Abstract
Background
Necrotizing enterocolitis (NEC) is the most common surgical emergency in neonates, with a mortality rate between 10 and 50%. The onset of necrotizing enterocolitis is highly variable and associated with numerous risk factors. Prior research has shown enteral supplementation with intestinal alkaline phosphatase (IAP) decreases the severity of NEC. The aim of this study is to investigate whether IAP is protective to the preterm intestine in the presence of formula feeding and in the absence of NEC.
Methods
Preterm rat pups were fed formula with or without supplementation with IAP, and intestine was obtained on day of life 3 for analysis of IAP activity, mRNA expression of TNF-a, IL-6 and iNOS and permeability and cytokine expression after LPS. exposure.
Results
There was no difference in the absolute and intestine specific alkaline phosphatase activity in both groups. Rat pups fed IAP had decreased mRNA expression of the inflammatory cytokines TNFα, IL-6 and iNOS. Pups supplemented with IAP had decreased permeability and inflammatory cytokine expression after exposure to LPS ex vivo when compared to formula fed controls.
Conclusions
Our results support that IAP is beneficial to preterm intestine and decreases intestinal injury and inflammation caused by LPS.
Keywords: Intestinal Alkaline Phosphatase, Necrotizing Enterocolitis, Toll like receptor 4, Lipopolysaccharide
Introduction
Necrotizing enterocolitis (NEC) is the most common surgical emergency in the neonate with an incidence of 0.3 to 2.4 per 1000 live births in the United States(1–5). It is a multifactorial disease that is very challenging to treat due to the fragility of the premature newborn. There are a myriad of identified risk factors including prematurity, formula feeding, bacterial colonization and ischemia. However, excluding supportive care, there are no interventions that have proven to prevent disease progression once NEC is diagnosed. Surgical interventions address only the complications of NEC and not the disease process. In either case, medical and surgical treatment of NEC is associated with enormous morbidity and mortality. Therefore, new strategies to prevent NEC are needed.
The immature neonatal gut is less able to handle insults such as bacterial colonization or formula feeding and is more susceptible to injury. Prior research has shown the preterm intestine to be more susceptible to inflammation compared to the term intestine even in absence of NEC(6). Novel therapies for premature newborns may be most effective in preventing NEC before clinical signs of NEC are identified. Despite known risk factors, it is difficult to identify which premature newborns will develop NEC. Therefore, preventative strategies must be effective but also not be harmful in order to treat the entire at risk population.
One such potential agent that has been shown to decrease intestinal inflammation is intestinal alkaline phosphatase (IAP), which is a member of a family of enzymes found throughout the body that dephosphorylate molecules. Importantly, IAP is an endogenous brush boarder enzyme of the intestine that is both incorporated into the membrane and secreted into the lumen where it is biologically active(7). Intestinal alkaline phosphatase is developmentally regulated and its expression and activity is decreased in the premature intestine and therefore may be deficient in the preterm newborn (6). While IAP has several proposed mechanisms of action, one mechanism of particular importance in NEC is its ability to dephosphorylate the lipid A moiety of lipopolysaccharide (LPS) thereby inactivating the molecule and preventing its interaction with toll like receptor 4 (TLR4) (8, 9).
Prior research has shown enterally supplemented IAP to be highly effective in decreasing the severity of NEC induced intestinal injury, down regulates numerous inflammatory cytokines and improves barrier function in a rat model of NEC(7–13). These beneficial effects of IAP appear to be dose dependent with 4u/kg/feed of enteral IAP to be the optimal dose at which a response is seen in a neonatal rodent NEC model(14). However, the effect of IAP on the premature intestine as a prophylactic measure, in absence of NEC, has not been examined. It is our hypothesis that replacement of the deficient IAP enzyme in the intestine will decrease baseline and LPS stimulated intestinal inflammation and permeability in the newborn intestine. This would further support the utility of IAP in the prevention and treatment of NEC in the neonate.
Methods
Animal Model
Animal experiments were approved by the animal care and use committee of the Medical College of Wisconsin (AUA number 0092). Control liters of full term Sprague-Dawley rat pups (Harlan laboratories, Madison, WI) were spontaneously born and dam-fed until they were sacrificed on day of life three. Preterm rat pups were delivered one day prematurely at estimated gestational age of 20 days (the gestational period of a rat is 21–23) via cesarean section, separated from the mother and maintained in an incubator at 37°C and 70% humidity. The premature rat pups were gavage fed formula via an orogastric tube three times daily. Premature rat pups were divided into two experimental groups. One half of the premature rat pups were supplemented with 4 units/kg IAP in each feed (Sigma-Aldrich) while the other half placebo. On day of life three, rat pups were euthanized using intraperitoneal injections of ketamine and xylazine (100 mg/kg, 10 mg/kg respectively) followed by the creation of a pneumothorax and intestinal tissue was harvested for analysis.
