Abstract
Vision impairment and blindness due to the loss of the light-sensing cells of the retina, i.e. photoreceptors, represents the main reason for disability in industrialized countries. Replacement of degenerated photoreceptors by cell transplantation represents a possible treatment option in future clinical applications. Indeed, recent preclinical studies demonstrated that immature photoreceptors, isolated from the neonatal mouse retina at postnatal day 4, have the potential to integrate into the adult mouse retina following subretinal transplantation. Donor cells generated a mature photoreceptor morphology including inner and outer segments, a round cell body located at the outer nuclear layer, and synaptic terminals in close proximity to endogenous bipolar cells. Indeed, recent reports demonstrated that donor photoreceptors functionally integrate into the neural circuitry of host mice. For a future clinical application of such cell replacement approach, purified suspensions of the cells of choice have to be generated and placed at the correct position for proper integration into the eye. For the enrichment of photoreceptor precursors, sorting should be based on specific cell surface antigens to avoid genetic reporter modification of donor cells. Here we show magnetic-associated cell sorting (MACS) - enrichment of transplantable rod photoreceptor precursors isolated from the neonatal retina of photoreceptor-specific reporter mice based on the cell surface marker CD73. Incubation with anti-CD73 antibodies followed by micro-bead conjugated secondary antibodies allowed the enrichment of rod photoreceptor precursors by MACS to approximately 90%. In comparison to flow cytometry, MACS has the advantage that it can be easier applied to GMP standards and that high amounts of cells can be sorted in relative short time periods. Injection of enriched cell suspensions into the subretinal space of adult wild-type mice resulted in a 3-fold higher integration rate compared to unsorted cell suspensions.
Keywords: Medicine, Issue 84, Photoreceptor Cells, Vertebrate, Retinal Degeneration, Regeneration, retina, magnetic associated cell sorting (MACS), transplantation, regenerative therapy
Introduction
Vision is one of the prime senses of humans. Impairment of this sense and blindness are one of the main reasons for disability in industrialized countries. The predominant cause for vision impairment or blindness is retinal degeneration, characterized by photoreceptor cell loss, as it can be observed in macular degeneration, retinitis pigmentosa, cone-rod dystrophy, and other conditions. To date, an effective therapy to restore lost vision is not available. In 2006 and 2008 two different labs reported, independent from each other, a successful transplantation of rod photoreceptor precursor cells into adult wild-type mice retinas1,2. Thus, arising the possibility of photoreceptor precursor cell transplantation also into a degenerated retina, to replace degenerated photoreceptors and restore vision. Indeed, it has been demonstrated recently, that such transplanted photoreceptor precursor cells elicit morphological criteria of mature wild-type photoreceptors, such as properly developed outer segments3, synaptic terminals in close proximity to endogenous bipolar cells and a round cell body located in the outer nuclear layer2-4, as well as the ability to integrate functionally into the host neural circuitry5-7. One of the main principles of this strategy is the use of post-natal day 4 (PN 4, PN0 is defined as day of birth) young mice retinas, resulting in a mixture of different cell types for transplantation. On the background of a future therapeutic application, this mixture has to be purified for photoreceptor precursor cells. CD73 has been described as the first cell surface marker specific for young photoreceptors in the retina8-10. Here, we demonstrate a photoreceptor precursor cell purification method based on this cell surface marker and with the use of the magnetic-associated cell sorting (MACS) technique. MACS might have advantages in comparison to fluorescent-activated cell sorting techniques, due to fast sorting times and the easier adjustment to GMP conditions. We could demonstrate a ~90% enrichment and an up to 3-fold higher integration rate when transplanting the enriched population to the subretinal space in adult wild-type retinas. Thus, MACS-based photoreceptor precursor cell enrichment and subretinal transplantation, are reliable and promising techniques for the development of a regenerative therapeutic strategy for the treatment of retinal degeneration.
Protocol
Ethical use and care of animals statement:
All animal experiments were carried out in strict accordance with European Union and German laws (Tierschutzgesetz) and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animal experiments were approved by the animal ethics committee of the TU Dresden and the Landesdirektion Dresden (approval number: 24D-9168.11-1/2008-33).
