Abstract
In cultured cortical and hippocampal neurons when intracellular pH drops from 6.6 to 6.1, yet unclear intracellular stores release micromolar amounts of Zn2+ into the cytosol. Mitochondria, acidic organelles, and/or intracellular ligands could release this Zn2+. Although exposure to the protonophore FCCP precludes re-loading of the mitochondria and acidic organelles with Zn2+, FCCP failed to compromise the ability of the intracellular stores to repeatedly release Zn2+. Therefore, Zn2+-releasing stores were not mitochondria or acidic organelles but rather intracellular Zn2+ ligands. To test which ligands might be involved, the rate of acid-induced Zn2+ release from complexes with cysteine, glutathione, histidine, aspartate, glutamate, glycine, and carnosine was investigated; [Zn2+] was monitored in vitro using the ratiometric Zn2+-sensitive fluorescent probe FuraZin-1. Carnosine failed to chelate Zn2+ but did chelate Cu2+; the remaining ligands chelated Zn2+ and upon acidification were releasing it into the medium. However, when pH was decreasing from 6.6 to 6.1, only zinc-cysteine complexes rapidly accelerated the rate of Zn2+ release. The zinc-cysteine complexes also released Zn2+ when a histidine-modifying agent, diethylpyrocarbonate, was applied at pH 7.2. Since the cytosolic zinc-cysteine complexes can contain micromolar amounts of Zn2+, these complexes may represent the stores responsible for an acid-induced intracellular Zn2+ release.
Keywords: FuraZin-1, Cysteine, Glutathione, Carnosine, Histidine, Diethylpyrocarbonate
Introduction
Brain tissue acidifies shortly after the onset of ischemia (Siemkowicz and Hansen 1981; Smith et al. 1986) and acidosis is neurotoxic (Goldman et al. 1989; Nedergaard et al. 1991). The mechanisms of acid-induced cell death include the activation of acid-sensitive ion channels (Xiong et al. 2004; Wemmie et al. 2006), facilitation of apoptosis (Barry and Eastman 1992; Gottlieb et al. 1996), and an enhancement of oxidative damage (Siesjöet et al. 1985; Ying et al. 1999). Recently, it has been demonstrated that acid-induced neuronal death is preceded by elevations of intracellular [Zn2+] ([Zn2+]i) (Isaev et al. 2010), which occur due to a Zn2+ release from intracellular stores (Sensi et al. 2003; Kiedrowski 2011, 2012). Since an elevation of [Zn2+]i compromises glycolysis (Ikeda et al. 1980; Krotkiewska and Banaś 1992) and mitochondrial function (Brown et al. 2000; Jiang et al. 2001), an acid-induced [Zn2+]i elevation may become a target for stroke therapies. Therefore, the mechanism of acid-induced intracellular Zn2+ release needs to be better understood. Although Sensi et al. (2003) suggested that Zn2+ is released from mitochondria, it could also be released from acidic organelles that use the H+ gradient to accumulate Zn2+ (Colvin 2002; Ohana et al. 2009), or from metallothioneins which chelate Zn2+, as suggested by Shuttleworth and Weiss (2011). Other yet unclear cytosolic Zn2+ ligands might also play a role. The purpose of this work was to clarify the mechanisms responsible for intracellular Zn2+ release in acidified neurons.
Materials and Methods
Primary cultures of mouse hippocampal and cortical neurons
Primary hippocampal and cortical cultures were prepared using SPOT™ kits from the University of Illinois at Chicago Research Resources Center (http://www.rrc.uic.edu/portal/SPOT_Culture_Kit). These kits contained brain tissue from c57/bl/6 mice at embryonic day 16. Kit production was approved by the Institutional Animal Care and Use Committee. Cultures were prepared as previously described (Kiedrowski, 2011) except that to improve long-term cell viability, glial proliferation was not prevented with cytostatic agents. Glial cells were identified after ion imaging experiments. To this end, the cells were fixed with 4% formaldehyde, exposed to a primary rabbit anti-GFAP (1:500, AB5804 Chemicon) antibody followed by a secondary TRITC-labeled goat anti-rabbit IgG (1:500, Sigma T6778), as described in Kiedrowski (2007). In many experiments, GFAP-positive cells could not be detected in the field that was being investigated (Fig. S1A and C). The cultures were used for experiments after two weeks in vitro.
Experimental media
For fluorescence imaging, cultures were transferred to Locke’s buffer containing (in mM) NaCl (157.6), KCl (2.0), KHCO3 (3.6), MgCl2 (1.0), CaCl2 (1.3), glucose (5), and piperazine-1,4-bis(2-ethanesulfonic acid), PIPES (10); pH was adjusted to 7.2 or 6.1 using NaOH or HCl, respectively. To create Ca2+-free and Zn2+-free buffers, CaCl2 was omitted and 100 μM EGTA was added.
Fluorescence imaging and superfusion
Fluorescence imaging was performed using two instruments. Initially, an Attofluor digital imaging system (Atto Instruments, Rockville, MD) connected to a Zeiss Axiovert 100 microscope (Carl Zeiss Mikroskopie, Jena, Germany) was used, as described earlier (Kiedrowski 2011). More recently, a digital fluorescence imaging system controlled by MetaFluor 7.1 software, equipped with a Zeiss Axio Observer D1 microscope with a motorized dichroic turret, a 4-position Sutter excitation filter wheel, a 4-position Sutter emission filter wheel, a Sutter XL xenon illumination unit, a Sutter LAMBDA 10-3 controller, and a RETIGA EXi Blue camera (QImaging, Surrey, BC, Canada) was used. Fluorescence was monitored using a Zeiss Fluar 20×, NA 0.75 objective. Superfusion media were delivered directly onto the cells via an 8-channel manifold (MPRE-8, Cell MicroControls, Norfolk, VA); flow was computer-controlled using an 8-channel valve switch (cFlow8, Cell MicroControls). Cells were superfused at a rate of 0.5 ml/min. Temperature was maintained at 37 °C using a bipolar temperature controller (TC2BIP, Cell MicroControls).
Simultaneous monitoring Fura2-FF and FluoZin-3 fluorescence
Cultures were loaded with Fura-2FF AM and FluoZin-3 AM and fluorescence was imaged in single cells as described in Kiedrowski (2012). Briefly, Fura-2FF data were collected as the ratio of fluorescence intensity measured after 340 and 380 nm excitations (F340/F380). FluoZin-3 data were collected as the fluorescence emitted after a 488 nm excitation (F488). At the end of the experiments, 20 μM 1-hydroxypyridine-2-thione zinc salt and 100 μM ZnCl2 (ZnPyr) were applied to measure the Fura-2FF signal and the FluoZin-3 signal after saturation with Zn2+ (Fig. S1 B). The Fura-2FF and FluoZin-3 data were expressed as a percentage of the respective ZnPyr signals.
Monitoring intracellular pH (pHi)
Cultures were loaded with 2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein acetoxymethyl ester (BCECF AM) and the fluorescence was monitored and calibrated as described in detail in Kiedrowski (2011).