Alkaline phosphatase activity
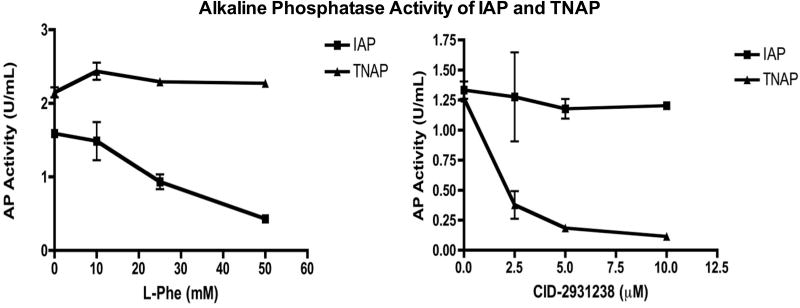
Alkaline phosphatase activity was measured from the ileal homogenate using a p-Nitrophenyl phosphate (pNPP) colorimetric assay (Thermo Scientific). Intestinal sections were placed in phosphate buffered saline and were homogenized using a bullet blender STORM (Next Advance, Averill Park, NY), centrifuged and the supernatant was used for the assay. After homogenization, the protein content was quantified using a Coomassie Plus Protein assay (Thermo Scientific). Absorbance of all samples was measured in duplicate at 450nm and compared to a blank well, which did not have pNPP. The alkaline phosphatase activity of each sample was measured in the presence of IAP and TNAP inhibitors (phenylalanine and 2,5-Dimethoxy-N-(quinolin-3-yl)benzene sulfonamide (CID-2931238), respectively) to determine the specific activity of each isoform. These inhibitors were tested in our laboratory for specificity using purified TNAP and IAP protein in the pNPP assay. Increasing concentrations of phenylalanine and CID-2931238 were added to purified IAP and TNAP in the pNPP assay. Figure 1 In vitro, both the TNAP and IAP inhibitors were selective for their respective isoform and almost completely inhibited all AP activity as illustrated in figure 1. A standard curve of pNPP dilutions was used to quantify the amount of alkaline phosphatase activity.
Figure 1.
Alkaline phosphatase activity of IAP and TNAP with their respective inhibitors (Phenylalanine and CID-2931238).
Real time PCR for iNOS, TNFα, IL-6, TNAP and IAP
Real time polymerase chain reaction (RT-PCR) was performed to evaluate for mean relative messenger RNA levels of IAP, TNAP, iNOS, TNFα, and IL-6 (IAP: Forward-CTGGGCGTCCATCAATADCGCCA, Reverse-CCTCCTCCACTGGGATGACACCA; TNAP: Qiagen Primer Assay Catalogue number PPR52402A; iNOS: GGCTGGAAGCCCCGCTATGG; TNFα: GTGTTTGAGATCCATGCCATTG; IL-6: CCTTCTGTGACTTAACTCTCC) in the terminal ileum(6). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control for reference. All intestine samples obtained for RNA analysis using RT-PCR were stored in RNA later until the RNA was isolated using the Qiagen RNeasy mini kit (Valencia, CA) with on column DNAse treatment. RNA concentration and purity was then determined using the Nanodrop spectrophotometer (SPEC-TRAmas PLUS; Molecular Devices, Sunnyvale, CA). Complementary DNA was synthesized from equal amounts of RNA using the EasyScript cDNA synthesis kit (Lambda Biotech, St. Louis, MO). Real-time polymerase chain reaction was performed using ABI Prism 7900HT software (Applied Biosystems) together with iQ SYBR Green RT-PCR Supermix (Bio-Rad). All gene amplification reactions were performed in triplicate.
Ex vivo intestinal loops and assessment of barrier function
The permeability of the terminal ileum was tested in a manner similar to previously described techniques(11, 15, 16). After the animals were sacrificed as described above, 1 to 1.5 cm sections of ileum were removed from the mesentery and filled with 10 mg/mL of 10kD fluorescein isothiocyanate dextran (FD-10, Sigma-Aldrich) mixed in phosphate buffered saline (Invitrogen). An adjacent loop of intestine was filled with FD-10 in PBS supplemented with 50 μg/mL LPS. The ends of the loops were secured with 5-0 silk suture and the loops were placed in 35 mm tissue culture treated dishes (Cell Treat, Shirley, MA) and filled with 2 mL of RPMI 1640 medium (Invitrogen) with 10% vol/vol heat inactivated fetal bovine serum (FBS). Loops were incubated for 1 hour in a cell culture incubator at 37°C and 5% CO2. Afterwards, 200 uL of the bathing solution was removed for measurement of translocation of FD-10. Fluorescence and concentration of FD-10 in the bathing solution was determined by a standard curve of known amounts of FD-10, normalized to intestinal length in millimeters.
Ex vivo model of intestinal injury
Sections of ileum were obtained from the above groups of pups and placed in six-well tissue culture plates (Becton Dickinson Labware, Franklin Lakes, NJ) with 2 mL of RPMI with 10% FBS and 50 μg/mL LPS. The intestine was incubated in a cell culture incubator at 37°C and 5% CO2 for six hours. After completing the incubation, the intestine was rinsed with PBS and placed in RNA later (Life Technologies).
Statistical Analysis
Using Prism software (version 6) data were analyzed using a Student’s t-test as the data is normally distributed. Individual comparisons were made between the control, formula fed and IAP-supplemented treatment groups. The data is reported as the mean and standard error of the mean and all tests were conducted as two-tailed tests. Differences were considered statistically significant if the p-value was ≤ 0.05.