1. Before Starting Cell Dissociation and Cell Sorting
Label three 15 ml reaction tubes with: Wash (W), Positive fraction (+) and Negative fraction (-).
2. Retina Dissociation
Decapitate the PN 4 pups using a scissor.
Rinse the head of the pups shortly in 70% ethanol followed by washing in PBS.
Transfer the head to cold HBSS and enucleate the eye (repeat this step for all of the heads). To enucleate, open the eyelids and fixate the eye with curved forceps at the optic nerve region. Then pull the eye carefully out of the orbit.
After enucleating all the eyes, isolate the retina: introduce a closed scissor into the optic nerve and open the blades. Peal the RPE/chorid out. Remove the lenses and blood vessels using curved forceps.
Transfer the isolated retinas into a 1.5 ml reaction tube containing the papain solution (provided by the papain dissociation kit).
- Incubate the retinas in the papain solution for 30-60 min in a water bath or shaker (400 rpm) at 37 °C.
- While retinas incubate in the papain solution, pipette 1 ml of ovomucoid solution to a 15 ml reaction tube (label "1").
- Add 60 µl of DNase I (10 mg/ml) + 60 µl of ovomucoid solution (provided by the kit) + 520 µl of EBSS to a 15 ml reaction tube (label "2").
After papain incubation, transfer the papain solution containing the partially digested retinas to reaction tube "2".
Using a fire polished pipette, perform mechanical dissociation (10x up and down).
Pipette the single cell suspension to reaction tube "1". Try to generate 2 layers by gently layering the cell suspension on top of the ovomucoid solution.
Centrifuge for 5 min at 300 x g (approximately 1,300 rpm).
Discard the supernatant and resuspend the cells in 500 µl of MACS buffer.
3. Cell Sorting Using Magnetic Associated Cell Sorting (MACS)
Add X μl rat anti-CD73 antibody to the 500 µl in order to achieve a final concentration of 10 μg/ml.
Incubate 5 min on ice.
Fill the 15 ml reaction tube to 10 ml with MACS buffer and centrifuge it for 5 min at a speed of 300 x g.
Remove the supernatant.
Resuspend the pellet in 480 μl MACS buffer and add 120 μl of goat anti-rat IgG microbeads.
Incubate 15 min on ice; do not agitate, shake or mix it.
Fill the 15 ml reaction tube to 5 ml with MACS buffer and centrifuge it for 5 min at 300 x g.
- As the reaction tube is been centrifuged:
- Adjust an LS Column into the magnetic stand.
- Put the preseparation filter on top of the LS Column.
- Hydrate the preseparation filter and the LS Column with 3 ml MACS buffer and collect the MACS buffer into the wash (W) tube.
Remove the supernatant.
Resuspend the pellet in 500 μl MACS buffer.
Load the 500 μl cell suspension to the filter, then add 1 ml MACS buffer to the filter and collect the negative fraction into the negative fraction (-) reaction tube.
Next, add 3 x 3 ml of MACS buffer to the column to wash off the cells that are not bound to the column
Once these 9 ml are through the LS Column, remove the column from the magnetic stand and install it on top of the positive fraction (+) reaction tube.
Quickly load 5 ml of MACS buffer into the LS Column and put the plunger in the LS Column and press it down until the entire buffer is through the column.
Centrifuge the reaction tube containing the positive fraction for 5 min at 300 x g.
Remove the supernatant, resuspend in 500 µl of MACS buffer and keep at 4 °C.
Count the total amount of cells using a common cell counting device.
Prepare a cell suspension from the positive sorted fraction containing 2 x 105 cells/µl in MACS buffer and keep at 4 °C
4. Transplantation of MAC-sorted Photoreceptor Precursor Cells into the Mouse Retina
Make sure to have all of the following solutions ready: 1 ml sterile PBS or HBSS, 10 µl DNAse I, 1-2 ml sterile deionized H2O, aliquots of the cell suspension fractions at 4 °C.