Simultaneous monitoring of mitochondrial membrane potential (Δψm) and Fura-2FF fluorescence
Cultures were loaded for 5 min at 37°C with 2 μM rhodamine 123 (Rh123) and 0.1 μM Fura-2FF AM. The Fura-2FF signal (F340/F380 ratio) was monitored as described above. Rh123 was excited at 488 nm and emissions over 520 nm were monitored. Δψm was assessed in the Rh123 quenching mode. In this mode, mitochondrial depolarization is detected as an increase of Rh123 fluorescence intensity due to an unquenching of Rh123 released from the mitochondria to the cytosol (Perry et al. 2011). To measure the Rh123 signal associated with maximal depolarization, 3 μM carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP) was applied to depolarize the mitochondria. Rh123 data were expressed as a percentage of the maximal signal.
Measuring the affinity of FuraZin-1 for Zn2+ at pH 7.2 and 6.1.
FuraZin-1 AM (1 mM in DMSO) was hydrolyzed with 1 M KOH, neutralized with 1 M HCl and diluted with H2O to obtain a 100 μM stock of FuraZin-1 potassium salt of which the aliquots were stored at −80 °C. The affinity of FuraZin-1 for Zn2+ at pH 7.2 versus 6.1 was characterized using the PTI QuantaMaster spectrofluorometer (Birmingham, NJ). In these experiments, fluorescence excitation spectra (320 – 420 nm excitation, 490 nm emission) of 100 nM FuraZin-1 in a solution containing 100 mM KCl and 50 mM PIPES with pH 7.2 or 6.1 (adjusted with KOH) and ZnCl2 ranging from 0.1 μM to 3 mM were measured at 37 °C. Continuous mixing was provided by a magnetic stirrer. The apparent FuraZin-1 Zn2+ dissociation constants were calculated using SigmaPlot 10 software (Systat Software Inc., Richmond, CA) from the F340 and F340/F380 data.
Assay of Zn2+ release from ligands
Using the PTI QuantaMaster spectrofluorometer, the F340/F380 ratio was measured at 37 °C in a cuvette containing 100 mM KCl, 50 mM PIPES, 100 nM FuraZin-1, and 100 μM ZnCl2, pH 7.2. Then 1 mM tested ligand (in some experiments 10 mM) was added to the cuvette and the F340/F380 ratios were measured while pH was gradually decreased (using 5 M HCl) until it reached 6.1; pH was monitored using a pH meter. To study Zn2+ chelation at pH 7.1 - 7.9, 3-(N-morpholino)propanesulfonic acid, MOPS, was used as a pH buffer.
The effects of diethylpyrocarbonate, DEPC, on Zn2+ chelation by cysteine were tested using the above described Metafluor 7.1-controlled imaging system. In these experiments, a custom-made imaging chamber filled with 200 nM FuraZin-1, 100 mM KCl, and 50 mM PIPES, pH 7.2, was placed in the LU-CB1 Leiden dish holder connected to the TC-102 temperature controller (Medical Systems Corp., Greenvale, NY) to maintain 37 °C. F340/380 ratios were measured during successive additions of 100 μM ZnCl2, 100 μM cysteine, and 1 mM DEPC to the chamber. Mixing was provided by a custom-made device.
Statistical analysis
The data were analyzed using the SigmaStat 3.5 software (Systat Software Inc., Richmond, CA). For multiple comparisons, ANOVA followed by the Newman-Student-Keuls test was used. The Student’s t-test was used to compare data from two groups. The differences were considered statistically significant if they reached at least p<0.05.
Reagents
Fura-2FF AM was obtained from Teflabs (Austin, TX, USA), FuraZin-1 AM from Molecular Probes (Eugene, OR), and BCECF AM, FluoZin-3 AM, rhodamine 123 from Invitrogen (Carlsbad, CA, USA). All other reagents were from Sigma-Aldrich (St Louis, MO, USA) unless stated otherwise.
Results
The idea that mitochondria represent a major Zn2+ store responsible for acid-induced [Zn2+]i elevations (Sensi et al. 2003) was examined. The driving force for mitochondrial Zn2+ accumulation is provided by the mitochondrial membrane potential (Δψm), which is compromised by protonophores such as FCCP. One may expect that as long as FCCP is present in the medium and depolarizes mitochondria, the latter cannot re-accumulate Zn2+ (assuming that mitochondria released Zn2+ when FCCP was applied). If mitochondria cannot re-accumulate Zn2+, it must be assumed that they cannot re-release it either. This logic was tested in cultured cortical and hippocampal neurons continuously exposed to FCCP while an impact of repetitive pulses of the extracellular pH (pHo) drop from 7.2 to 6.1 on [Zn2+] was investigated. To monitor [Zn2+]i, FluoZin-3, a Zn2+-specific fluorescent probe (Zhao et al. 2009), was used. Since intracellular acidification may trigger a Ca2+ release from intracellular stores (Ou-yang et al. 1994), [Ca2+] was also monitored. To measure [Ca2+]i, Fura-2FF was used. As the excitation spectra of FluoZin-3 and Fura-2FF do not overlap, [Zn2+]i and [Ca2+]i could be monitored simultaneously. However, the Fura-2FF signal (F340/F380 ratio) increase may indicate a [Ca2+]i or [Zn2+]i elevation. To determine the extent to which the Fura-2FF signal reported [Ca2+]i, at the end of each experiment, a plasma-membrane permeable heavy metal chelator, 10 μM N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN), was applied. Such TPEN application obliterates the [Zn2+]i increase without affecting [Ca2+]i (Kiedrowski 2011). To ensure that the observed [Zn2+]i or [Ca2+]i elevations represented a Zn2+ or Ca2+ release from intracellular stores, the extracellular medium was free of Zn2+ and Ca2+.