Results
Intestinal Alkaline Phosphatase Activity is Decreased in Premature Formula-fed Rat Pups
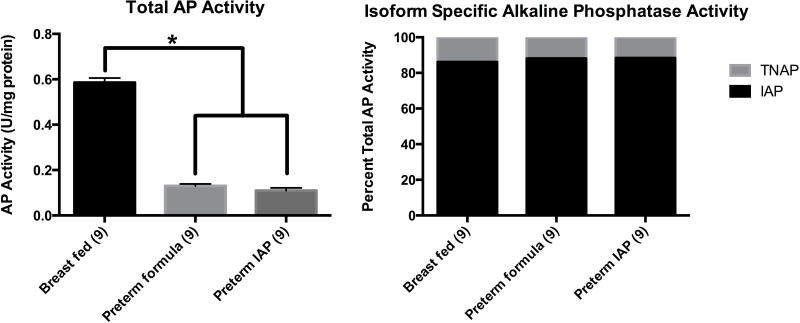
Total AP activity was measured from isolated segments of terminal ileum in the three groups of neonatal rat pups on the third day of life using the pNPP assay (Figure 2A). The breast-fed full-term control pups had a 6-fold increase in total AP activity in the terminal ileum versus the formula-fed preterm pups (Breast fed=0.59 U/mg vs. Preterm formula=0.13 U/mg, p<0.00001). The supplemental IAP had no significant effect on the total AP activity measured in the terminal ileum homogenates (Preterm IAP=0.11 U/mg). Adding phenylalanine and CID-2931238 ex vivoto the pNPP assay we were able to determine the relative fraction of IAP and TNAP in the intestinal homogenates of the terminal ileum from our three groups of rat pups (Figure 2B). Intestinal alkaline phosphatase accounted for 86% of total AP activity while TNAP only 14% of total AP activity in breast-fed full-term rat pups. Even though the total alkaline phosphatase activity was decreased in the preterm rat pups, IAP activity still accounted for 88% of total AP activity. Supplementation with enteral IAP had no effect on Total AP, IAP or TNAP activity in the terminal ileal intestinal homogenates (0.11, 0.10, 0.01, respectively).
Figure 2.
Figure 2A Total alkaline phosphatase activity of the terminal ileum in breast-fed, formula fed and IAP supplemented rat pups as measured by the pNPP assay. Results are expressed as means ± SEM, p<0.01 indicated by an asterisk. Figure 2B is the percentage of IAP and TNAP specific alkaline phosphatase activity in the terminal ileum.
Intestinal Alkaline Phosphatase Expression is Decreased in Premature Formula-fed Rats Pups
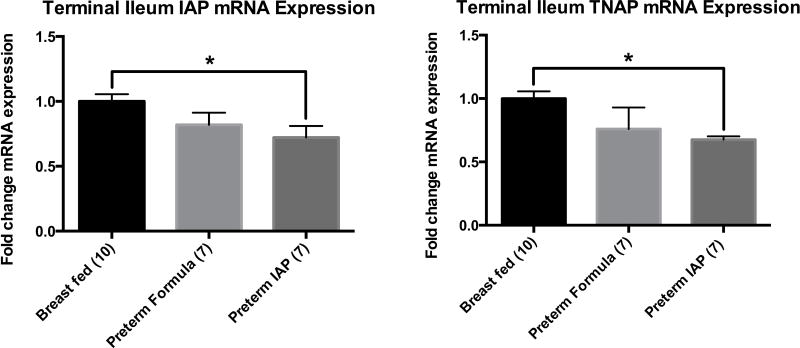
We also used RT-PCR to determine the mRNA expression of IAP and TNAP in the terminal ileal homogenates of our three groups of rat pups (Figure 3). On day three of life, relative to the expression of the breast-fed full-term rat pups, the formula-fed preterm rat pups had an 18% decrease in IAP mRNA expression (p=0.10) and 24% decrease in TNAP mRNA expression (p=0.15). The mRNA expression of IAP and TNAP in the formula-fed preterm rats supplemented with enteral IAP was significantly decreased compared to controls (IAP 0.72, p=0.01 and TNAP 0.68, p<0.0004).
Figure 3.
Relative mean expression mRNA expression of IAP and TNAP in the terminal ileum as measured by RT-PCR. Results are presented as mean expression ± SEM, P ≤ 0.01 indicated by an asterisk (*).
