculation (min., max. = 1, 6) and the median time for infection in historical controls was 2 inoculations (min., max. = 1, 12) (P = 0.39, Mann–Whitney test). All procedures and animal care were reviewed and approved by the CDC Institutional Animal Care and Use Committee.
SHIV infection was assessed with weekly SHIV RNA assays using a real-time PCR assay with a sensitivity of 50 copies/mL.11 Virus-specific antibodies were tested using a synthetic peptide enzyme immunoassay. Animals in the PrEP arms were considered protected from systemic SHIV infection if they remained seronegative and negative for SHIV plasma RNA and SHIV DNA in PBMCs during PrEP and during the 70 days of washout in the absence of any drug treatment. An eclipse phase of 7 days was used and the time of infection was defined as the week before the first evidence of SHIV RNA positivity in plasma.
Viably cryopreserved PBMCs were processed from the blood that was collected from all treated macaques at the time of each weekly virus challenge. These viral challenge procedures generally lasted 40–60 min, during which time the blood was collected. The blood collections were the same time post-dose for all macaques (3 days after drug dosing); however, the −3 day/+2 h group had two doses per week whereas the −3 day group had just one dose per week.
Tenofovir diphosphate and emtricitabine triphosphate were measured in the viably cryopreserved PBMC samples using methods and procedures described previously.4,14,15 Briefly, the PBMC sample was thawed quickly in a 37°C water bath followed by rapid dilution with pre-warmed PBS. Red blood cells were lysed if contamination was visible. Cells were counted with an automated haemocytometer (Countess, Invitrogen) and viability and total cell count were recorded. Cells were washed and lysed with 70% methanol in water and stored at −80°C until LC-MS/MS analysis. Tenofovir diphosphate and emtricitabine triphosphate were quantified in the lysed cellular matrix using a validated LC-MS/MS assay, as described previously.15 The quantifiable linear range for tenofovir diphosphate was 2.5–2000 fmol per sample and that for emtricitabine triphosphate was 0.1–200 pmol per sample. Approximately 4 million total cells were extracted, constituting the sample, and results were reported as fmol or pmol per million viable cells.
Data analysis
The sample size of n = 6 for the dosing experiments was selected based on power calculation methods adapted from Regoes et al.16 This sample size maintains sufficient power (>80% power and 5% significance level) within a wide range of infection probabilities in treated animals and has been extensively used in PrEP evaluation with the repeat low-dose model as described here.11,17–19
The concentration–effect data were analysed using a Cox proportional hazards model with the Efron method for ties. Alternative approaches were evaluated for tied infection times (multiple animals infected at the same challenge week), including ‘exact’ methods,20 but this did not meaningfully change the results. The effect of tenofovir diphosphate concentration on the rate (hazard) of HIV infection for the kth challenge was modelled as:
where β represents the ln hazard ratio associated with the level of tenofovir diphosphate and λ0 (tk) represents the rate of HIV infection at the kth challenge in a control animal with no PrEP. The concentration of tenofovir diphosphate associated with a γ% reduction in risk of HIV infection was estimated as:
The results were evaluated for sensitivity to the log-linear link between the hazard function and tenofovir diphosphate concentration. Exploration using cubic splines did not result in a statistically significant improvement in model fit. All confidence intervals and P values were based on the Wald test. The same methods were also used to evaluate concentration–effect relationships with emtricitabine triphosphate.
Single and multiple imputations were applied to missing tenofovir diphosphate concentrations. The single imputation used a value predicted by the fit of a mono-exponential curve to the animal's existing data (see below). Multiple imputation started with the same initial fitted value but added as uncertainty a 30% coefficient of variation.
The drug concentrations were averaged for each study day then fitted to a mono-exponential curve [Ct = Css × (1 − e−K × t)] to estimate half-life using least squares regression with GraphPad Prism, where Ct is the averaged drug concentration at time t, Css is the fitted steady-state drug concentration and K is the fitted first-order elimination rate constant. The half-life was derived as ln(2)/K. For the pharmacokinetic analyses, drug concentrations that were below the limit of quantification were set to one half the lower limit of quantification (i.e. 0.05 pmol/sample). This was applied to emtricitabine triphosphate only.