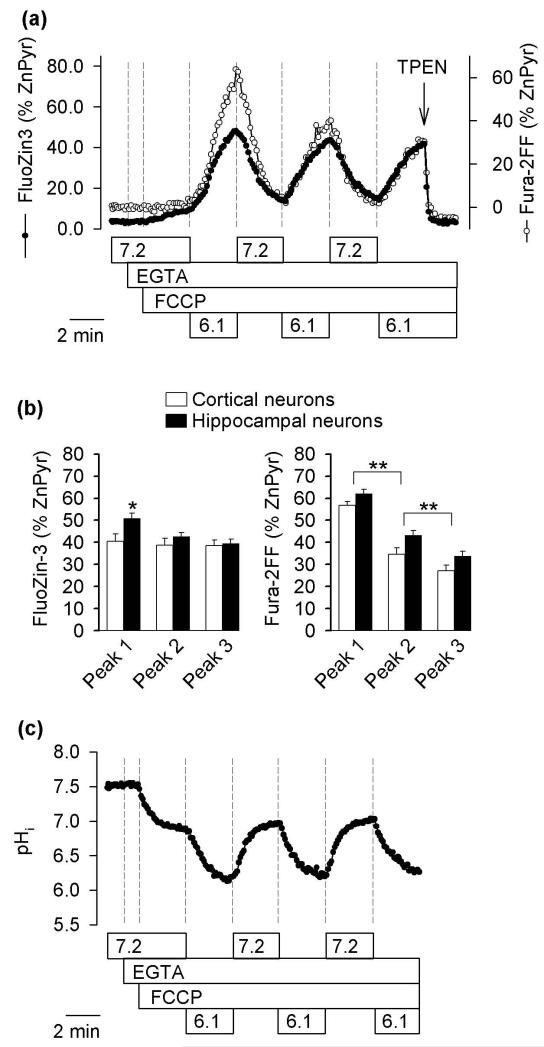
In cortical and hippocampal neurons, upon application of 3 μM FCCP at pH 7.2, only a minor (less than 5%) increase of the FluoZin-3 signal was observed. However, in 6% of cortical (4 of 67) and 4% of hippocampal (9 of 229) neurons, the Fura-2FF signal rapidly increased to apparently probe-saturating levels (Fig. S1, neurons 1, 2). In the remaining neurons, the Fura-2FF signal behaved similar to the FluoZin-3 signal; as shown in Fig. 1a, both signals started to rapidly increase only after the pHo was lowered from 7.2 to 6.1. When the pHo was returned to 7.2, the signals decreased to their original levels. Triple repetitions of this maneuver resulted in three peaks in the FluoZin-3 and Fura-2FF signals in both cortical (Fig. 1a) and hippocampal neurons (Fig. S2). A statistical analysis of these peaks is shown in Fig. 1b. The three peaks of Fura-2FF signal, in cortical and hippocampal neurons, were progressively smaller, with the first peak by 84% and the second peak by 28% larger than the third peak (Fig. 1b, right). The three peaks of FluoZin-3 signals in cortical neurons were, however, approximately the same size, whereas in hippocampal neurons, the first peak was only about 16% larger (p<0.05) than the second peak and there was no statistically significant difference between the last two peaks (Fig. 1b, left). An application of 10 μM TPEN quenched the last peak of both FluoZin-3 and Fura-2FF signals to background levels. The data suggest that only [Zn2+]i was responsible for FluoZin-3 and Fura-2FF signal elevation in the third peak whereas [Ca2+]i elevation significantly contributed to the first peak.
Fig. 1.
Effects of repetitive applications of low pH on [Zn2+]i, [Ca2+]i, and pHi in neurons. (a) Effects of repetitive acidifications on FluoZin-3 and Fura-2FF signals in cortical neurons. Indicated in the legend are: pHo (7.2 or 6.1), superfusion with Ca2+- free and Zn2+-free medium (EGTA), and applications of 3 μM FCCP and 10 μM TPEN. The data are percentages of FluoZin-3 and Fura-2FF signals measured after an application of 100 μM ZnCl2 plus 10 μM pyrithione (ZnPyr) to saturate the probes with Zn2+ (for details see Methods). The plots are scaled such that the third peak of Fura-2FF signal overlaps the FluoZin-3 signal. The data are means (12 neurons) from a single experiment that was repeated five times with similar results. (b) Average peaks of FluoZin-3 and Fura-2FF signals in cortical and hippocampal neurons from experiments analogous to that shown in (a). The data are means ± SEM from five experiments on cortical neurons and six experiments on hippocampal neurons; ** p <0.01, * p <0.05, one-way ANOVA followed by Student-Newman-Keuls test. (c) Effects of repetitive acidifications on pHi in cortical neurons. The data are means (14 neurons) from a single experiment that was repeated three times with similar results. The experimental conditions are the same as described in (a).
Parallel experiments on neurons loaded with BCECF showed that when FCCP was applied at pHo 7.2, pHi decreased from 7.5 to about 7.0. When the pHo was adjusted to 6.1, the pHi dropped to 6.2. When the pHo was increased to 7.2, the pHi increased to 7.0, and so on (Fig. 1c). Together, the [Zn2+]i and pHi data suggest that the Zn2+ release from intracellular stores (Fig. 1a) was associated with a drop in pHi. As the mitochondria in these neurons were constantly depolarized with FCCP, the Δψm collapse per se played no role in the mechanism of Zn2+ release.
The idea that FCCP causes intracellular Zn2+ release because it depolarizes mitochondria was explored further; if this is the case, all agents that depolarize mitochondria should affect [Zn2+]i the same way FCCP does. Mitochondria can be depolarized by an application of rotenone plus oligomycin (Rot/Oligo) (Budd and Nicholls 1996). In preliminary experiments performed on hippocampal neurons, it was established that after increasing the rotenone and oligomycin concentrations to 10 μM and 5 μg/ml, respectively, the rate of Rot/Oligo-induced Δψm collapse approached that induced by an application of 3 μM FCCP. It was then tested how Rot/Oligo versus FCCP affects [Zn2+]i and Δψm at pHo 7.2 and 6.1. Δψm was monitored with Rh123, and [Zn2+]i with Fura-2FF (using TPEN to chelate Zn2+, it was established mostly Zn2+ was responsible for the Fura-2FF signal increase). Since the excitation spectra of Rh123 and Fura-2FF do not overlap, Δψm and [Zn2+]i could be monitored simultaneously.
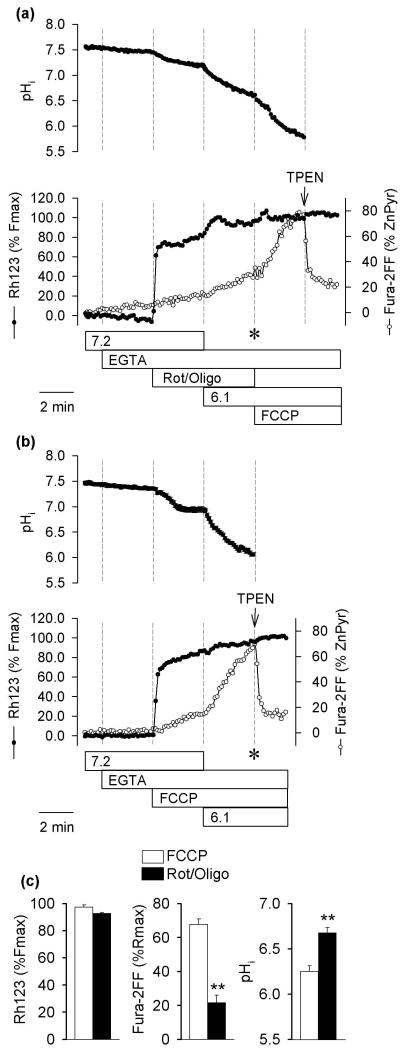
As shown in Fig. 2a, although an application of Rot/Oligo at pHo 7.2 promptly depolarized mitochondria, the rate of the Fura-2FF signal increase remained unchanged even after the pHo was decreased to 6.1. Parallel experiments on neurons loaded with BCECF showed that Rot/Oligo at pHo 6.1 caused only a small drop in pHi (to 6.7). When FCCP was applied after Rot/Oligo, an additional pHi drop was observed. This additional pHi drop coincided with an acceleration of the Fura-2FF signal increase. A large part of this signal increase was quenched by TPEN and therefore represented [Zn2+]i rather than [Ca2+]i elevations (Fig. 2a). These data again show that the [Zn2+]i increase is associated with a pHi drop rather than a Δψm collapse.