Anesthetize the adult mouse (i.e. 2-4 months old) with an intraperitoneal injection of medetomidine hydrochloride (0.01 mg/10 g body weight), ketamine (0.75 mg/10 g body weight). Then do a subcutaneous injection of Buprenorphine (0.05 mg/kg body weight) for pain relief.
Dilate pupils with a drop of Phenylephrin 2.5%-Tropicamid 0.5%.
Fix mouse in mouse head holder and place under stereo microscope.
Apply a drop of Visidic gel to prevent drying of the eye.
Make a small hole at the border between sclera and cornea (respectively ora serrata) using a sterile 30 G ½ in needle.
Cut a common cover slide into small (approximately 5 mm x 5 mm) pieces using a diamond pen, place one of these pieces on top of the cornea, allowing direct visualization of the retina.
Flush the presterilized microliter syringe several times with deionized water.
Load the microliter syringe with 1 µl of cell suspension, direct needle tangentially through the conjunctiva and sclera and place under visual control in the nasal half of the retina.
Punch gently a hole into the retina until reaching the subretinal space, inject the cell suspension into the subretinal space.
Observe the bullous detachment of retina, which should be uniform, covering approximately ¼ of the retinal space without any bleeding.
Withdraw the syringe gently, the retinal hole seals automatically.
Flush the microliter syringe several times with deionized water.
Release mouse from head holder.
Wake up mouse by injecting atipamozole hydrochloride for reversal of the medetomidine hydrochloride effect.
Place mouse for wake up time in warm dark chamber (approximately 25 °C).
The next 2 days after transplantation, Buprenorphine (0.05 mg/kg of body weight) should be administered by subcutaneous injection in the morning and in the afternoon for pain relief.
Representative Results
In order to assess the ability of rod photoreceptors to integrate into the mouse retina, a mouse reporter line was used, in which GFP is driven by the neural retina leucine zipper (Nrl, Nrl-GFP) promoter11. Nrl is the earliest marker of rod photoreceptors starting its expression at E12.5 throughout adulthood, allowing a specific labeling of donor rod photoreceptor cells.
PN 4 Nrl-GFP pups were decapitated and eyes were enucleated. Retinas were then isolated and dissociated using the method described above. The resulting cell suspension was then sorted using CD73-based MACS (Figures 1 and 2). Following MAC sorting, we analyzed the enrichment achieved during this procedure.
As shown in Figure 3A, the initial cell suspension (Input) contained 30.4% GFP-positive cells, i.e. rod photoreceptors. Following CD73-based MAC-sorting, an enrichment of up to 86.9% in the CD73-positive fraction (CD73+) was observed by flow cytometry. In the CD73-negative fraction (CD73-) only 9.9% of all cells were positively detected for GFP (Figure 3A). The enrichment of Nrl-GFP positive cells following CD73-based MACS is additionally visualized after plating in vitro (Figure 3B).
After MAC-sorting, donor cells in the CD73-positive fraction were spun down and resuspended to a final concentration of 200,000 cells/µl. This cell suspension was further used for transplantation into the subretinal space of wild-type host retinas (Figure 4). Three to four weeks following transplantation, the host retinas were fixed, isolated and sectioned. Several donor cells integrated into the outer nuclear layer of the hosts and acquired the morphology of mature photoreceptors with localization of the cell body in the outer nuclear layer and formation of synaptic spherules and inner segments (Figure 5). Detailed pictures have been published before by our group3,8 showing an outcome comparable with FAC-sorted cells transplanted to host retinae by other groups4,6,10. Consistent in all these studies is, that the transplanted cells stay at the site of injection, defined by the bullous detached host retina, and do not migrate away. Some integrate into the host retina (i.e. outer nuclear layer), and some remain in the subretinal space3. Additionally, it has been shown, that the distribution of donor cells at the site of integration depends on the type of mouse model used5. No acute host/graft rejection signs were detected in transplantations between C57BL/6J mice.