Results
Virological outcomes
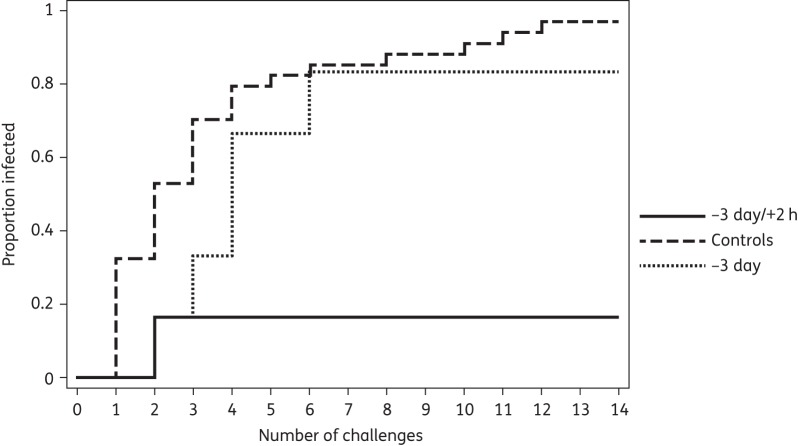
Thirty-three of 34 (97%) control animals acquired SHIV infection, 18 (55%) within the first two weekly inoculations. This compared with five out of six (83%) animals acquiring SHIV in the −3 day group, one (20%) at the second inoculation and four (80%) after the second inoculation. Virus inoculations were stopped in this group after seven exposures because of lack of efficacy in an interim analysis. Only one out of six (17%) animals in the −3 day/+2 h group acquired SHIV, at the second inoculation. The remaining five animals in this group remained seronegative and virus RNA and DNA negative after a total of 14 inoculations and the entire follow-up period. The Kaplan–Meier analysis is shown in Figure 1.
Figure 1.
Kaplan–Meier analysis of the proportion infected with SHIV in relation to number of inoculations (challenges) in the three groups of macaques.
Drug concentrations
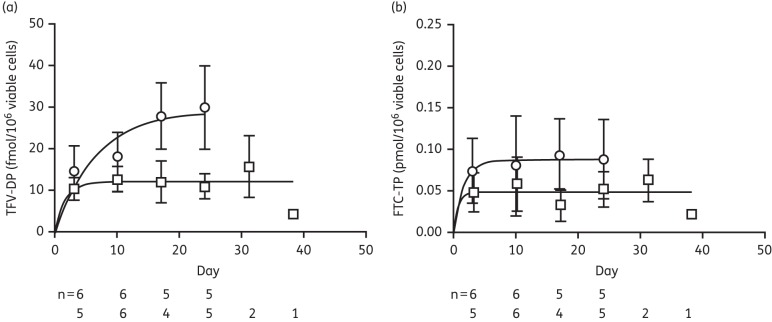
In the −3 day/+2 h group, the mean (SD) tenofovir diphosphate concentration increased ∼2-fold, from 14.9 (5.8) fmol/106 cells after week 1 (i.e. the first dose) to 30.7 (10.1) fmol/106 cells after 4 weeks. Concentrations in the −3 day group ranged from 10.4 (2.6) after dose 1 to 15.8 (7.6) fmol/106 cells at the 5th week (Figure 2a). No tenofovir diphosphate concentrations were below the limit of quantification (BLQ). For imputations, a single tenofovir diphosphate value of 16.9 was imputed at week 6 for the one uninfected animal in the −3 day group. For each of the five uninfected animals in the −3 day/+2 h group, 10 tenofovir diphosphate values were imputed to account for weeks 5–14 (a total of 50 imputations). The single imputations ranged from 17.4 to 50.5 fmol/106 cells. The multiple imputations added 30% variability to the single imputation values.
Figure 2.
Tenofovir diphosphate (TFV-DP) (a) and emtricitabine triphosphate (FTC-TP) (b) accumulation in viable PBMCs over time in groups given two doses per week (open circles) and one dose per week (open squares). Data points represent mean values and bars represent SDs, with the number of macaques contributing to the data shown below the graphs.
The mean (SD) emtricitabine triphosphate concentrations in the −3 day/+2 h group ranged from ∼0.075 (0.039) to 0.094 (0.043) pmol/106 cells, with evidence of a modest accumulation of drug (∼20%). Three of 22 emtricitabine triphosphate concentrations were BLQ. The concentrations in the −3 day group ranged from ∼0.049 (0.024) to 0.064 (0.026) pmol/106 cells with little or no evidence of drug accumulation (Figure 2b). Eight of 23 concentrations were BLQ. The mono-exponential regression fit to these means is also depicted. The estimated accumulation half-lives were ∼110 h for tenofovir diphosphate and 26 h for emtricitabine triphosphate. The median (IQR) viability of the cryopreserved PBMC samples was 71% (64%–75%).