Fig. 2.
pHi, Δψm, and [Zn2+]i in hippocampal neurons exposed to low pH and agents that depolarize mitochondria. (a) Upper panel shows effects of Rot/Oligo (10 μM rotenone plus 5 μg/ml oligomycin) on pHi monitored using BCECF; lower panel shows effects of Rot/Oligo on Δψm and [Zn2+]i monitored using Rh123 and Fura-2FF, respectively. Other treatments were the same as described in Fig. 1a. TPEN (10 μM) was applied to determine the extent of Zn2+ contribution to the Fura-2FF signal. The data are means from a single experiment on 29 neurons (BCECF) and 24 neurons (Rh123 + Fura-2FF) and represent four to six such experiments. (b) Experiments analogous to those shown in (a) but FCCP was applied to depolarize mitochondria. The data are means from a single experiment on 28 neurons (BCECF) and 26 neurons (Rh123 + Fura-2FF) and represent four to six such experiments. c) Average Δψm (Rh123 signal), [Zn2+]i (Fura-2FF signal), and pHi measured at the time that is indicated by the asterisks in (a) and (b). The data are means ± SEM from four to six experiments; ** p<0.01 Student’s t-test.
Fig. 2b shows another set of experiments in which FCCP was applied at pHo 7.2, similar to the application of Rot/Oligo in Fig. 2a. FCCP promptly depolarized mitochondria, as expected, and pHi stabilized at about 7.0. When the pHo was decreased to 6.1, the pHi gradually dropped below 6.6 and during this pHi decrease, an acceleration of the Fura-2FF signal increase took place. Again, after TPEN was applied to chelate Zn2+, the Fura-2FF signal rapidly decreased (Fig. 2b).
Fig. 2c shows a statistical analysis of the Rh123, Fura-2FF, and pHi data at the time points indicated by the asterisks in Figs. 2 a and b. While both Rot/Oligo and FCCP depolarized mitochondria, the pHi drop and [Zn2+]i elevation were significantly larger in the presence of FCCP than in the presence of Rot/Oligo. These data rule out the idea that FCCP causes an intracellular Zn2+ release because it depolarizes mitochondria.
One may envision that acidic organelles that use H+ gradient as the driving force to accumulate Zn2+ (Colvin 2002; Ohana et al. 2009) could release Zn2+ upon a drop in pHi. However, these organelles cannot re-accumulate Zn2+ after the H+ gradient has collapsed. Since an application FCCP promptly dissipates H+ gradients in all acidic organelles tested to date: lysosomes (Ohkuma et al. 1982), endosomes (Maranda et al. 2001), chromaffin granules (Cidon et al. 1983), and synaptic vesicles (Cidon and Sihra 1989), these organelles could not play major role in the repetitive acid-induced [Zn2+]i elevations taking place in the presence of FCCP (Fig. 1a).
It has been suggested that acid-induced [Zn2+]i elevations result from a Zn2+ release from metallothioneins (Shuttleworth and Weiss 2011). However, pH drop alone (without coinciding oxidation) induces Zn2+ release from metallothioneins only if pH drops below 5.0 (Jiang et al. 2000). In neurons, acid-induced intracellular Zn2+ release takes place when the pHi drops from 6.6 to 6.1 (Fig. 1c); therefore, Zn2+ might be released from ligands that bind Zn2+ with a lower than metallothionein affinity. To identify such ligands, one could attempt to use Zn2+-sensitive fluorescent probes. But a caveat is that when pH drops from 7.2 to 6.1, high affinity probes such as FluoZin-3 or Fura-2FF show about a10-fold decrease in their affinity for Zn2+ (Kiedrowski 2012). Fortunately, this problem could be minimized using a low affinity ratiometric Zn2+ probe, FuraZin-1 (Gee et al. 2002); although acidification did affect FuraZin-1 fluorescence (Fig. S3 A-C), the plots of FuraZin-1 F340/F380 ratios as a function of [Zn2+] at pH 7.2 and 6.1 nearly overlapped (Fig. S3 D). Therefore, FuraZin-1 could be used to study the impact of a pH drop on Zn2+ release from various agents considered to be physiological Zn2+ ligands: cysteine, histidine, glutathione (GSH), aspartate, glutamate, glycine, and carnosine (β-alanyl-L-histidine). Carnosine resides only in a subpopulation of olfactory neurons (Margolis 1974; Neidle and Kandera 1974) and in astrocytes (Biffo et al. 1990). The reason carnosine was tested is that this dipeptide contains a histidine whose amino group forms a peptide bond; this histidine binds Zn2+ without the participation of the amino group, the same way the histidines in proteins do. Therefore, using carnosine, one can address the impact of pH on Zn2+ binding by the histidines in proteins.
In these experiments after measuring the FuraZin-1 F340/F380 ratio at 100 μM [Zn2+] and pH 7.2, 1 mM or 10 mM ligands were added to the cuvette to chelate Zn2+. Depending on the affinity of the ligands for Zn2+, a variable FuraZin-1 signal drop was observed. Then, to determine the impact of pH on the Zn2+ chelation, the pH in the cuvette was manipulated by adding HCl or KOH.
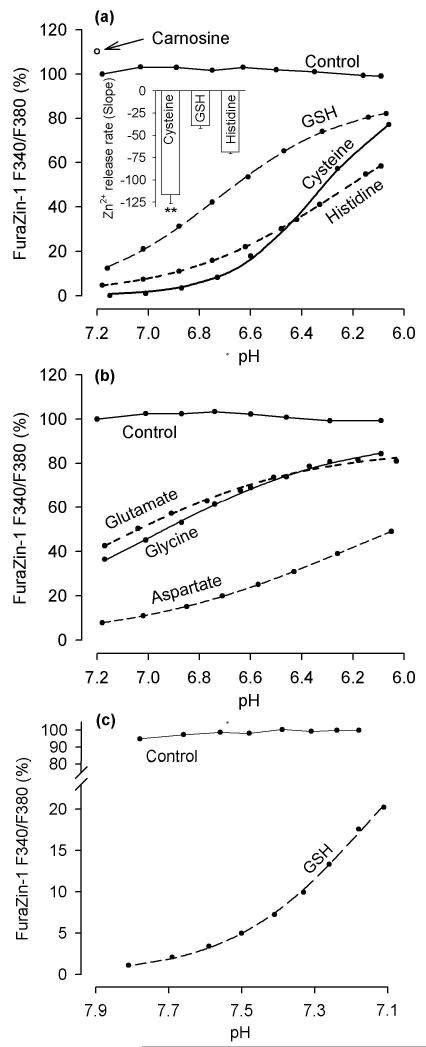
All tested ligands with the exception of carnosine caused a drop in the FuraZin-1 signal indicating that the ligands chelated Zn2+; when the pH was decreasing from 7.2 to 6.1, the ligands were releasing Zn2+ (Fig. 3 a and b). However, a rapid acceleration of the rate of Zn2+ release coinciding with a pH drop from 6.6 to 6.1 was only observed in the cysteine solution (Fig. 3a, inset).
Fig. 3.