Supplemental, enteral intestinal alkaline phosphatase inhibits the increased expression of inflammatory cytokines in the terminal ileum of formula-fed preterm rat pups
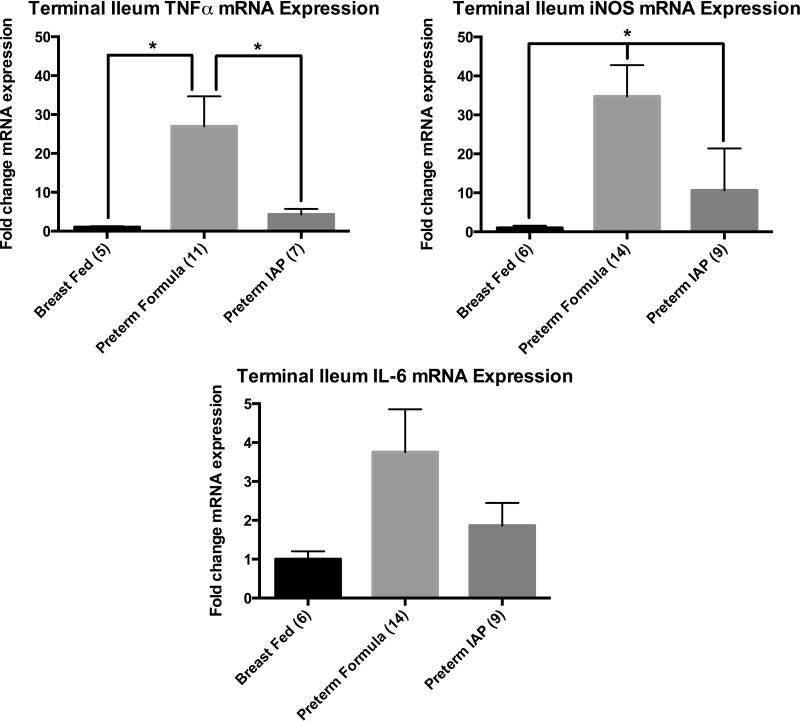
To further clarify the role of IAP in attenuation of inflammation in absence of additional stressors, expression of the inflammatory mediators IL-6, TNFα, and iNOS were chosen for study as they have been shown to be integral in the inflammation underlying NEC. As shown in figure 4, relative to the breast-fed full-term rat pups, the premature formula-fed pups had over a twenty-fold increase in bothTNF α (26.9 vs. 1, p=0.04) and iNOS (34.7 vs. 1, p=0.01) and a three-fold increase in IL-6 (3.8 vs. 1, p=0.12) mRNA expression compared to controls. Supplemental, enteral IAP significantly inhibited the increased inflammation in the formula-fed preterm rat pups. The mRNA expression of iNOS was decreased three-fold (p=0.03 vs. premature formula-fed) and TNFα five-fold (p=0.03 vs. premature formula-fed) in the ileum of premature rat pups fed IAP compared to the preterm formula-fed pups. There was no significant difference in the expression of IL-6 when comparing the control breast-fed pups to the preterm formula-fed pups or the preterm formula-fed pups supplemented with IAP.
Figure 4.
Relative mean mRNA expression of iNOS, IL-6 and TNFα measured by RT-PCR. Results are presented as mean expression ± SEM, P ≤ 0.02 indicated by an asterisk (*).
Supplemental, enteral IAP mitigates the increased intestinal permeability in formula-fed preterm rat pups
We sought to determine if supplemental, enteral IAP could prevent increased permeability of the small intestine in preterm rat pups using isolated ileal loops to measure flux of FITC-dextran 10 in the presence and absence of LPS exposure ex vivo (Figure 5). Compared to breast-fed full-term rat pups, the formula-fed preterm group had a significant increase in permeability at baseline (1.7×0^-3 vs. 1.1×10^-3, premature vs. breast-fed, respectively, p=0.03). After the intestinal loops were further challenged by the addition of LPS (50 μg/mL) ex vivo, we found a 30% increase in FITC-dextran flux detected in the formula-fed preterm pups compared to the breast-fed full-term rat pups (2.4×10^−3 vs. 1.7×10^−3, respectively, p=0.04). Importantly, the supplemental, enteral IAP fed to the formula-fed preterm rat pups resulted in a significant decrease in FITC-dextran flux when the terminal ileal loops were exposed ex vivoto LPS (1.6×10^−3 vs. 2.4×10^−3, preterm IAP-fed vs. premature formula-fed, respectively, p=0.01). Although there was a decrease in the baseline intestinal permeability in the formula-fed preterm rat pups supplemented with IAP compared to the formula-fed preterm rat pups this was not statistically significant.
Figure 5.
Permeability of rat pup intestine from breast-fed, preterm formula fed and preterm formula fed with IAP as measured by flux of FD-10. Results expressed as mean flux ± SEM. Difference is significant in every group except the breast-fed exposed to LPS when compared to the formula fed with LPS (p=0.1 for Breast fed with LPS vs. Formula fed with LPS, p ≤ 0.03 for all other groups compared to formula fed with LPS).
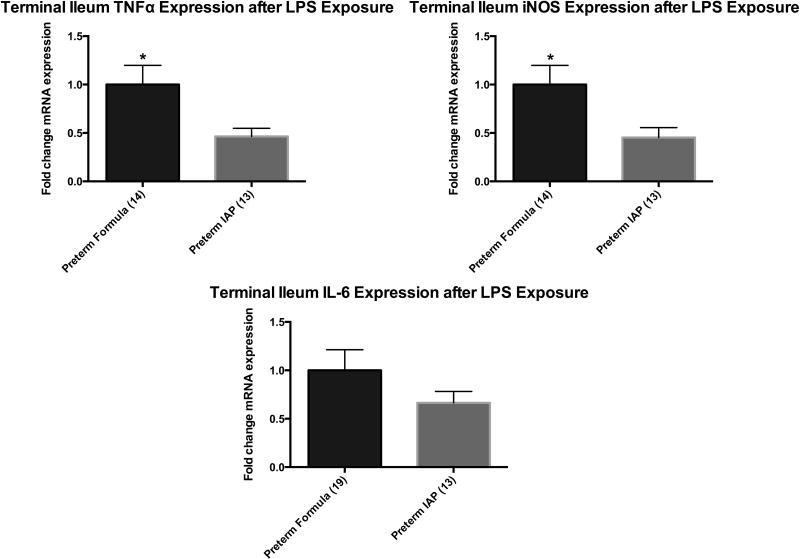
Supplemental, enteral IAP decreased LPS stimulated intestinal inflammation in the formula-fed preterm rat pup
We sought to determine if supplemental, enteral IAP could decrease LPS stimulated intestinal inflammation in the preterm intestine. The terminal ileum was obtained from preterm formula-fed rat pups and preterm rat pups supplemented with IAP on day of life 3 and stimulated ex vivo with 50 μg/mL of LPS for 6 hours. RT-PCR was used to quantify the mRNA expression of iNOS, TNFα and IL-6. The formula-fed preterm rat pups that received supplemental, enteral IAP experienced a decrease in LPS stimulated iNOS (p=0.02) and TNFα (p=0.02) mRNA expression when compared to preterm formula-fed rats. The expression of IL-6 was decreased in the intestine of animals supplemented with IAP, but this did not reach statistical significance (1 vs. 0.66, p=0.23).