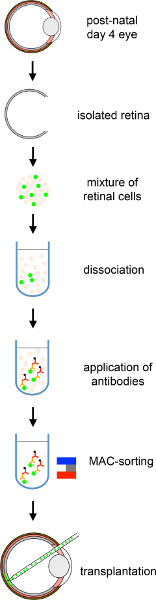 Figure 1. Overall scheme of subretinal transplantation of MACS purified photoreceptor precursors into adult mouse retina. Donor cells from PN 4 Nrl-GFP pups are isolated, followed by CD73-based MAC-sorting and transplanted into the subretinal space of mouse host retinas. Click here to view larger figure.
Figure 1. Overall scheme of subretinal transplantation of MACS purified photoreceptor precursors into adult mouse retina. Donor cells from PN 4 Nrl-GFP pups are isolated, followed by CD73-based MAC-sorting and transplanted into the subretinal space of mouse host retinas. Click here to view larger figure.
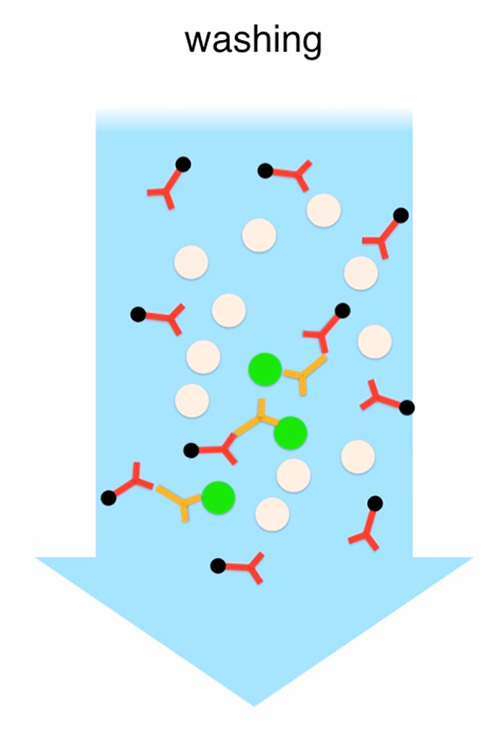 Figure 2. Enrichment of transplantable photoreceptors by MACS. The cell suspension generated from all collected PN4 retinas is incubated with primary rat anti-CD73 antibodies followed by washing and incubation with anti-rat antibodies conjugated with microbeads. Unbound antibodies are washed away. The cell suspension is passed through a LS-column that is connected with a magnetic stand. Cells that are bound by antibodies stay attached to the column while the remaining cells are eluted and collected (CD73-negative fraction). Finally, the LS-column is removed from the magnetic stand and the remaining cells are eluted and collected (CD73-positive fraction). Click here to view full movie.
Figure 2. Enrichment of transplantable photoreceptors by MACS. The cell suspension generated from all collected PN4 retinas is incubated with primary rat anti-CD73 antibodies followed by washing and incubation with anti-rat antibodies conjugated with microbeads. Unbound antibodies are washed away. The cell suspension is passed through a LS-column that is connected with a magnetic stand. Cells that are bound by antibodies stay attached to the column while the remaining cells are eluted and collected (CD73-negative fraction). Finally, the LS-column is removed from the magnetic stand and the remaining cells are eluted and collected (CD73-positive fraction). Click here to view full movie.
 Figure 3. Analysis of Nrl-GFP positive cell enrichment following CD73-based MAC-sorting. (A) Before sorting, the initial population of donor cells contained 30.4% of Nrl-GFP positive cells. Following MAC-sorting, an enrichment of GFP-positive cells could be detected in the CD73+ fraction (86.9%) while the CD73- fraction contained only low amounts of GFP+ cells (9.9%). (B) Representative image demonstrating the result of CD73-based MAC-sorting of PN4 Nrl-GFP cells. 1 million cells from every fraction were plated on laminin-coated coverslips. Cells were fixed 4 hr after plating with 4% PFA for 10 min. Cells were stained with DAPI (4',6-diamidino-2-phenylindole, 1:20,000). A significant enrichment of GFP-positive cells in the CD73+ fraction was observed when compared to the input fraction or the CD73- fraction. Click here to view larger figure.