Effects of pH on Zn2+ chelation by tested ligands. (a) Acid-induced Zn2+ release from GSH, cysteine, and histidine. The ligands (1 mM) were added to a solution containing 100 nM FuraZin-1, 100 mM KCl, and 50 mM PIPES (37 °C) and the FuraZin-1 signal was measured while pH was manipulated. The inset shows the slopes in the pH range 6.6 - 6.1 (these slopes reflect the rates of Zn2+ release from the ligands when pH was decreasing from 6.6 to 6.1); the data are means ± SEM from five to seven experiments identical to those shown in the main figure; ** p < 0.01, Cysteine vs GSH, and Cysteine vs Histidine, one way ANOVA followed by Student-Newman-Keuls test. (b) Acid-induced Zn2+ release from glutamate, glycine, and aspartate. The ligands (10 mM) were tested the same way as described in (a). (c) The effect of pH drop from 7.9 to 7.1 on Zn2+ chelation by 1 mM GSH. The experiment was performed as described in (a) except that MOPS (instead of PIPES) was used as pH buffer. In all panels, the data are expressed as a percentage of the FuraZin-1 F340/F380 signal measured at pH 7.2 prior to the addition of the ligands; the Control refers to the FuraZin-1 signals measured in the absence of the ligands.
The extent of the ligand-induced FuraZin-1 signal drop should be proportional to the ligand’s affinity for Zn2+ (log K). The respective log K values are as follows: cysteine, 9.11; GSH, 7.98; histidine, 6.51; aspartate, 5.87; glycine, 4.96; and glutamate, 4.72 (Martell et al. 2004). Unexpectedly, although GSH has a higher than histidine affinity for Zn2+, at pH 7.2, 1 mM GSH chelated less Zn2+ than 1 mM histidine (Fig. 3a). To clarify this inconsistency, the effect of pH in the 7.9 - 7.1 range on Zn2+ binding by GSH was investigated. As shown in Fig. 3c, GSH started to lose its affinity for Zn2+ at a pH as high as 7.7.
Interestingly, carnosine, an agent reported to chelate Zn2+ (Corona et al. 2011), not only did not decrease, but consistently increased the FuraZin-1 F340/380 ratio by about 10% (Fig. 3a, arrow). These data indicate that carnosine did not chelate Zn2+ but could chelate a contaminating ion that affected FuraZin-1 fluorescence. To test this possibility, the impact of EDTA (to chelate the contaminating ion) on the FuraZin-1 excitation spectra was examined. It was found that the addition of up to 200 nM EDTA boosted FuraZin-1 fluorescence. When more than 200 nM EDTA was applied, no further fluorescence intensity increase was observed (Fig. S4 A). This outcome indicates that the solution in which FuraZin-1 was dissolved (100 mM KCl and 50 mM PIPES) was contaminated with an ion that quenched FuraZin-1 fluorescence. As this ion was completely chelated by 200 nM EDTA, its concentration was not higher than 200 nM. To determine the identity of the contaminating ion, the impact of Ca2+, Mg2+, Fe2+, and Cu2+ on the FuraZin-1 excitation spectra was explored. It was found that only Cu2+ quenched FuraZin-1 fluorescence (Fig. S4 B) and that this quenching was dose-dependently removed by carnosine (Fig. S4 C). These data indicate that carnosine is a potent Cu2+ (but not Zn2+) chelator.
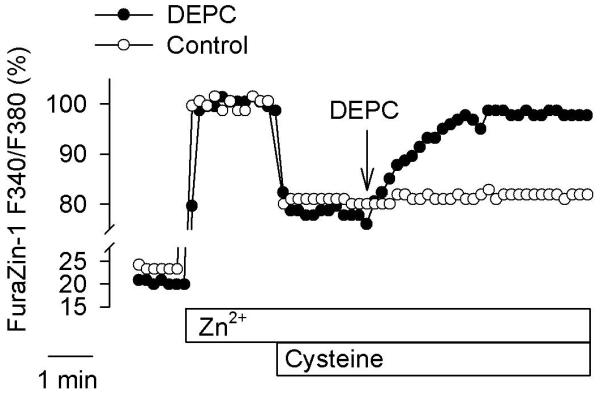
The data in Fig. 3a show that the profile of the acid-induced Zn2+ release from cysteine resembles that observed in acidified neurons in which there is a rapid acceleration of [Zn2+]i increase (Fig. 1 a) at the time when the pHi drops below 6.6 (Fig. 1 c). Although these data indicate that, of the ligands tested, cysteine was the most likely source of Zn2+, it has been suggested that histidine is the primary source of the acid-induced [Zn2+]i elevations (McCranor et al. 2012). The importance of histidine as a source of Zn2+ stems from the fact that an agent that covalently modifies the imidazole ring of histidine, diethylpyrocarbonate (DEPC), causes robust [Zn2+]i elevations (Haase and Beyersmann 2002). However, DEPC is a highly reactive agent which might also affect the ability of cysteine to bind Zn2+. To test whether this is the case, the impact of 1 mM DEPC on Zn2+ chelation by 100 μM cysteine was investigated using FuraZin-1. As shown in Fig. 4, within five minutes after DEPC application, virtually all the Zn2+ chelated by cysteine was released back into the medium.
Fig. 4.
Diethylpyrocarbonate (DEPC) counteracts Zn2+ chelation by cysteine. The F340/F380 ratio was measured in vitro in a solution containing 200 nM FuraZin-1, 100 mM KCl, and 50 mM PIPES, pH 7.2. Where indicated, 100 μM ZnCl2, 100 μM cysteine, and 1 mM DEPC were added to the solution; the Control shows data from an experiment without DEPC.
Discussion
This work addressed the mechanism of an acid-induced intracellular Zn2+ release in neurons (Kiedrowski 2011, 2012). Although this release was promoted by FCCP, it did not coincide with the FCCP-induced Δψm collapse but instead coincided with the FCCP-induced pHi drop below 6.6. Therefore, Zn2+ release was triggered not by mitochondrial depolarization but by intracellular acidification. The latter resulted from the FCCP-mediated H+ influx from the extracelluar medium into the cytosol. The driving force for this H+ influx was provided by the pH gradient across the plasma membrane.
FCCP easily penetrates biological membranes; the FCCP-induced H+ fluxes, by depolarizing mitochondria and dissipating H+ gradients in acidic organelles, preclude Zn2+ re-accumulation in acidic organelles and mitochondria. Since FCCP failed to compromise the ability of the intracellular stores to repeatedly release Zn2+, the Zn2+-releasing stores were neither mitochondria nor acidic organelles but rather intracellular Zn2+ ligands. When the pH was decreasing from 6.6 to 6.1, only the zinc-cysteine complexes showed a rapid acceleration in the rate of Zn2+ release (Fig. 3a). Therefore, the acid-induced [Zn2+]i elevations that took place in a similar range of the pHi drop (Fig. 1a) most likely resulted from a Zn2+ release from the cytosolic zinc-cysteine complexes.
In the cytosol, cysteine-bound Zn2+ exists in three fractions: large molecular weight proteins (proteome), 10kDa proteins (metallothioneins), and low molecular weight ligands (Nowakowski and Petering 2012). Since metallothioneins are not likely to release Zn2+ when the pH drops from 6.6 to 6.1, Zn2+ must be released from the proteomic or low molecular weight fraction or from both. The low molecular weight fraction contains molecules with an average molecular weight slightly larger than GSH, probably ZnGSH (Rana et al. 2008). Since the rate of Zn2+ release from ZnGSH does not accelerate when pH drops from 6.6 to 6.1 (Fig. 3a), Zn2+ is probably released from proteomic cysteines.
