Abstract
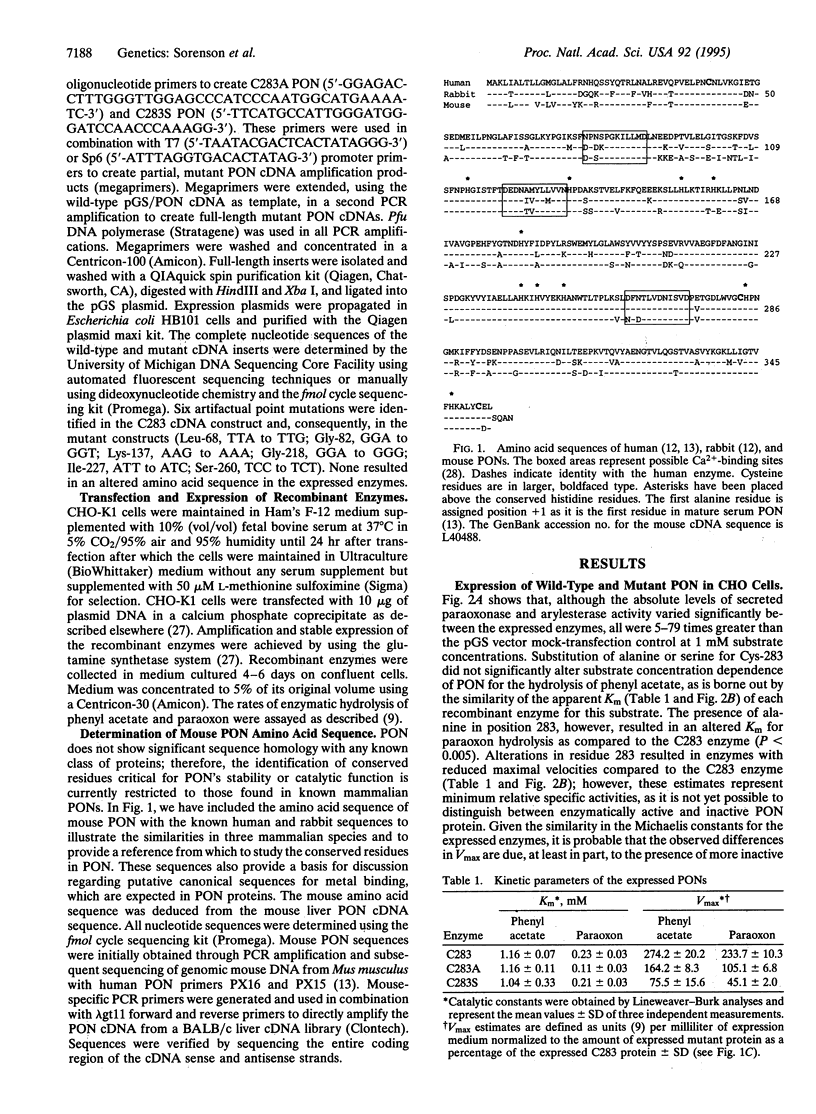
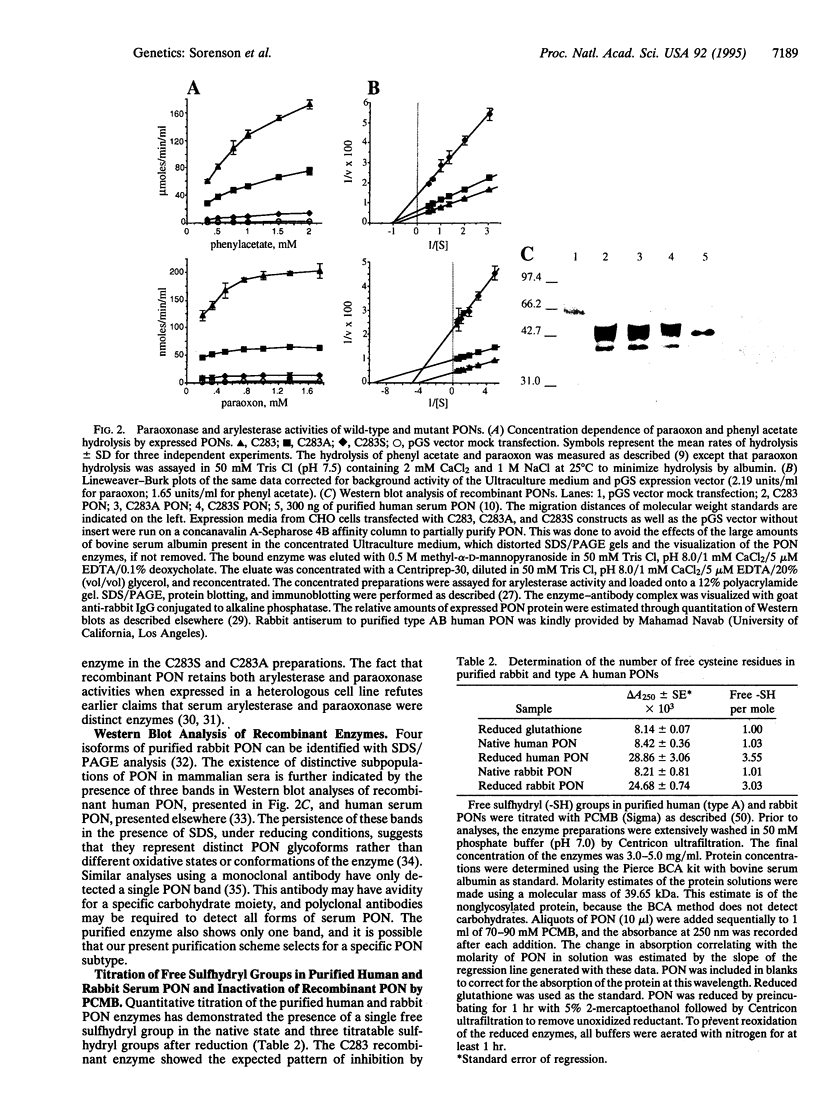
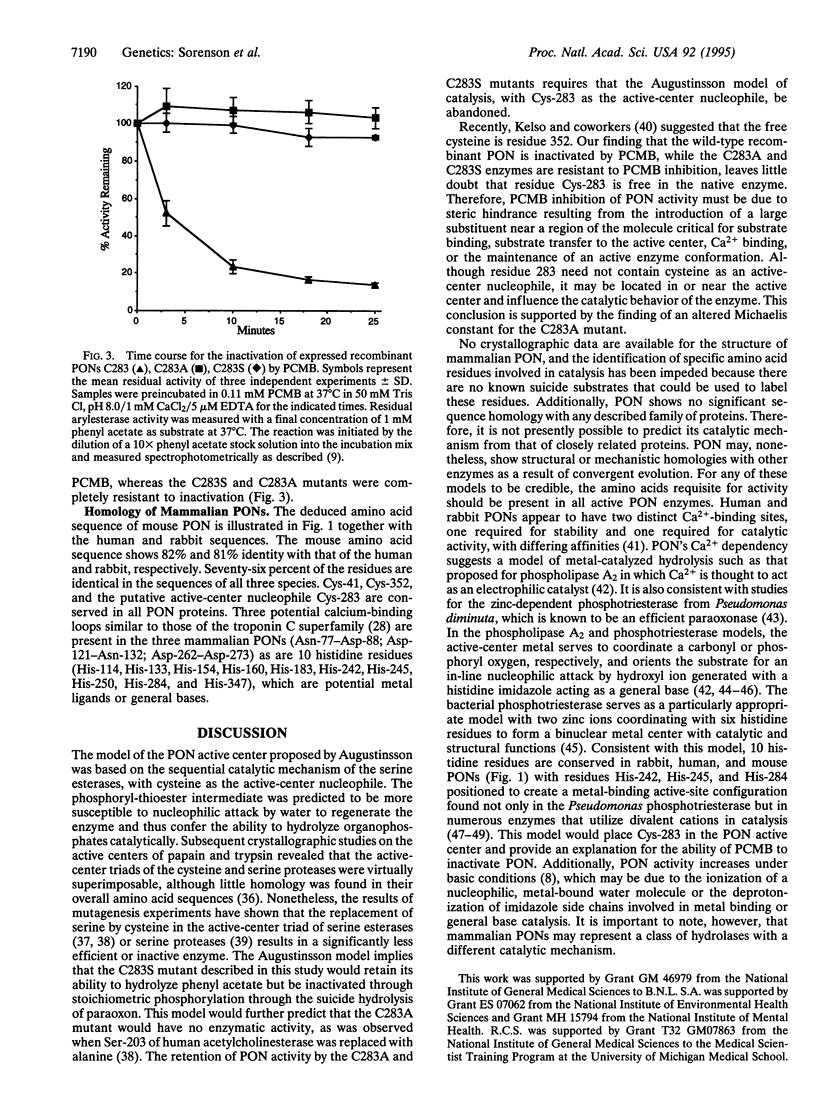
For three decades, mammalian paraoxonase (A-esterase, aromatic esterase, arylesterase; PON, EC 3.1.8.1) has been thought to be a cysteine esterase demonstrating structural and mechanistic homologies with the serine esterases (cholinesterases and carboxyesterases). Human, mouse, and rabbit PONs each contain only three cysteine residues, and their positions within PON have been conserved. In purified human PON, residues Cys-41 and Cys-352 form an intramolecular disulfide bond and neither could function as an active-center cysteine. Highly purified, enzymatically active PON contains a single titratable sulfhydryl group. Thus, Cys-283 is the only probable candidate for an active-center cysteine. Through site-directed mutagenesis of the human cDNA, Cys-283 was replaced with either serine (C283S) or alanine (C283A). The expressed C283 (wild-type) enzyme was inactivated by para-hydroxymercuribenzoate, but the C283S and C283A mutant enzymes were not inactivated. C283A and C283S mutant enzymes retained both paraoxonase and arylesterase activities, and the Km values for paraoxon and phenyl acetate were similar to those of the wild type. Clearly, residue Cys-283 is free in active PON, but a free sulfhydryl group is not required for either paraoxonase or arylesterase activities. Consequently, it is necessary to examine other models for the active-site structure and catalytic mechanism of PON.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGUSTINSSON K. B. ARYLESTERASES. J Histochem Cytochem. 1964 Oct;12:744–747. doi: 10.1177/12.10.744. [DOI] [PubMed] [Google Scholar]
- Adkins S., Gan K. N., Mody M., La Du B. N. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: glutamine or arginine at position 191, for the respective A or B allozymes. Am J Hum Genet. 1993 Mar;52(3):598–608. [PMC free article] [PubMed] [Google Scholar]