Concentration–effect analysis
An analysis of tenofovir diphosphate that used single imputation (without adding 30% variability) demonstrated that each 5 fmol/106 cells of tenofovir diphosphate was associated with a 40% reduction in risk of SHIV acquisition (95% CI 22%–60%, P < 0.0001). The concentration associated with a 90% reduction in risk (EC90) was 22.6 (95% CI 12.7–47.5) fmol/106 cells. Multiple imputation gave similar estimates: each 5 fmol/106 cells of tenofovir diphosphate was associated with a 40% (95% CI 17%–56%) reduction in risk of SHIV acquisition (P = 0.002) and the EC90 was 22.6 (95% CI 13.8–60.8) fmol/106 cells. The relationship between SHIV risk reduction and tenofovir diphosphate concentration is depicted in Figure 3. For emtricitabine triphosphate, each 0.20 pmol/106 cells was associated with a 41% (95% CI 9%–61%) reduction in risk of SHIV acquisition (P = 0.0032). The concentration associated with a 90% reduction in risk (EC90) was 0.089 (95% CI 0.045–0.28) pmol/106 cells. However, only three animals that contracted SHIV had quantifiable emtricitabine triphosphate (0.03, 0.05 and 0.07 pmol/106 cells). In a model that combined both emtricitabine triphosphate and tenofovir diphosphate, neither was independently significant.
Figure 3.
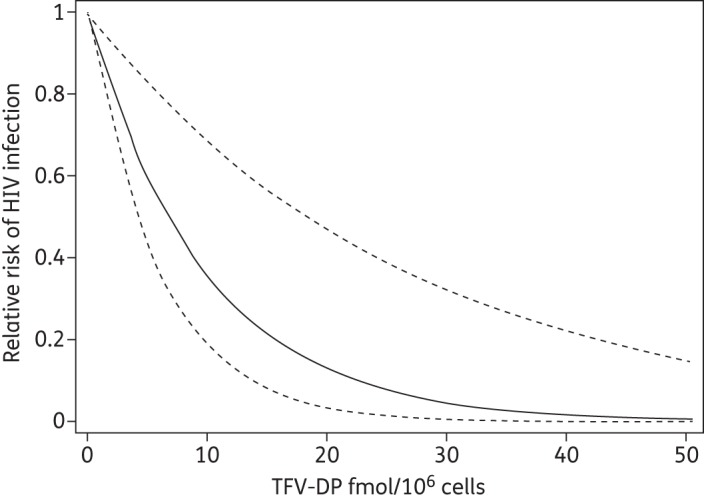
Relative risk of SHIV acquisition in relation to tenofovir diphosphate (TFV-DP) concentration (continuous line). Broken lines represent the 95% CI.
Discussion
This study identified a significant relationship between tenofovir diphosphate concentrations in viably cryopreserved PBMCs with risk of SHIV acquisition in an NHP model, such that each increase of 5 fmol/106 cells corresponded to a 40% reduction in SHIV infection risk. Although the superior efficacy in the −3/+2 group was explained by higher tenofovir diphosphate concentrations, the effect of dose timing relative to the viral challenge (i.e. the timing of the +2 h dose) could not be separated from that of the higher tenofovir diphosphate concentration. Further studies are needed to determine the independent effect of dose timing on efficacy.