Figure 3. Analysis of Nrl-GFP positive cell enrichment following CD73-based MAC-sorting. (A) Before sorting, the initial population of donor cells contained 30.4% of Nrl-GFP positive cells. Following MAC-sorting, an enrichment of GFP-positive cells could be detected in the CD73+ fraction (86.9%) while the CD73- fraction contained only low amounts of GFP+ cells (9.9%). (B) Representative image demonstrating the result of CD73-based MAC-sorting of PN4 Nrl-GFP cells. 1 million cells from every fraction were plated on laminin-coated coverslips. Cells were fixed 4 hr after plating with 4% PFA for 10 min. Cells were stained with DAPI (4',6-diamidino-2-phenylindole, 1:20,000). A significant enrichment of GFP-positive cells in the CD73+ fraction was observed when compared to the input fraction or the CD73- fraction. Click here to view larger figure.
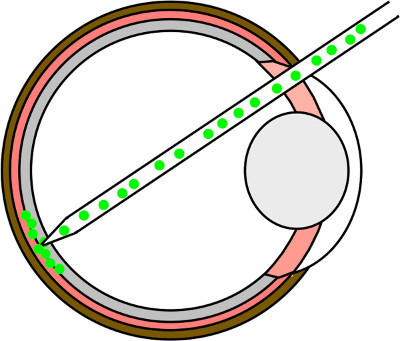 Figure 4. Subretinal injection. Schematic drawing of the transplantation process into the adult mouse retina. The needle is inserted through a hole in the ora serrata, navigated through the vitreal space by avoiding touching the lens and placed at the retina under visual control. By gentle pushing, the needle is punched through the retina and placed in the subretinal space. Eventually, the cell suspension is injected carefully under visual control by evaluating the detachment process.
Figure 4. Subretinal injection. Schematic drawing of the transplantation process into the adult mouse retina. The needle is inserted through a hole in the ora serrata, navigated through the vitreal space by avoiding touching the lens and placed at the retina under visual control. By gentle pushing, the needle is punched through the retina and placed in the subretinal space. Eventually, the cell suspension is injected carefully under visual control by evaluating the detachment process.
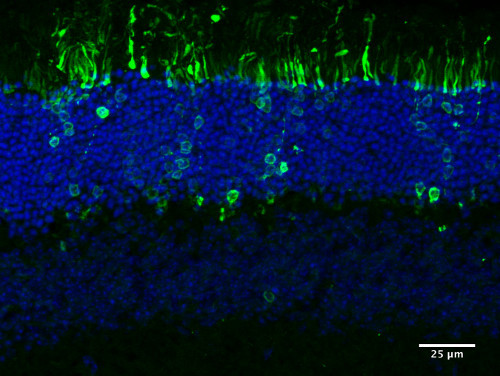 Figure 5. Integration of transplanted photoreceptor cells. Representative picture depicting integrated CD73-based MACS sorted PN4 Nrl-GFP cells following transplantation into the adult mouse retina. Transplanted photoreceptor precursors correctly integrate into the outer nuclear layer (ONL) and generate mature photoreceptor morphology.
Figure 5. Integration of transplanted photoreceptor cells. Representative picture depicting integrated CD73-based MACS sorted PN4 Nrl-GFP cells following transplantation into the adult mouse retina. Transplanted photoreceptor precursors correctly integrate into the outer nuclear layer (ONL) and generate mature photoreceptor morphology.
Discussion
Subretinal transplantation of photoreceptor precursor cells represents a reliable tool to achieve integration of these light-sensitive cells into host retinas in significant numbers1,2. This might allow the establishment of a cell therapy for the treatment of retinal degenerative diseases in future6. The donor population of cells, currently isolated from PN 4 retinas, is a mixture of different cell types, from which only the photoreceptor precursor cells integrate after subretinal injection. By using CD73-based MAC-sorting, the proportion of photoreceptors within the donor cell suspension can be increased to ≈ 90% that allows an approximately 3-fold higher integration rate in wild-type host retinas when compared to the unsorted cell population8. In comparison to flow cytometry, MACS has the advantage of a fast sorting procedure and that it can be relatively easy applied to GMP standards. The purification of transplantable photoreceptors is of specific interest in light of recent advantages for the in vitro generation of photoreceptors from pluripotent stem cells12-14. Cultures of differentiated pluripotent stem cells are composed of diverse cell-types making the availability of specific sorting procedures an essential prerequisite for their future use in cell transplantation therapies.