The proteomic cysteines not involved in disulphide bonds are able to chelate Zn2+ and 21% of these cysteines reside in the evolutionally preserved CXXC motifs where X can be any coded amino acid (Miseta and Csutora 2000). Two CXXC motifs are often spatially arranged to form a zinc finger in which four cysteines coordinate one Zn2+ (Ye et al. 2007). In such tetrahedral complexes, Zn2+ is bound very tightly and probably does not dissociate at pH 6.1 (similar to the Zn2+ in metallothioneins).
EGTA, is able to chelate 32% of proteomic Zn2+ (Rana et al. 2008). The Zn2+ log K value of EGTA is 12.6. Therefore, EGTA may remove Zn2+ from the complexes in which one cysteine chelates one Zn2+, ZnCys (log K = 9.1) (Martell et al. 2004). Acidification destabilizes the ZnCys complexes. The log K value of ZnHCys complexes is only 4.6 (Martell et al. 2004); in these complexes, Zn2+ is bound so weakly that probes such as FluoZin-3 or Fura-2FF with affinity for Zn2+ in the nanomolar range may strip it. When pH drops below 6.5, Zn2+ dissociates from these complexes (Perrin and Sayce 1968).
A previous study performed on hippocampal neurons exposed to pHo 6.0 in the presence of gramicidin (to facilitate proton influx) addressed the amount of Zn2+ released from intracellular Zn2+ stores; it was estimated that about 1.3 μM Zn2+ was released (Kiedrowski 2012). If this Zn2+ had been released primarily from ZnCys complexes and considering that these complexes had released about 80% of Zn2+ (Fig. 3a), the concentration of the ZnCys complexes prior to acidification would have to be 1.3 μM/0.8 = 1.6 μM. To approximate the amount of cysteine needed to form 1.6 μM ZnCys complex, a classic steady-state equilibrium binding equation can be used:
| (1) |
where Kd is the dissociation constant, [Zn] is the concentration of free Zn2+, and [CysT] is the total concentration of cysteine. After rearranging equation (1) to:
| (2) |
and entering 1.6 μM for [ZnCys], 0.000776 μM for Kd (this corresponds to log K = 9.11), and 0.0006 μM for the basal [Zn2+] (Krężel and Maret 2006), [CysT] = 3.6 μM. This means that when 3.6 μM cysteine and 0.0006 μM Zn2+ are in equilibrium, the concentration of ZnCys complex is about 1.6 μM. However, the Kd value used in equation (2) does not apply to all cytosolic cysteines because the affinity of proteomic cysteines for Zn2+ may vary depending on a local environment and pH. Therefore, the result indicating that 3.6 μM cysteine can bind 1.6 μM Zn2+ may not accurately reflect the amount of Zn2+ chelated in all cytosolic ZnCys complexes.
Nevertheless, in the cytosol, there is much more than 3.6 μM cysteine available for Zn2+ chelation; the concentration of free cysteine alone is 80 – 140 μM (Anderson and Meister 1989). Therefore there must be much more than 1.6 μM Zn2+ chelated in ZnCys complexes, and upon acidification much more than the previously estimated 1.3 μM Zn2+ (Kiedrowski 2012) must be released. A likely reason that the released Zn2+ escaped detection is that most of it was intercepted by various cytosolic buffers that at pH close to 6 could chelate Zn2+, for example by metallothioneins. Considering that metallothioneins themselves release Zn2+ when exposed to oxidants (Malaiyandi et al. 2004) or nitric oxide (St Croix et al. 2002), it is possible that if oxidative and/or nitrosative stress coincides with intracellular acidosis, which happens during ischemia/reperfusion, a mild oxidative/nitrosative stimulus normally insufficient to induce Zn2+ release from metallothioneins could be sufficient under such conditions. Further research is necessary to clarify these possibilities.
Using equation 2, it has been calculated that 3.6 μM cysteine could chelate 1.6 μM Zn2+; when analogous calculations are performed for other ligands, it can be found that 29.5 μM GSH, 825 μM histidine, 3.6 mM aspartate, 29.2 mM glycine, or 50.8 mM glutamate is necessary to chelate 1.6 μM Zn2+. One may notice, that of all of these ligands, only the intracellular GSH concentration, 2.5 – 15 mM (Rice and Russo-Menna 1998; Mari et al. 2009), is high enough to chelate this much and even more Zn2+. However, GSH chelated Zn2+ well only when the pH was close to 8 (Fig. 3c). Considering that 8 is the pH of the mitochondrial matrix (Llopis et al. 1998) and that this matrix contains large amounts of GSH (Mari et al. 2009), GSH may play a more important role in Zn2+ chelation in the mitochondrial matrix than in the cytosol. One may envision the following scenario: when the mitochondrial matrix pH drops from 8 to 7, GSH starts to release Zn2+ (Fig. 3c) and this released Zn2+ leaks into the cytosol; at pH 7 ligands such as metallothioneins and cysteine intercept the released Zn2+; only after the pH drops below 6.6 and cysteine loses its affinity for Zn2+, does [Zn2+]i elevation become evident. This way, the Zn2+ released from the mitochondrial GSH could contribute to acid-induced [Zn2+] elevations in the cytosol.
The idea that intracellular acidification may affect [Ca2+]i was addressed in several previous studies with the conclusion that a pHi drop can trigger a Ca2+ release from intracellular stores. Some of these studies were conducted using Fura-2 (Ou-yang et al. 1994), which responds to Ca2+ and Zn2+ (Grynkiewicz et al. 1985). Therefore, it is not clear to what extent [Ca2+] versus [Zn2+]i elevations affected the Fura-2 signal. Nevertheless, in snail neurons, acid-induced [Ca2+]i elevations were detected using Ca2+-sensitive microelectrodes (Thomas 2002). The present data confirm that intracellular acidification can trigger Ca2+ release from an internal store. In contrast to the store that releases Zn2+, the store that releases Ca2+ becomes depleted during repeated exposures to a low pH. Most likely this is because the released Ca2+ is extruded to the extracellular medium more efficiently than Zn2+.
As shown in Fig. 3a, carnosine failed to chelate Zn2+ in an assay in which even the weak Zn2+ chelators, glutamate and glycine, were effective. This result is consistent with the fact that carnosine has a lower than glutamate or glycine affinity for Zn2+, log K= 4.0 (Martell et al. 2004). Most likely, single proteomic histidines, similar to carnosine, bind Zn2+ poorly. Whereas in many proteins histidines are involved in Zn2+ coordination (Vallee and Falchuk 1993), the latter is always mediated simultaneously by several histidines (or other amino acids) and such Zn2+ binding is tight and resists acidification. For example, in a zinc metalloenzyme, alkaline phosphatase, a pH drop to 4.9 does not affect Zn2+ coordination by histidines (Gettins and Coleman 1983).