An estimated EC90 of 22.6 (95% CI 13.8–60.8) fmol/106 cells was identified in this NHP model. This value compares well with the EC90 value estimated in participants in the iPrEx study of 16 (95% CI 3–28) fmol/106 cells.14 The cell samples and tenofovir diphosphate concentrations from both iPrEx and this study were processed and analysed in the same laboratory with the same procedures, assays and instruments. The median and IQR of PBMC viability were also very similar in this study (71%, 64%–75%) compared with the iPrEx analysis (66%, 56%–76%), further supporting the comparability of the data.14
HIV acquisition in MSM and this macaque model are comparable in other ways, relevant to these new findings. The repeated virus challenges to the rectal mucosa in the macaques were designed to simulate sexual activity over time in humans, such as might be expected in the iPrEx trial.4 The SHIV virion encodes an R5-tropic HIV envelope that resembles most transmitted HIV.21 The susceptibilities to tenofovir in cell systems are in the same range for SHIV (IC50 ∼0.3–1.7 μM) and HIV (IC50 ∼0.2–3.6 μM).22–25 Susceptibilities to emtricitabine are also similar for SHIV (IC50 ∼0.1 μM) and HIV (IC50 ∼0.4 μM).22,24 Values from cell-free assays for tenofovir diphosphate are also similar for SHIV (IC50 ∼0.3 μM) and HIV (IC50 ∼0.2–0.45 μM).22–24
In addition to these similarities, there are some important differences to consider.1 First, virus inoculations in the macaque model are atraumatic, the inocula are relatively homogeneous and exposures are done in the absence of semen. Second, the virus doses in the macaques (∼760 000 copies per dose) were slightly higher than HIV loads in human semen during acute HIV infection (∼32 000 copies/mL, although the volume of ejaculate determines the total virus load), such that macaque infection was ensured after a median of only two exposures as opposed to HIV acquisition in humans, which is generally less efficient.26 Modelling studies suggest that higher tenofovir diphosphate concentrations are needed for larger viral inoculum sizes.27 Future studies should evaluate the effect of varying viral inoculum sizes on the tenofovir diphosphate EC90.
This study was not designed to define tenofovir diphosphate and emtricitabine triphosphate pharmacokinetics in macaques, so an assumed first-order pharmacokinetic model was fitted to the averaged concentration over time (Figure 2). The fitted half-lives for tenofovir diphosphate and emtricitabine triphosphate were 110 and 26 h, respectively, similar to previous studies in macaques, as well as humans (60–160 h for tenofovir diphosphate and 30–50 h for emtricitabine triphosphate).11,28–33 However, the fitted steady-state tenofovir diphosphate concentrations in this study were moderately different from concentrations in humans. The results of the present study can be compared with human concentrations arising from the STRAND study, which evaluated two, four and seven tenofovir disoproxil fumarate doses per week of directly observed dosing in 24 HIV-negative volunteers.14 At steady state, tenofovir diphosphate was measured in viably cryopreserved PBMCs with the same procedures and assay as in this study. The corresponding median (IQR) tenofovir diphosphate concentrations in the STRAND study were 11 (6–13), 32 (25–39) and 42 (31–47) fmol/106 cells, respectively. An important difference between the studies was that in the two doses per week arm in STRAND the doses were given on two consecutive days (Tuesday and Wednesday) and the PBMCs were collected up to 140 h post-dose, whereas the present study separated the doses by 3 days, which will result in reduced fluctuation in concentrations.34 Nevertheless, it appears that the median (IQR) concentrations in macaques for two doses per week were about the same as the concentrations in humans for four doses per week: 30.9 (22.2–39.2) and 32 (25–39) fmol/106 cells, respectively. It is important to recognize that this study and the iPrEx/STRAND studies assayed tenofovir diphosphate/emtricitabine triphosphate from viably cryopreserved PBMCs.4,14 Traditionally, PBMC samples for intracellular drug analysis are immediately processed and lysed. Some drug loss and increased variability occurs with cell processing for viably cryopreserved cells.14 For these reasons, we suggest that additional studies be conducted to compare the cellular pharmacology of tenofovir/emtricitabine in macaques versus humans.
Emtricitabine triphosphate was also associated with antiviral effect in this study, but the effect was not independent of tenofovir diphosphate and fewer emtricitabine triphosphate concentrations were above the lower limit of quantification compared with tenofovir diphosphate, consistent with the shorter emtricitabine triphosphate half-life. This led to uncertainty in the emtricitabine triphosphate EC90 estimate, suggesting that further study is needed that focuses on the relationship of emtricitabine triphosphate with efficacy.