According to the MACS-purification procedure, several points are noteworthy, ensuring a workflow without major disturbances. The higher the number of retinas to be sorted, the more time is needed for their digestion. After digestion, during the sorting procedure, aggregation of cells has to be avoided. This occurs preferably before addition of the secondary antibody and during its incubation. In this case, the aggregate has to be removed immediately with a pipette. During the elution of MACS buffer while collecting the MACS-negative fraction, it is absolutely necessary to not add more than 3 ml to the column. The pressure of the buffer to the column will be too high if more buffer than 3 ml is added. This will disturb the binding of the antibody to the cells and lowers sorting efficiency. When removing the column from the magnetic stand to start collecting the MACS-positive fraction, this step has to be done quickly. The 5 ml of MACS buffer should be added to the column as fast as possible. Also, the plunge should be put and pressed quickly until the entire buffer is through the column. It is not necessary to worry about the applied pressure. The MACS buffer is isotonic and can be varied. It is possible to use FACS buffer, cell culture medium, PBS, EBSS, or HBSS. Sterility of the buffer can be achieved by filtering through a 0.4 µm filter membrane.
After enrichment by MACS, a successful transplantation of donor cells to the subretinal space can be achieved by keeping the following points in mind. Since photoreceptor cells seem to be very sensitive to treatment ex vivo, it is not advisable to keep them on 4 °C longer than necessary. Proceed for transplantation as fast as possible. When inserting the injection needle into the eyeball, it is important to avoid touching the lens as the relatively sharp metal needle might damage the lens resulting in induction of lens-induced uveitis. The last step on the way to the subretinal space, the penetration of the retina, is crucial. Since it cannot visually observed whether the subretinal space is reached it is important to develop a feeling for the right pushing pressure and tissue resistance. To test, if the needle head is placed correctly, a small volume could be applied and the detachment of the retina at the needle head should be carefully observed. If a round, bullous detachment without bleeding is forming, the position is correct and the rest of the solution can be applied carefully. During injection, the needle should remain in its position to avoid further damage to the tissue. The retinal hole created by the injection needle will seal by itself if the needle is retracted slowly. The whole procedure should be done in the minimal amount of time possible to avoid further stress and damage to the animal. During transplantation, the cell suspension tends to clump and block the microliter syringe. If this happens, a dilution of the cell suspension using sterile PBS or HBSS is suggested. Another possibility to overcome this problem is to add 1 µl of DNAse I to the cell suspension, to dissolve DNA clumps from dead cells, which tend to glue living cells together.
This protocol is limited to the magnetic sorting of cells. Therefore, the cells could be sorted for only one marker per sorting round. In comparison to flow cytometry, where cells could be sorted using different markers (including physiological properties as well as different cell surface markers) at the same time, this is a major disadvantage of this technique. Whenever the sorting for different markers in a short time window is needed, flow cytometry might be the better solution. Also, sorting for fluorescent properties of cells, like transgenically expressed GFP or other life cell markers is not possible with this technique. It is currently restricted to the use of cell surface antibodies that can be conjugated with magnetic beads. Nevertheless, this technique could be scaled up based on the technical equipment used.
Since Miltenyi Biotec is currently the only provider of magnetic cell sorting tools, there is no alternative available to date. For cell dissociation, other enzymes than papain (i.e. trypsin) could be used. Of note, our lab achieved the best results with papain, since this seems to be the most gentle dissociation enzyme in our hands. The time window of papain digestion (30-60 min) is variable. In this time window optimal digestion was achieved in our lab. Shorter or longer incubation time might be necessary depending on sample size, sample quality and enzyme used. Individual testing is suggested.