Discussion
In this study, we evaluated the effect of IAP on premature rat pup intestine under the stress of formula feeding. We hypothesized that IAP would decrease intestinal inflammation and permeability in the newborn intestine, even in the absence of NEC. Our data demonstrate that formula-fed preterm rat pups have decreased IAP activity and, when compared to full-term breast-fed rat pups, premature formula-fed rat pups have increased intestinal inflammation and permeability and are more susceptible to LPS stimulated inflammation. When the formula is supplemented with IAP, however, the observed increase in inflammation and permeability is significantly decreased approximating levels seen in full-term, breast-fed control rats.
Our data showed the total AP activity and IAP specific activity in the formula-fed preterm rat pups was decreased compared to the breast-fed, full-term rat pups. Furthermore, in the newborn intestine, IAP specific activity accounts for over 80% of total AP activity in the terminal ileum. While there was some decrease in IAP gene expression, this was not statistically significant and may indicate that inhibition of IAP activity in the preterm intestine occurs downstream of gene expression. We believe this is one of the first reports to demonstrate that IAP expression and activity is decreased in premature and formula-fed rat pups. This is an important observation in that there is a clear association of a deficiency in IAP and increased intestinal inflammation in both human and animal models of intestinal inflammation (23–25). And, as was shown here, providing supplemental enteral IAP has been demonstrated to decrease intestinal inflammation when the endogenous production of IAP is inhibited (23, 25, 26).
The dose of IAP used in our study was chosen based on our previous results using a dose range of IAP to try to mitigate intestinal injury and inflammation in a neonatal rat model of NEC that demonstrated 4u/kg per feed to be the lowest dose effective in significantly preventing NEC (10). We previously observed in the NEC model that supplemental, enteral IAP increased the total AP activity in the intestine in rat pups with NEC. However, we did not observe an increase in either total AP or IAP specific activity. The conflicting results from these two experiments may be the result of several possibilities. The previously published data was measured in the mid-small intestine while our data is measured in the distal ileum. Additionally, the cause of the decreased expression of IAP in NEC compared to prematurity may be different. The NEC model is known to cause intestinal ischemia and necrosis, which likely depletes the endogenous expression of IAP. However, supplemental, enteral IAP significantly diminished the severity of intestinal injury in the NEC model, potentially allowing for the observed increased AP activity. Here we used a formula-fed preterm newborn rat and the cause of decreased IAP expression and activity is likely different from the NEC model. Intestinal alkaline phosphatase is developmentally regulated and its expression is decreased by times of starvation (27, 28). Premature weaning of piglets has been demonstrated to significantly decrease IAP expression and activity (29). Therefore, the decrease in production of IAP in the formula-fed preterm rats may be a result of developmental regulation as well as the lack of some induction agent in breast milk not found in neonatal formula. Another possible explanation for the difference is LPS has previously been demonstrated to stimulate the expression of IAP (23). In our experiments presented here the animals were not exposed to LPS as they are in our prior reports utilizing a NEC model. Lastly, it remains to be determined if the supplemental IAP remains intraluminal, becomes incorporated in the mucosal matrix or is absorbed by the intestinal epithelium. This is the subject of future investigation. By measuring IAP in the intestinal homogenate we lose the portion of IAP that remains intraluminal.
The inflammatory cytokine cascades have been well studied in patients with sepsis and NEC. Prior research has shown that neonates developing sepsis or severe NEC have increased transcription of IL-6, TNFα and iNOS (18, 30). These cytokines were shown to increase within 1.5 hours of starting enteral formula feeds in rat pups and remain elevated for 24 hours (6). Additionally, when rat pups were exposed to hypoxia and LPS, there is a significant increase in these cytokines (6, 10). Therefore we focused on these cytokines to determine whether enteral supplementation with IAP affects the baseline level of inflammation caused by formula feeding and can blunt the inflammatory response caused by an LPS challenge. Our data show that by day of life three, prematurity and formula feeding is associated with an increase in the expression of iNOS, TNFα and IL-6 compared to breast fed controls. The addition of IAP to the formula led to a decrease in the expression of iNOS, TNFα and IL-6 when compared to formula fed animals. This data supports our hypothesis that IAP would be effective in preventing increased intestinal inflammation in the newborn intestine. Our results are inline with those recently shown in an adult rodent model of metabolic syndrome (31). This study again demonstrated that both the inhibition of IAP with phenylalanine and an IAP knockout mouse was associated with increased intestinal inflammation and permeability that lead to metabolic syndrome. As shown in our results, the supplementation of enteral IAP prevented this increased inflammation in this model as well.