The present study did not include drug concentrations in the mucosal or lymphatic tissue, where initiation of SHIV and HIV infection likely takes place.35 It has been hypothesized that drug concentrations in mucosal tissues may better reflect prophylactic effect, particularly for topical administration, such as vaginal tenofovir gel or tenofovir disoproxil fumarate vaginal ring dosing.36–40 For vaginal tenofovir dosing, several human and macaque studies have identified concentration–effect relationships. A cervicovaginal tenofovir concentration of ≥1000 ng/mL was associated with protection against HIV or SHIV acquisition.38–40 Studies in macaques have identified an EC90 for tenofovir diphosphate in vaginal lymphocytes of ∼700–900 fmol/106 cells.39,40 However, the need for these vaginal tenofovir concentrations with oral dosing is less clear.36,41 For example, the vaginal drug concentrations achieved by oral dosing are ∼100-fold lower than those from topical gels and far below the thresholds 1000 ng/mL and 700–900 fmol/106 cells achieved with gel,30,42 but oral tenofovir disoproxil fumarate was shown to be highly effective in women,43 suggesting an important role for the higher systemic drug concentrations achieved with oral dosing in women.
Both tenofovir and tenofovir diphosphate accumulate in rectal tissue and rectal mononuclear cells following oral dosing in macaques and humans at levels higher than those achieved in PBMCs.11,30,44,45 As one example in humans, steady-state tenofovir diphosphate was 1846 fmol/106 cells in rectal mononuclear cells versus 98 fmol/106 cells in PBMCs among 17 HIV-negative individuals receiving daily oral tenofovir disoproxil fumarate/emtricitabine for 30 days (note that cells were immediately processed and freshly lysed in this study).30 This PBMC concentration (98 fmol/106 cells) is ∼2.5-fold higher than the estimated EC90 from iPrEx, which was estimated at 40 fmol/106 cells for freshly lysed cells (and 16 fmol/106 for viable cells).14 The corresponding rectal mononuclear cell concentration is 738 fmol/106 cells (1846 fmol/106 cells/2.5), which is similar to the value identified in vaginal tissue cells from macaques.39 A similar value was also identified using an ex vivo tissue infectivity approach in humans.46 Taken together, these findings suggest that oral dosing provides high tenofovir diphosphate concentrations both systemically as well as in rectal tissue, providing a two-pronged barrier to HIV and SHIV infection. Further studies are needed to better understand the importance of route of administration and tissue concentrations on PrEP efficacy.
In conclusion, this study found a similar EC90 for tenofovir diphosphate in macaques compared with humans (23 versus 16 fmol/106 cells) for prevention of rectal SHIV/HIV acquisition. Additional studies are needed to validate this finding and to extend the research to macaque models of vaginal transmission and to other animal models in the field that were not evaluated in this study, such as RAG-hu and humanized BLT mice.1,47,48 Future work should attempt to demonstrate that the pharmacological conditions achieved in the animal model can be (or are) achieved in humans. Such studies can help support these models, which play an important role in the development and evaluation of PrEP therapies.
Funding
This work was supported by NIH U01AI84735, CDC intramural funds and Interagency Agreement Y1-Al-0681-02 between CDC and NIH.
Transparency declarations
P. L. A. receives study drug from Gilead Sciences for research protocols. CDC (W. H. and J. G. G.-L.) received tenofovir disoproxil fumarate and emtricitabine from Gilead through a Material Transfer Agreement. D. V. G. and L. R. B.: none to declare.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention or NIH.
Acknowledgements
We wish to thank L. Anthony Guida, Brandon Klein, Jia-Hua Zheng, Mian-er Cong and Qi Zheng for analytical contributions to the work.