The idea that histidine is a major Zn2+-releasing ligand comes from the fact that the DEPC-mediated modification of imidazole is associated with an intracellular Zn2+ release (Haase and Beyersmann 2002). Since DEPC also promoted a Zn2+ release from cysteine (Fig. 4), the DEPC-induced [Zn2+]i elevations most likely result from a DEPC-mediated Zn2+ release from zinc-cysteine complexes.
That large amounts of Zn2+ are stored in zinc-cysteine complexes is indicated by the fact that an application of a sulfhydryl reactive agent, N-ethylmaleimide, leads to a major intracellular Zn2+ release (Haase and Beyersmann 2002; Nowakowski and Petering 2012). Interestingly, the released Zn2+ was shown to accumulate in Zn2+-storing organelles, which prior to the N-ethylmaleimide application contained only small amounts of labile Zn2+ (Haase and Beyersmann 2002). If such organelles are not loaded with large amounts of Zn2+ under basal conditions, they are not likely to play a major role in an acid-induced intracellular Zn2+ release. This observation supports the general conclusion of the present report that acid-induced [Zn2+] elevations are caused by a Zn2+i release from cytosolic zinc-cysteine complexes.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grants 1R03NS077095 and 1R21NS082786 and a part of the work was presented on poster 350.04 at the 2012 Society for Neuroscience Meeting. The author is grateful to Drs. Peter Gettins, Thomas O’Halloran, and C. William Shuttleworth for inspiring discussions on the subject and additionally to Dr. Gettins for access to the PTI QuantaMaster spectrofluorometer. The author declares his involvement in SPOT™ culture kit production at the University of Illinois at Chicago Research Resources Center.
Abbreviations used
- BCECF
2′,7′-bis-(2-carboxyethyl)-5(6)-carboxyfluorescein
- [Ca2+]i and [Zn2+]i
intracellular Ca2+ and Zn2+i concentration
- DEPC
diethylpyrocarbonate
- FCCP
carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone
- GSH
glutathione
- pHi and pHo
intracellular and extracellular pH
- ZnPyr signal
Fura-2FF or FluoZin-3 signal after application of zinc + pyrithione
- MOPS
3-(N-morpholino)propanesulfonic acid
- PIPES
piperazine-1,4-bis(2-ethanesulfonic acid)
- Rh123
rhodamine 123
- TPEN
N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine
Footnotes
Conflicts of interest
The author declares his involvement in SPOT culture kit production at the University of Illinois at Chicago Research Resources Center.
=> if ‘none’, insert “The authors have no conflict of interest to declare.”
=> otherwise insert info unless it is already include
References
- Anderson ME, Meister A. Marked increase of cysteine levels in many regions of the brain after administration of 2-oxothiazolidine-4-carboxylate. FASEB J. 1989;3:1632–1636. doi: 10.1096/fasebj.3.5.2920877. [DOI] [PubMed] [Google Scholar]
- Barry MA, Eastman A. Endonuclease activation during apoptosis: the role of cytosolic Ca2+ and pH. Biochem. Biophys. Res. Commun. 1992;186:782–789. doi: 10.1016/0006-291x(92)90814-2. [DOI] [PubMed] [Google Scholar]
- Biffo S, Grillo M, Margolis FL. Cellular localization of carnosine-like and anserine-like immunoreactivities in rodent and avian central nervous system. Neuroscience. 1990;35:637–651. doi: 10.1016/0306-4522(90)90335-2. [DOI] [PubMed] [Google Scholar]
- Brown AM, Kristal BS, Effron MS, Shestopalov AI, Ullucci PA, Sheu KF, Blass JP, Cooper AJ. Zn2+ inhibits alpha-ketoglutarate-stimulated mitochondrial respiration and the isolated alpha-ketoglutarate dehydrogenase complex. J. Biol. Chem. 2000;275:13441–13447. doi: 10.1074/jbc.275.18.13441. [DOI] [PubMed] [Google Scholar]
- Budd SL, Nicholls DG. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J. Neurochem. 1996;66:403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Cidon S, Ben-David H, Nelson N. ATP-driven proton fluxes across membranes of secretory organelles. J. Biol. Chem. 1983;258:11684–11688. [PubMed] [Google Scholar]
- Cidon S, Sihra TS. Characterization of a H+-ATPase in rat brain synaptic vesicles. Coupling to L-glutamate transport. J. Biol. Chem. 1989;264:8281–8288. [PubMed] [Google Scholar]
- Colvin RA. pH dependence and compartmentalization of zinc transported across plasma membrane of rat cortical neurons. Am. J. Physiol. Cell Physiol. 2002;282:C317–329. doi: 10.1152/ajpcell.00143.2001. [DOI] [PubMed] [Google Scholar]
- Corona C, Frazzini V, Silvestri E, Lattanzio R, La Sorda R, Piantelli M, Canzoniero LM, Ciavardelli D, Rizzarelli E, Sensi SL. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS One. 2011;6:e17971. doi: 10.1371/journal.pone.0017971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Ton-That D, Sensi SL, Weiss JH. Measuring zinc in living cells. A new generation of sensitive and selective fluorescent probes. Cell Calcium. 2002;31:245–251. doi: 10.1016/S0143-4160(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Gettins P, Coleman JE. 31P nuclear magnetic resonance of phosphoenzyme intermediates of alkaline phosphatase. J. Biol. Chem. 1983;258:408–416. [PubMed] [Google Scholar]
- Goldman SA, Pulsinelli WA, Clarke WY, Kraig RP, Plum F. The effects of extracellular acidosis on neurons and glia in vitro. J. Cereb. Blood Flow Metab. 1989;9:471–477. doi: 10.1038/jcbfm.1989.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb RA, Nordberg J, Skowronski E, Babior BM. Apoptosis induced in Jurkat cells by several agents is preceded by intracellular acidification. Proc. Natl. Acad. Sci. USA. 1996;93:654–658. doi: 10.1073/pnas.93.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Haase H, Beyersmann D. Intracellular zinc distribution and transport in C6 rat glioma cells. Biochem. Biophys. Res. Commun. 2002;296:923–928. doi: 10.1016/s0006-291x(02)02017-x. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Kimura K, Morioka S, Tamaki N. Inhibitory effects of Zn2+ on muscle glycolysis and their reversal by histidine. J. Nutr. Sci. Vitaminol. (Tokyo) 1980;26:357–366. doi: 10.3177/jnsv.26.357. [DOI] [PubMed] [Google Scholar]
- Isaev NK, Stelmashook EV, Lukin SV, Freyer D, Mergenthaler P, Zorov DB. Acidosis-induced zinc-dependent death of cultured cerebellar granule neurons. Cell. Mol. Neurobiol. 2010;30:877–883. doi: 10.1007/s10571-010-9516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn2+ induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J. Biol. Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- Jiang LJ, Vasak M, Vallee BL, Maret W. Zinc transfer potentials of the alpha - and beta-clusters of metallothionein are affected by domain interactions in the whole molecule. Proc. Natl. Acad. Sci. U S A. 2000;97:2503–2508. doi: 10.1073/pnas.97.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski L. Critical role of sodium in cytosolic [Ca2+] elevations in cultured hippocampal CA1 neurons during anoxic depolarization. J. Neurochem. 2007;100:915–923. doi: 10.1111/j.1471-4159.2006.04308.x. [DOI] [PubMed] [Google Scholar]