Prior hypotheses about the beneficial effects of IAP suggest a mechanism by which IAP dephosphorylates LPS, thereby inactivating the molecule (10). This data shows that IAP still has significant beneficial effects on the premature pup intestine in the absence of enteral supplemented LPS thereby suggesting an additional mechanism by which enteral IAP affects the intestine. The mechanism by which IAP works in the intestine is incompletely understood. One hypothesis regarding a mechanism by which IAP alters the intestinal epithelium’s inflammation is that there is a change in the TLR4 receptor caused by IAP (17, 21). Toll like receptor 4 expression was shown to decrease in intestinal epithelial cell lines following exposure to butyrate. Butyrate is known to increase the expression of IAP and following exposure to butyrate, TLR4 expression decreased while IAP increased in vitro (32). Prior research has shown that TLR4 stimulation is important in the development of NEC. The TLR4 receptor expression is increased shortly after birth and remains increased with stimulation by LPS (17, 33). Additionally, TLR4 knockout mice and enterocyte specific deletion of TLR4 in mice was protective from the development of NEC suggesting that the TLR4 signaling cascade is integral to the development of NEC (34). We also measured the expression of inflammatory cytokines in the pup intestine after exposure to LPS ex vivo. We found that, compared to preterm controls, the rat pups fed IAP had decreased permeability and expression of iNOS, TNFα, and IL-6 after exposure to LPS ex vivo. As discussed earlier, these cytokines are pivotal in the development of NEC and their increased expression in NEC is regulated through the TLR4 signaling pathway (19, 35, 36). While this ex vivo model has some limitations, it allowed for more direct control of the secondary insult. Importantly, this data suggests that supplemental, enteral IAP not only decreases the baseline intestinal inflammation associated with prematurity and formula feeding, but also to a secondary insult from LPS. Our data also support a hypothesis that one of the major effects of IAP in the newborn intestine is by inhibition of the LPS-TLR4 signaling cascade. While we believe IAP affects the LPS-TLR4 signaling cascade, further work is needed to examine if IAP does this entirely by inactivating LPS or if it also has some effect on the TLR4 signaling pathway as well. Further investigation is necessary to measure TLR4 protein expression, localization as well as the effect of IAP downstream of the TLR4 receptor.
Recent research has shown IAP to influence the intestinal microbiome by improving the bacterial heterogeneity (37, 40). This influence is thought to promote colonization with commensal bacteria and prevent over-population with pathogenic bacteria. While the presence of bacteria in the newborn intestine has been a long held belief as necessary for NEC, it is only recently that we are beginning to have an understanding that dysbiosis rather than colonization may be a contributor to the development of NEC (41–43). A recent study has shown that IAP can dephosphorylate nucleotide tri-phosphates and the dephosphorylation of UDP may have a role in promoting intestinal health. This is another potential mechanism by which IAP may mitigate intestinal inflammation (44). All of these mechanisms warrant further investigation.
Clinically, numerous strategies have been evaluated to lessen the risk of NEC including the use of antenatal steroids, enteral feeding schedules, breast milk, and probiotics (45–47). Unfortunately, none of these interventions have eliminated the risk NEC. Our prior research has shown that, in an animal model, IAP decreases the severity of intestinal injury and the expression of inflammatory cytokines in the presence of NEC-like stress (6, 11, 12, 14, 48). The findings presented here support our hypothesis that the premature intestine is at increased risk of intestinal inflammation and NEC due to a relative absence of IAP. Furthermore, we showed that supplementing premature newborns with enteral IAP may prevent the development of NEC.
Figure 6.
Relative mean mRNA expression of TNFα, iNOS and IL-6 measured by RT-PCR after exposure of the intestine to LPS for 6 hours ex vivo. Results are presented as mean expression ± SEM, P=0.02 indicated by an asterisk (*).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christensen RD, Gordon PV, Besner GE. Can we cut the incidence of necrotizing enterocolitis in half--today? Fetal Pediatr Pathol. 2010;29(4):185–98. doi: 10.3109/15513815.2010.483874. [DOI] [PubMed] [Google Scholar]
- 2.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, et al. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2006 Feb;117(2):e137–42. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 3.Holman RC, Stoll BJ, Curns AT, Yorita KL, Steiner CA, Schonberger LB. Necrotising enterocolitis hospitalisations among neonates in the united states. Paediatr Perinat Epidemiol. 2006 Nov;20(6):498–506. doi: 10.1111/j.1365-3016.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 4.Llanos AR, Moss ME, Pinzon MC, Dye T, Sinkin RA, Kendig JW. Epidemiology of neonatal necrotising enterocolitis: A population-based study. Paediatr Perinat Epidemiol. 2002 Oct;16(4):342–9. doi: 10.1046/j.1365-3016.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- 5.Sankaran K, Puckett B, Lee DS, Seshia M, Boulton J, Qiu Z, et al. Variations in incidence of necrotizing enterocolitis in canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004 Oct;39(4):366–72. doi: 10.1097/00005176-200410000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Rentea RM, Welak SR, Fredrich K, Donohoe D, Pritchard KA, Oldham KT, et al. Early enteral stressors in newborns increase inflammatory cytokine expression in a neonatal necrotizing enterocolitis rat model. Eur J Pediatr Surg. 2013 Feb;23(1):39–47. doi: 10.1055/s-0032-1329704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beumer C, Wulferink M, Raaben W, Fiechter D, Brands R, Seinen W. Calf intestinal alkaline phosphatase, a novel therapeutic drug for lipopolysaccharide (LPS)-mediated diseases, attenuates LPS toxicity in mice and piglets. J Pharmacol Exp Ther. 2003 Nov;307(2):737–44. doi: 10.1124/jpet.103.056606. [DOI] [PubMed] [Google Scholar]