References
- 1.Romano J, Kashuba A, Becker S, et al. Pharmacokinetics and pharmacodynamics in HIV prevention; current status and future directions: a summary of the DAIDS and BMGF sponsored think tank on pharmacokinetics (PK)/pharmacodynamics (PD) in HIV prevention. AIDS Res Hum Retroviruses. 2013;29:1418–27. doi: 10.1089/aid.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 6.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367:411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381:2083–90. doi: 10.1016/S0140-6736(13)61127-7. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Lerma JG, Paxton L, Kilmarx PH, et al. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol Sci. 2010;31:74–81. doi: 10.1016/j.tips.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 9.García-Lerma JG, Heneine W. Animal models of antiretroviral prophylaxis for HIV prevention. Current Opinion in HIV and AIDS. 2012;7:505–13. doi: 10.1097/COH.0b013e328358e484. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Lerma J, Cong M, Masciotra S, et al. Intermittent pre-exposure prophylaxis (PrEP) with oral Truvada protects macaques against repeated rectal SHIV exposures; Abstracts of the Third International Workshop on HIV Transmission, Mexico City, Mexico, 2008 Abstract 40. [Google Scholar]
- 11.Garcia-Lerma JG, Cong ME, Mitchell J, et al. Intermittent prophylaxis with oral Truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2:14ra4. doi: 10.1126/scitranslmed.3000391. [DOI] [PubMed] [Google Scholar]
- 12.Subbarao S, Otten RA, Ramos A, et al. Chemoprophylaxis with tenofovir disoproxil fumarate provided partial protection against infection with simian human immunodeficiency virus in macaques given multiple virus challenges. J Infect Dis. 2006;194:904–11. doi: 10.1086/507306. [DOI] [PubMed] [Google Scholar]
- 13.Grant RM, Wainberg MA. Chemoprophylaxis of HIV infection: moving forward with caution. J Infect Dis. 2006;194:874–6. doi: 10.1086/507314. [DOI] [PubMed] [Google Scholar]
- 14.Anderson PL, Glidden D, Liu A, et al. Emtricitabine-tenofovir exposure and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012;4:151ra125. doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bushman LR, Kiser JJ, Rower JE, et al. Determination of nucleoside analog mono-, di-, and tri-phosphates in cellular matrix by solid phase extraction and ultra-sensitive LC-MS/MS detection. J Pharm Biomed Anal. 2011;56:390–401. doi: 10.1016/j.jpba.2011.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regoes RR, Longini IM, Jr, Feinberg MB, et al. Preclinical assessment of HIV vaccines and microbicides by repeated low-dose virus challenges. PLoS Med. 2005;2:e249. doi: 10.1371/journal.pmed.0020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Lerma JG, Aung W, Cong ME, et al. Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors. J Virol. 2011;85:6610–7. doi: 10.1128/JVI.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Lerma JG, Otten RA, Qari SH, et al. Prevention of rectal SHIV transmission in macaques by daily or intermittent prophylaxis with emtricitabine and tenofovir. PLoS Med. 2008;5:e28. doi: 10.1371/journal.pmed.0050028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massud I, Aung W, Martin A, et al. Lack of prophylactic efficacy of oral maraviroc in macaques despite high drug concentrations in rectal tissues. J Virol. 2013;87:8952–61. doi: 10.1128/JVI.01204-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Therneau TM, Grambsch PM. eds. Modeling Survival Data: Extending the Cox Model. Statistics for Biology and Health. New York: Springer; 2000. [Google Scholar]
- 21.Harouse JM, Gettie A, Eshetu T, et al. Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIVSF162P3. J Virol. 2001;75:1990–5. doi: 10.1128/JVI.75.4.1990-1995.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cihlar T, Ray AS, Boojamra CG, et al. Design and profiling of GS-9148, a novel nucleotide analog active against nucleoside-resistant variants of human immunodeficiency virus type 1, and its orally bioavailable phosphonoamidate prodrug, GS-9131. Antimicrob Agents Chemother. 2008;52:655–65. doi: 10.1128/AAC.01215-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivas RV, Fridland A. Antiviral activities of 9-R-2-phosphonomethoxypropyl adenine (PMPA) and bis(isopropyloxymethylcarbonyl)PMPA against various drug-resistant human immunodeficiency virus strains. Antimicrob Agents Chemother. 1998;42:1484–7. doi: 10.1128/aac.42.6.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cong ME, Youngpairoj AS, Zheng Q, et al. Protection against rectal transmission of an emtricitabine-resistant simian/human immunodeficiency virus SHIV162p3 M184 V mutant by intermittent prophylaxis with Truvada. J Virol. 2011;85:7933–6. doi: 10.1128/JVI.00843-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Witvrouw M, Pannecouque C, Switzer WM, et al. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir Ther. 2004;9:57–65. [PubMed] [Google Scholar]