- Kiedrowski L. Cytosolic zinc release and clearance in hippocampal neurons exposed to glutamate - the role of pH and sodium. J. Neurochem. 2011;117:231–243. doi: 10.1111/j.1471-4159.2011.07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski L. Cytosolic acidification and intracellular zinc release in hippocampal neurons. J. Neurochem. 2012;121:438–450. doi: 10.1111/j.1471-4159.2012.07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krężel A, Maret W. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J. Biol. Inorg. Chem. 2006;11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- Krotkiewska B, Banaś T. Interaction of Zn2+ and Cu2+ ions with glyceraldehyde-3-phosphate dehydrogenase from bovine heart and rabbit muscle. Int. J. Biochem. 1992;24:1501–1505. doi: 10.1016/0020-711x(92)90078-f. [DOI] [PubMed] [Google Scholar]
- Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaiyandi LM, Dineley KE, Reynolds IJ. Divergent consequences arise from metallothionein overexpression in astrocytes: zinc buffering and oxidant-induced zinc release. Glia. 2004;45:346–353. doi: 10.1002/glia.10332. [DOI] [PubMed] [Google Scholar]
- Maranda B, Brown D, Bourgoin S, Casanova JE, Vinay P, Ausiello DA, Marshansky V. Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J. Biol. Chem. 2001;276:18540–18550. doi: 10.1074/jbc.M011577200. [DOI] [PubMed] [Google Scholar]
- Margolis FL. Carnosine in the primary olfactory pathway. Science. 1974;184:909–911. doi: 10.1126/science.184.4139.909. [DOI] [PubMed] [Google Scholar]
- Mari M, Morales A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009;11:2685–2700. doi: 10.1089/ars.2009.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AE, Smith RM, Motekaitis RJ. NIST Critically Selected Stability Constants of Metal Complexes. NIST Standard Reference Database 46 Version 8. 2004 [Google Scholar]
- McCranor BJ, Bozym RA, Vitolo MI, Fierke CA, Bambrick L, Polster BM, Fiskum G, Thompson RB. Quantitative imaging of mitochondrial and cytosolic free zinc levels in an in vitro model of ischemia/reperfusion. J. Bioenerg. Biomembr. 2012;44:253–263. doi: 10.1007/s10863-012-9427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miseta A, Csutora P. Relationship between the occurrence of cysteine in proteins and the complexity of organisms. Mol Biol Evol. 2000;17:1232–1239. doi: 10.1093/oxfordjournals.molbev.a026406. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA, Desai S, Pulsinelli WA. Acid-Induced death in neurons and glia. J. Neurosci. 1991;11:2489–2497. doi: 10.1523/JNEUROSCI.11-08-02489.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle A, Kandera J. Carnosine - an olfactory bulb peptide. Brain Res. 1974;80:359–364. doi: 10.1016/0006-8993(74)90701-x. [DOI] [PubMed] [Google Scholar]
- Nowakowski A, Petering D. Sensor specific imaging of proteomic Zn2+ with zinquin and TSQ after cellular exposure to N-ethylmaleimide. Metallomics. 2012;4:448–456. doi: 10.1039/c2mt00189f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I. Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J. Biol. Chem. 2009;284:17677–17686. doi: 10.1074/jbc.M109.007203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S, Moriyama Y, Takano T. Identification and characterization of a proton pump on lysosomes by fluorescein-isothiocyanate-dextran fluorescence. Proc. Natl. Acad. Sci. U S A. 1982;79:2758–2762. doi: 10.1073/pnas.79.9.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou-yang YB, Mellergård P, Kristián T, Kristánova V, Siesjö BK. Influence of acid-base changes on the intracellular calcium concentration of neurons in primary culture. Exp. Brain Res. 1994;101:265–271. doi: 10.1007/BF00228746. [DOI] [PubMed] [Google Scholar]
- Perrin DD, Sayce IG. Complex formation by nickel and zinc with penicillamine and cysteine. J. Chem. Soc. (A) 1968:53–57. [Google Scholar]
- Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50:98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana U, Kothinti R, Meeusen J, Tabatabai NM, Krezoski S, Petering DH. Zinc binding ligands and cellular zinc trafficking: apo-metallothionein, glutathione, TPEN, proteomic zinc, and Zn-Sp1. J. Inorg. Biochem. 2008;102:489–499. doi: 10.1016/j.jinorgbio.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice ME, Russo-Menna I. Differential compartmentalization of brain ascorbate and glutathione between neurons and glia. Neuroscience. 1998;82:1213–1223. doi: 10.1016/s0306-4522(97)00347-3. [DOI] [PubMed] [Google Scholar]
- Sensi SL, Ton-That D, Sullivan PG, Jonas EA, Gee KR, Kaczmarek LK, Weiss JH. Modulation of mitochondrial function by endogenous Zn2+ pools. Proc. Natl. Acad. Sci. U S A. 2003;100:6157–6162. doi: 10.1073/pnas.1031598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol. Sci. 2011;32:480–486. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemkowicz E, Hansen AJ. Brain extracellular ion composition and EEG activity following 10 minutes ischemia in normo- and hyperglycemic rats. Stroke. 1981;12:236–240. doi: 10.1161/01.str.12.2.236. [DOI] [PubMed] [Google Scholar]
- Siesjö BK, Bendek G, Koide T, Westerberg E, Wieloch T. Influence of acidosis on lipid peroxidation in brain tissues in vitro. J. Cereb. Blood Flow Metab. 1985;5:253–258. doi: 10.1038/jcbfm.1985.32. [DOI] [PubMed] [Google Scholar]
- Smith ML, von Hanwehr R, Siesjö BK. Changes in extra- and intracellular pH in the brain during and following ischemia in hyperglycemic and in moderately hypoglycemic rats. J. Cereb. Blood Flow Metab. 1986;6:574–583. doi: 10.1038/jcbfm.1986.104. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Wasserloos KJ, Dineley KE, Reynolds IJ, Levitan ES, Pitt BR. Nitric oxide-induced changes in intracellular zinc homeostasis are mediated by metallothionein/thionein. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L185–192. doi: 10.1152/ajplung.00267.2001. [DOI] [PubMed] [Google Scholar]
- Thomas RC. The effects of HCl and CaCl2 injections on intracellular calcium and pH in voltage-clamped snail (Helix aspersa) neurons. J. Gen. Physiol. 2002;120:567–579. doi: 10.1085/jgp.20028665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Ye J, Cho SH, Fuselier J, Li W, Beckwith J, Rapoport TA. Crystal structure of an unusual thioredoxin protein with a zinc finger domain. J. Biol. Chem. 2007;282:34945–34951. doi: 10.1074/jbc.M704044200. [DOI] [PubMed] [Google Scholar]
- Ying W, Han SK, Miller JW, Swanson RA. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J. Neurochem. 1999;73:1549–1556. doi: 10.1046/j.1471-4159.1999.0731549.x. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bertoglio BA, Devinney MJ, Jr., Dineley KE, Kay AR. The interaction of biological and noxious transition metals with the zinc probes FluoZin-3 and Newport Green. Anal. Biochem. 2009;384:34–41. doi: 10.1016/j.ab.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.