- 8.Koyama I, Matsunaga T, Harada T, Hokari S, Komoda T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem. 2002 Sep;35(6):455–61. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]
- 9.Tuin A, Huizinga-Van der Vlag A, van Loenen-Weemaes AM, Meijer DK, Poelstra K. On the role and fate of LPS-dephosphorylating activity in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006 Feb;290(2):G377–85. doi: 10.1152/ajpgi.00147.2005. [DOI] [PubMed] [Google Scholar]
- 10.Whitehouse JS, Riggle KM, Purpi DP, Mayer AN, Pritchard KA, Jr, Oldham KT, et al. The protective role of intestinal alkaline phosphatase in necrotizing enterocolitis. J Surg Res. 2010 Sep;163(1):79–85. doi: 10.1016/j.jss.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Rentea RM, Liedel JL, Welak SR, Cassidy LD, Mayer AN, Pritchard KA, Jr, et al. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg. 2012 Jun;47(6):1135–42. doi: 10.1016/j.jpedsurg.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Rentea RM, Liedel JL, Fredrich K, Welak SR, Pritchard KA, Jr, Oldham KT, et al. Intestinal alkaline phosphatase administration in newborns decreases systemic inflammatory cytokine expression in a neonatal necrotizing enterocolitis rat model. J Surg Res. 2012 Oct;177(2):228–34. doi: 10.1016/j.jss.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM. Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect Immun. 2005 Jul;73(7):4309–14. doi: 10.1128/IAI.73.7.4309-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rentea RM, Liedel JL, Fredrich K, Pritchard K, Jr, Oldham KT, Simpson PM, et al. Enteral intestinal alkaline phosphatase administration in newborns decreases iNOS expression in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg. 2013 Jan;48(1):124–8. doi: 10.1016/j.jpedsurg.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, et al. Intestinal barrier failure during experimental necrotizing enterocolitis: Protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol. 2006 Nov;291(5):G938–49. doi: 10.1152/ajpgi.00090.2006. [DOI] [PubMed] [Google Scholar]
- 16.Liedel JL, Guo Y, Yu Y, Shiou SR, Chen S, Petrof EO, et al. Mother’s milk-induced Hsp70 expression preserves intestinal epithelial barrier function in an immature rat pup model. Pediatr Res. 2011 May;69(5 Pt 1):395–400. doi: 10.1203/PDR.0b013e3182114ec9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gribar SC, Sodhi CP, Richardson WM, Anand RJ, Gittes GK, Branca MF, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol. 2009 Jan 1;182(1):636–46. doi: 10.4049/jimmunol.182.1.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanthakumar N, Meng D, Goldstein AM, Zhu W, Lu L, Uauy R, et al. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS One. 2011 Mar 21;6(3):e17776. doi: 10.1371/journal.pone.0017776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, Meldrum DR. Cytokines in necrotizing enterocolitis. Shock. 2006 Apr;25(4):329–37. doi: 10.1097/01.shk.0000192126.33823.87. [DOI] [PubMed] [Google Scholar]
- 20.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, et al. A critical role for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol. 2007 Oct 1;179(7):4808–20. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 21.Soliman A, Michelsen KS, Karahashi H, Lu J, Meng FJ, Qu X, et al. Platelet-activating factor induces TLR4 expression in intestinal epithelial cells: Implication for the pathogenesis of necrotizing enterocolitis. PLoS One. 2010 Oct 15;5(10):e15044. doi: 10.1371/journal.pone.0015044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfs TG, Derikx JP, Hodin CM, Vanderlocht J, Driessen A, de Bruine AP, et al. Localization of the lipopolysaccharide recognition complex in the human healthy and inflamed premature and adult gut. Inflamm Bowel Dis. 2010 Jan;16(1):68–75. doi: 10.1002/ibd.20995. [DOI] [PubMed] [Google Scholar]
- 23.Bates JM, Akerlund J, Mittge E, Guillemin K. Intestinal alkaline phosphatase detoxifies lipopolysaccharide and prevents inflammation in zebrafish in response to the gut microbiota. Cell Host Microbe. 2007 Dec 13;2(6):371–82. doi: 10.1016/j.chom.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Molnar K, Vannay A, Szebeni B, Banki NF, Sziksz E, Cseh A, et al. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J Gastroenterol. 2012 Jul 7;18(25):3254–9. doi: 10.3748/wjg.v18.i25.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ramasamy S, Nguyen DD, Eston MA, Alam SN, Moss AK, Ebrahimi F, et al. Intestinal alkaline phosphatase has beneficial effects in mouse models of chronic colitis. Inflamm Bowel Dis. 2011 Feb;17(2):532–42. doi: 10.1002/ibd.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):7003–8. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg RF, Austen WG, Jr, Zhang X, Munene G, Mostafa G, Biswas S, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3551–6. doi: 10.1073/pnas.0712140105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lalles JP. Intestinal alkaline phosphatase: Multiple biological roles in maintenance of intestinal homeostasis and modulation by diet. Nutr Rev. 2010 Jun;68(6):323–32. doi: 10.1111/j.1753-4887.2010.00292.x. [DOI] [PubMed] [Google Scholar]