Given that single-step MACS does not reach purity levels as high as flow cytometry, a possible further application of this technique might be negative sorting of unwanted and potential harmful cells. There, the cell fraction positive for a given cell surface marker but not of interest can be sorted out, leaving the fraction of interest isolated in the flow through. This could be done in advance or after a positive sorting step and allows the further discrimination of cells using different cell surface markers as well as the removal of unwanted cells, like feeder layer cells or undifferentiated cells from pluripotent stem cell cultures with their potential tumorgenic risk. Therefore, MACS might be suitable for purification of photoreceptors from ES or iPS cell cultures in possible future therapeutic applications as it represents a gentle, fast and simple treatment for the purification of high amounts of cultured cells. MACS could be easily applied to GMP-conditions since the details of sorting steps, i.e. mechanics, fluids and procedures are reduced compared to flow cytometry. It is noteworthy, that MACS is already routinely used in clinical trials for the enrichment of cells from blood or bone marrow. Interestingly, MACS can be even applied on cells when no suitable cell surface marker is present, by genetically introducing a surface marker to make cells “competent” for subsequent magnetic cell sorting steps15.
In summary, MACS represents a reliable and fast tool to enrich photoreceptor precursor cells using cell surface markers for transplantation studies. Additionally, it is an easily employable method for future clinical applications that require GMP-conditions.
Disclosures
The authors declare no competing financial interests.
Acknowledgments
We like to thank Anand Swaroop for providing Nrl-GFP mice, Jochen Haas for technical support, and Sindy Böhme and Emely Lessmann for animal husbandry.
This work was supported by the Deutsche Forschungsgemeinschaft (DFG): FZT 111 - Center of Regenerative Therapies Dresden, the CRTD Seed Grant Program, the SFB 655, and the ProRetina e.V. foundation, the DIGS-BB Graduate Program Dresden, and the Fundação para a Ciência e Tecnologia (SFRH/BD/60787/2009)
References
- Bartsch U, et al. Retinal cells integrate into the outer nuclear layer and differentiate into mature photoreceptors after subretinal transplantation into adult mice. Exp. Eye Res. 2008;86:691–700. doi: 10.1016/j.exer.2008.01.018. [DOI] [PubMed] [Google Scholar]
- MacLaren RE, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Eberle D, et al. Outer segment formation of transplanted photoreceptor precursor cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakowski J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum. Mol. Genet. 2010;19:4545–4559. doi: 10.1093/hmg/ddq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AC, et al. Repair of the degenerate retina by photoreceptor transplantation. Proc. Natl. Acad. Sci. U.S.A. 2013;110:354–359. doi: 10.1073/pnas.1212677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012. [DOI] [PMC free article] [PubMed]
- Singh MS, et al. Reversal of end-stage retinal degeneration and restoration of visual function by photoreceptor transplantation. Proc. Natl. Acad. Sci. U.S.A. 2013;110 doi: 10.1073/pnas.1119416110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle D, Schubert S, Postel K, Corbeil D, Ader M. Increased integration of transplanted CD73-positive photoreceptor precursors into adult mouse retina. Invest. Ophthalmol. Vis. Sci. 2011;52:6462–6471. doi: 10.1167/iovs.11-7399. [DOI] [PubMed] [Google Scholar]
- Koso H, et al. CD73, a novel cell surface antigen that characterizes retinal photoreceptor precursor cells. Invest. Ophthalmol. Vis. Sci. 2009;50:5411–5418. doi: 10.1167/iovs.08-3246. [DOI] [PubMed] [Google Scholar]
- Lakowski J, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29:1391–1404. doi: 10.1002/stem.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto M, et al. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc. Natl. Acad. Sci. U.S.A. 2006;103:3890–3895. doi: 10.1073/pnas.0508214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiraku M, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–56. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- Nakano T, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Osakada F, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Lee MY, Lufkin T. Development of the "Three-step MACS": a novel strategy for isolating rare cell populations in the absence of known cell surface markers from complex animal tissue. J. Biomol. Tech. 2012;23:69–77. doi: 10.7171/jbt.12-2302-003. [DOI] [PMC free article] [PubMed] [Google Scholar]