- Blatter Garin M. C., Abbott C., Messmer S., Mackness M., Durrington P., Pometta D., James R. W. Quantification of human serum paraoxonase by enzyme-linked immunoassay: population differences in protein concentrations. Biochem J. 1994 Dec 1;304(Pt 2):549–554. doi: 10.1042/bj3040549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter M. C., James R. W., Messmer S., Barja F., Pometta D. Identification of a distinct human high-density lipoprotein subspecies defined by a lipoprotein-associated protein, K-45. Identity of K-45 with paraoxonase. Eur J Biochem. 1993 Feb 1;211(3):871–879. doi: 10.1111/j.1432-1033.1993.tb17620.x. [DOI] [PubMed] [Google Scholar]
- Chen B., Przybyla A. E. An efficient site-directed mutagenesis method based on PCR. Biotechniques. 1994 Oct;17(4):657–659. [PubMed] [Google Scholar]
- Dumas D. P., Caldwell S. R., Wild J. R., Raushel F. M. Purification and properties of the phosphotriesterase from Pseudomonas diminuta. J Biol Chem. 1989 Nov 25;264(33):19659–19665. [PubMed] [Google Scholar]
- Eckerson H. W., Romson J., Wyte C., La Du B. N. The human serum paraoxonase polymorphism: identification of phenotypes by their response to salts. Am J Hum Genet. 1983 Mar;35(2):214–227. [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Costa L. G., Hassett C., Richter R. J., Sundstrom J. A., Adler D. A., Disteche C. M., Omiecinski C. J., Chapline C., Crabb J. W. Human and rabbit paraoxonases: purification, cloning, sequencing, mapping and role of polymorphism in organophosphate detoxification. Chem Biol Interact. 1993 Jun;87(1-3):35–48. doi: 10.1016/0009-2797(93)90023-r. [DOI] [PubMed] [Google Scholar]
- Furlong C. E., Richter R. J., Chapline C., Crabb J. W. Purification of rabbit and human serum paraoxonase. Biochemistry. 1991 Oct 22;30(42):10133–10140. doi: 10.1021/bi00106a009. [DOI] [PubMed] [Google Scholar]
