Abstract
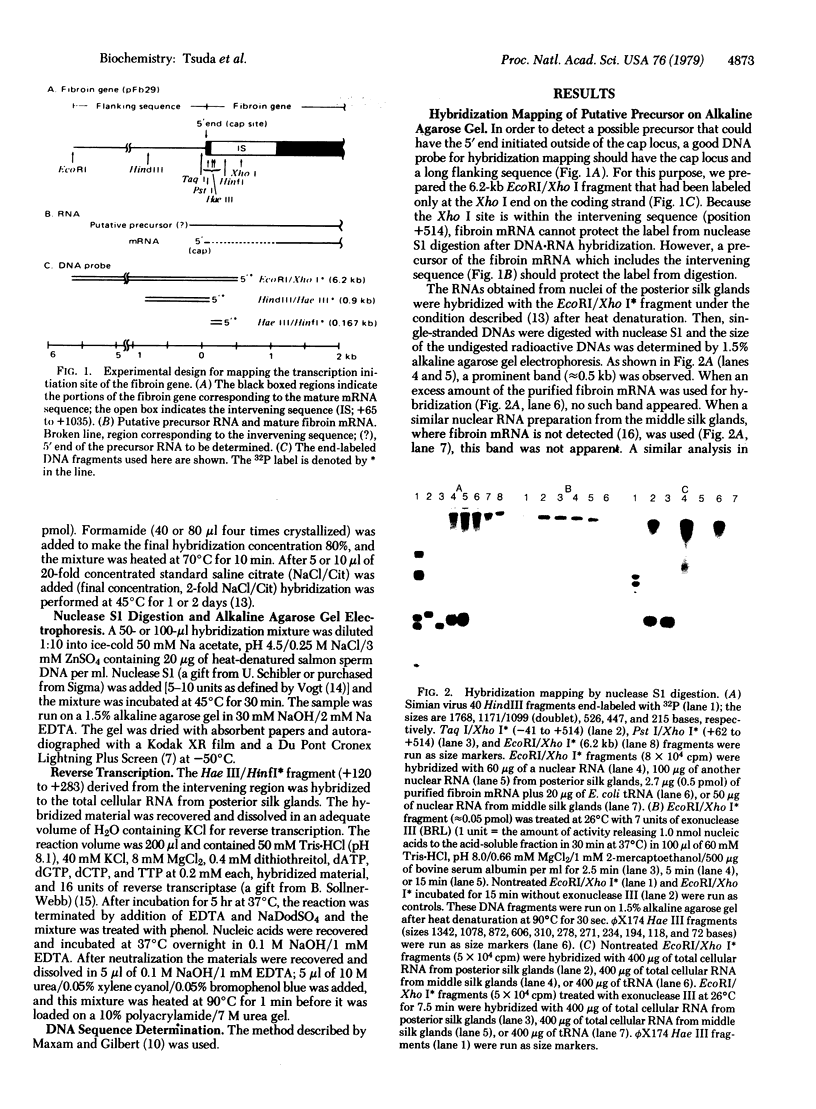
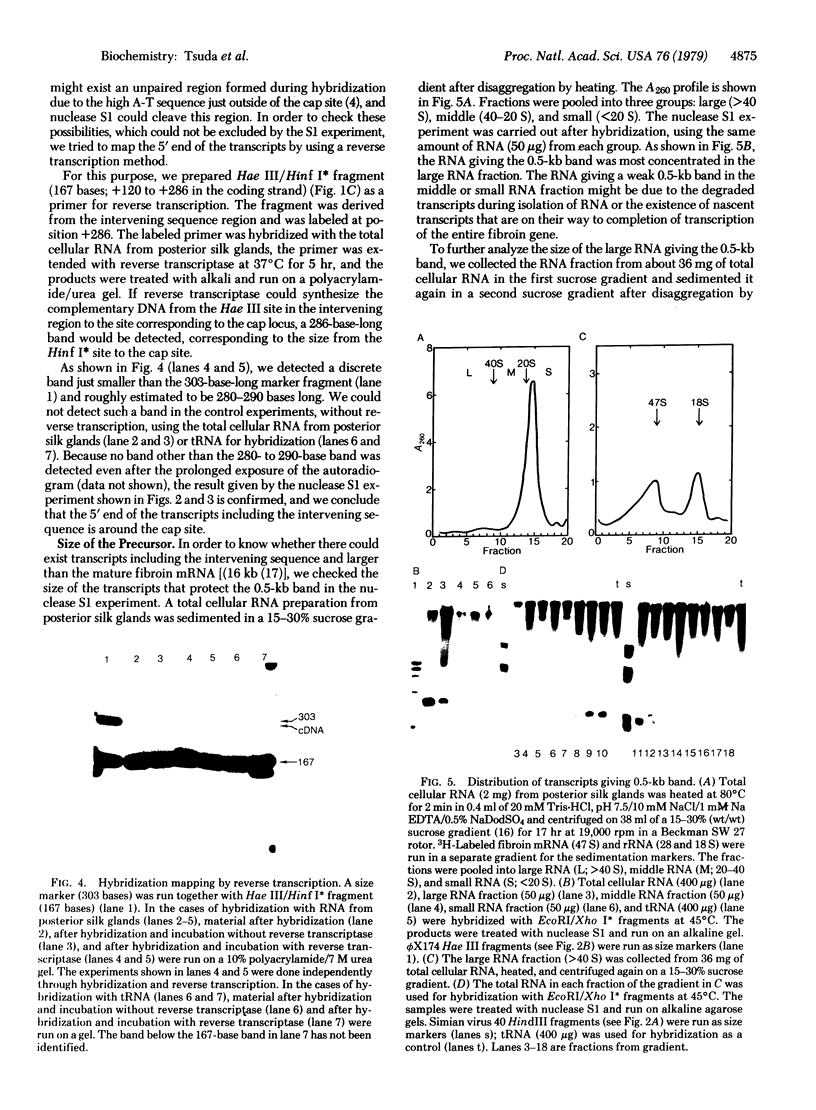
To map the initiation site of fibroin gene transcription on cloned genomic fibroin DNA (pFb29), we looked for precursors of fibroin mRNA, first by applying the hybridization mapping method to the RNA from the posterior silk gland of Bombyx mori. The 6.2-kilobase (kb) DNA fragment used for the assay, from 5.7 kb upstream to 0.5 kb downstream from the cap site, was end-labeled at the Xho I site (position +514) within the intervening sequence of the fibroin gene. By analyzing the labeled DNA protected by the RNA from nuclease S1 treatment, we have shown that the intervening sequence is transcribed together with the surrounding gene sequences in the posterior silk gland (producer) but not in the middle silk gland (nonproducer). Large transcripts that could include entire intervening (1 kb) and fibroin mRNA (16 kb) sequences were found. The 5' end of such transcripts was located around or near the cap locus. Synthesis and analysis of the DNA complementary to the transcripts primed within the intervening sequence region also confirmed that the 5' end of the transcripts is near the cap locus. These results strengthen the possibility that the cap site is the transcription initiation site of fibroin gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Excision of DNA segments introduced into cloning vectors by the poly(dA-dT) joining method. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1763–1767. doi: 10.1073/pnas.75.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung C. S. On the size relationship between nuclear and cytoplasmic RNA in sea urchin embryos. Dev Biol. 1974 Feb;36(2):343–356. doi: 10.1016/0012-1606(74)90056-6. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M., Williamson R., Brown D. D. The size of fibroin messenger RNA and its polyadenylic acid content. Cell. 1975 Mar;4(3):199–205. doi: 10.1016/0092-8674(75)90168-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Myers J. C., Spiegelman S., Kacian D. L. Synthesis of full-length DNA copies of avian myeloblastosis virus RNA in high yields. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2840–2843. doi: 10.1073/pnas.74.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Suzuki Y. Cloning of the silk fibroin gene and its flanking sequences. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5363–5367. doi: 10.1073/pnas.74.12.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G. E., Walkinshaw M. D., Arnott S. A computer aided oligonucleotide analysis provides a model sequence for RNA polymerase-promoter recognition in E.coli. Nucleic Acids Res. 1978 Oct;5(10):3759–3773. doi: 10.1093/nar/5.10.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Brown D. D. Isolation and identification of the messenger RNA for silk fibroin from Bombyx mori. J Mol Biol. 1972 Feb 14;63(3):409–429. doi: 10.1016/0022-2836(72)90437-8. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Gage L. P., Brown D. D. The genes for silk fibroin in Bombyx mori. J Mol Biol. 1972 Oct 14;70(3):637–649. doi: 10.1016/0022-2836(72)90563-3. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Giza P. E. Accentuated expression of silk fibroin genes in vivo and in vitro. J Mol Biol. 1976 Nov 5;107(3):183–206. doi: 10.1016/s0022-2836(76)80001-0. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Ohshima Y. Isolation and characterization of the silk fibroin gene with its flanking sequences. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):947–957. doi: 10.1101/sqb.1978.042.01.096. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki E. Quantitative measurements of fibroin messenger RNA synthesis in the posterior silk gland of normal and mutant Bombyx mori. J Mol Biol. 1974 Sep 15;88(2):393–407. doi: 10.1016/0022-2836(74)90490-2. [DOI] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Suzuki Y. Structural analysis of the fibroin gene at the 5' end and its surrounding regions. Cell. 1979 Feb;16(2):425–436. doi: 10.1016/0092-8674(79)90018-7. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]