- 29.Lackeyram D, Yang C, Archbold T, Swanson KC, Fan MZ. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J Nutr. 2010 Mar;140(3):461–8. doi: 10.3945/jn.109.117267. [DOI] [PubMed] [Google Scholar]
- 30.Sharma R, Tepas JJ, 3rd, Hudak ML, Mollitt DL, Wludyka PS, Teng RJ, et al. Neonatal gut barrier and multiple organ failure: Role of endotoxin and proinflammatory cytokines in sepsis and necrotizing enterocolitis. J Pediatr Surg. 2007 Mar;42(3):454–61. doi: 10.1016/j.jpedsurg.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 31.Kaliannan K, Hamarneh SR, Economopoulos KP, Nasrin Alam S, Moaven O, Patel P, et al. Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Natl Acad Sci U S A. 2013 Apr 23;110(17):7003–8. doi: 10.1073/pnas.1220180110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bocker U, Yezerskyy O, Feick P, Manigold T, Panja A, Kalina U, et al. Responsiveness of intestinal epithelial cell lines to lipopolysaccharide is correlated with toll-like receptor 4 but not toll-like receptor 2 or CD14 expression. Int J Colorectal Dis. 2003 Jan;18(1):25–32. doi: 10.1007/s00384-002-0415-6. [DOI] [PubMed] [Google Scholar]
- 33.Hackam DJ, Afrazi A, Good M, Sodhi CP. Innate immune signaling in the pathogenesis of necrotizing enterocolitis. Clinical and Developmental Immunology. 2013;2013:1–10. doi: 10.1155/2013/475415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sodhi CP, Neal MD, Siggers R, Sho S, Ma C, Branca MF, et al. Intestinal epithelial toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology. 2012 Sep;143(3):708, 18. e1–5. doi: 10.1053/j.gastro.2012.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Claud EC, Zhang X, Petrof EO, Sun J. Developmentally regulated tumor necrosis factor-alpha induced nuclear factor-kappaB activation in intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2007 May;292(5):G1411–9. doi: 10.1152/ajpgi.00557.2006. [DOI] [PubMed] [Google Scholar]
- 36.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997 Feb;32(2):275–82. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 37.Malo MS, Alam SN, Mostafa G, Zeller SJ, Johnson PV, Mohammad N, et al. Intestinal alkaline phosphatase preserves the normal homeostasis of gut microbiota. Gut. 2010 Nov;59(11):1476–84. doi: 10.1136/gut.2010.211706. [DOI] [PubMed] [Google Scholar]
- 38.Chen KT, Malo MS, Moss AK, Zeller S, Johnson P, Ebrahimi F, et al. Identification of specific targets for the gut mucosal defense factor intestinal alkaline phosphatase. Am J Physiol Gastrointest Liver Physiol. 2010 Aug;299(2):G467–75. doi: 10.1152/ajpgi.00364.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moss AK, Hamarneh SR, Mohamed MM, Ramasamy S, Yammine H, Patel P, et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am J Physiol Gastrointest Liver Physiol. 2013 Mar 15;304(6):G597–604. doi: 10.1152/ajpgi.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen KT, Malo MS, Beasley-Topliffe LK, Poelstra K, Millan JL, Mostafa G, et al. A role for intestinal alkaline phosphatase in the maintenance of local gut immunity. Dig Dis Sci. 2011 Apr;56(4):1020–7. doi: 10.1007/s10620-010-1396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlisle EM, Morowitz MJ. The intestinal microbiome and necrotizing enterocolitis. Curr Opin Pediatr. 2013 Jun;25(3):382–7. doi: 10.1097/MOP.0b013e3283600e91. [DOI] [PubMed] [Google Scholar]
- 42.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. doi: 10.1371/journal.pone.0020647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart CJ, Marrs EC, Nelson A, Lanyon C, Perry JD, Embleton ND, et al. Development of the preterm gut microbiome in twins at risk of necrotising enterocolitis and sepsis. PLoS One. 2013 Aug 30;8(8):e73465. doi: 10.1371/journal.pone.0073465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss AK, Hamarneh SR, Mohamed MM, Ramasamy S, Yammine H, Patel P, et al. Intestinal alkaline phosphatase inhibits the proinflammatory nucleotide uridine diphosphate. Am J Physiol Gastrointest Liver Physiol. 2013 Mar 15;304(6):G597–604. doi: 10.1152/ajpgi.00455.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin HY, Chang JH, Chung MY, Lin HC. Prevention of necrotizing enterocolitis in preterm very low birth weight infants: Is it feasible? J Formos Med Assoc. 2013 May 20; doi: 10.1016/j.jfma.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 46.Probiotics for the prevention of necrotizi. J clin pharm ther. 2013 PubMed - NCBI [Internet].; cited 8/18/2013]. Available from: http://www.ncbi.nlm.nih.gov.proxy.lib.mcw.edu/pubmed/23865733.
- 47.Necrotizing enterocolitis: Pathophysiolog. semin pediatr surg. 2013 PubMed - NCBI [Internet].; cited 8/18/2013]. Available from: http://www.ncbi.nlm.nih.gov.proxy.lib.mcw.edu/pubmed/23611612.
- 48.Riggle KM, Rentea RM, Welak SR, Pritchard KA, Jr, Oldham KT, Gourlay DM. Intestinal alkaline phosphatase prevents the systemic inflammatory response associated with necrotizing enterocolitis. J Surg Res. 2013 Mar;180(1):21–6. doi: 10.1016/j.jss.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]