- Gan K. N., Smolen A., Eckerson H. W., La Du B. N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab Dispos. 1991 Jan-Feb;19(1):100–106. [PubMed] [Google Scholar]
- Garavito R. M., Rossmann M. G., Argos P., Eventoff W. Convergence of active center geometries. Biochemistry. 1977 Nov 15;16(23):5065–5071. doi: 10.1021/bi00642a019. [DOI] [PubMed] [Google Scholar]
- Gibney G., Camp S., Dionne M., MacPhee-Quigley K., Taylor P. Mutagenesis of essential functional residues in acetylcholinesterase. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7546–7550. doi: 10.1073/pnas.87.19.7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett C., Richter R. J., Humbert R., Chapline C., Crabb J. W., Omiecinski C. J., Furlong C. E. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: the mature protein retains its signal sequence. Biochemistry. 1991 Oct 22;30(42):10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- Higaki J. N., Evnin L. B., Craik C. S. Introduction of a cysteine protease active site into trypsin. Biochemistry. 1989 Nov 28;28(24):9256–9263. doi: 10.1021/bi00450a004. [DOI] [PubMed] [Google Scholar]
- Huang Y. S., Woods L., Sultatos L. G. Solubilization and purification of A-esterase from mouse hepatic microsomes. Biochem Pharmacol. 1994 Sep 15;48(6):1273–1280. doi: 10.1016/0006-2952(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Humbert R., Adler D. A., Disteche C. M., Hassett C., Omiecinski C. J., Furlong C. E. The molecular basis of the human serum paraoxonase activity polymorphism. Nat Genet. 1993 Jan;3(1):73–76. doi: 10.1038/ng0193-73. [DOI] [PubMed] [Google Scholar]
- Kelso G. J., Stuart W. D., Richter R. J., Furlong C. E., Jordan-Starck T. C., Harmony J. A. Apolipoprotein J is associated with paraoxonase in human plasma. Biochemistry. 1994 Jan 25;33(3):832–839. doi: 10.1021/bi00169a026. [DOI] [PubMed] [Google Scholar]