- 26.Pilcher CD, Joaki G, Hoffman IF, et al. Amplified transmission of HIV-1: comparison of HIV-1 concentrations in semen and blood during acute and chronic infection. AIDS. 2007;21:1723–30. doi: 10.1097/QAD.0b013e3281532c82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duwal S, Schutte C, von Kleist M. Pharmacokinetics and pharmacodynamics of the reverse transcriptase inhibitor tenofovir and prophylactic efficacy against HIV-1 infection. PLoS ONE. 2012;7:e40382. doi: 10.1371/journal.pone.0040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J, Flexner C, Liberman RG, et al. Biphasic elimination of tenofovir diphosphate and nonlinear pharmacokinetics of zidovudine triphosphate in a microdosing study. JAIDS. 2012;61:593–9. doi: 10.1097/QAI.0b013e3182717c98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baheti G, Kiser JJ, Havens PL, et al. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5294–9. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson PL, Meditz A, Zheng JH, et al. Cellular pharmacology of tenofovir and emtricitabine in blood, rectal, and cervical cells from HIV-negative volunteers; Alexandria, VA, USA: Foundation for Retrovirology and Human Health; Abstracts of the Nineteenth Conference on Retroviruses and Opportunistic Infections, Seattle, WA, 2012. Abstract 587. [Google Scholar]
- 31.Wang LH, Begley J, St Claire RL, 3rd, et al. Pharmacokinetic and pharmacodynamic characteristics of emtricitabine support its once daily dosing for the treatment of HIV infection. AIDS Res Hum Retroviruses. 2004;20:1173–82. doi: 10.1089/aid.2004.20.1173. [DOI] [PubMed] [Google Scholar]
- 32.Jackson A, Moyle G, Watson V, et al. Tenofovir, emtricitabine intracellular and plasma, and efavirenz plasma concentration decay following drug intake cessation: implications for HIV treatment and prevention. JAIDS. 2013;62:275–81. doi: 10.1097/QAI.0b013e3182829bd0. [DOI] [PubMed] [Google Scholar]
- 33.Radzio J, Aung W, Holder A, et al. Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS ONE. 2012;7:e50632. doi: 10.1371/journal.pone.0050632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson PL, Kiser JJ, Gardner EM, et al. Pharmacological considerations for tenofovir and emtricitabine to prevent HIV infection. J Antimicrob Chemother. 2011;66:240–50. doi: 10.1093/jac/dkq447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–39. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 36.Hendrix CW. Exploring concentration response in HIV pre-exposure prophylaxis to optimize clinical care and trial design. Cell. 2013;155:515–8. doi: 10.1016/j.cell.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Thompson CG, Cohen MS, Kashuba AD. Antiretroviral pharmacology in mucosal tissues. JAIDS. 2013;63(Suppl 2):S240–7. doi: 10.1097/QAI.0b013e3182986ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim SS, Kashuba AD, Werner L, et al. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279–81. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dobard C, Sharma S, Martin A, et al. Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue. J Virol. 2012;86:718–25. doi: 10.1128/JVI.05842-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith JM, Rastogi R, Teller RS, et al. Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci USA. 2013;110:16145–50. doi: 10.1073/pnas.1311355110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baeten J, Celum C. Systemic and topical drugs for the prevention of HIV infection: antiretroviral pre-exposure prophylaxis. Annu Rev Med. 2013;64:219–32. doi: 10.1146/annurev-med-050911-163701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS ONE. 2013;8:e55013. doi: 10.1371/journal.pone.0055013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS. 2013;27:2155–60. doi: 10.1097/QAD.0b013e3283629037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Louissaint NA, Cao YJ, Skipper PL, et al. Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue. AIDS Res Hum Retroviruses. 2013;29:1443–50. doi: 10.1089/aid.2013.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patterson KB, Prince HA, Kraft E, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3:112re4. doi: 10.1126/scitranslmed.3003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anton PA, Cranston RD, Kashuba A, et al. RMP-02/MTN-006: a phase 1 rectal safety, acceptability, pharmacokinetic, and pharmacodynamic study of tenofovir 1% gel compared with oral tenofovir disoproxil fumarate. AIDS Res Hum Retroviruses. 2012;28:1412–21. doi: 10.1089/aid.2012.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neff CP, Ndolo T, Tandon A, et al. Oral pre-exposure prophylaxis by anti-retrovirals raltegravir and maraviroc protects against HIV-1 vaginal transmission in a humanized mouse model. PLoS ONE. 2010;5:e15257. doi: 10.1371/journal.pone.0015257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Denton PW, Estes JD, Sun Z, et al. Antiretroviral pre-exposure prophylaxis prevents vaginal transmission of HIV-1 in humanized BLT mice. PLoS Med. 2008;5:e16. doi: 10.1371/journal.pmed.0050016. [DOI] [PMC free article] [PubMed] [Google Scholar]