- Kuo J. M., Raushel F. M. Identification of the histidine ligands to the binuclear metal center of phosphotriesterase by site-directed mutagenesis. Biochemistry. 1994 Apr 12;33(14):4265–4272. doi: 10.1021/bi00180a022. [DOI] [PubMed] [Google Scholar]
- La Du B. N., Adkins S., Kuo C. L., Lipsig D. Studies on human serum paraoxonase/arylesterase. Chem Biol Interact. 1993 Jun;87(1-3):25–34. doi: 10.1016/0009-2797(93)90022-q. [DOI] [PubMed] [Google Scholar]
- Lai K., Dave K. I., Wild J. R. Bimetallic binding motifs in organophosphorus hydrolase are important for catalysis and structural organization. J Biol Chem. 1994 Jun 17;269(24):16579–16584. [PubMed] [Google Scholar]
- Lewis V. E., Donarski W. J., Wild J. R., Raushel F. M. Mechanism and stereochemical course at phosphorus of the reaction catalyzed by a bacterial phosphotriesterase. Biochemistry. 1988 Mar 8;27(5):1591–1597. doi: 10.1021/bi00405a030. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Bartels C. F., Vaughan T. A., Wong C. K., Norton S. E., Johnson L. L. Complete amino acid sequence of human serum cholinesterase. J Biol Chem. 1987 Jan 15;262(2):549–557. [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Abbott C., Durrington P. N. Protection of low-density lipoprotein against oxidative modification by high-density lipoprotein associated paraoxonase. Atherosclerosis. 1993 Dec;104(1-2):129–135. doi: 10.1016/0021-9150(93)90183-u. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Arrol S., Durrington P. N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991 Jul 29;286(1-2):152–154. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- Mackness M. I., Thompson H. M., Hardy A. R., Walker C. H. Distinction between 'A'-esterases and arylesterases. Implications for esterase classification. Biochem J. 1987 Jul 1;245(1):293–296. doi: 10.1042/bj2450293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden B. J., Shaw G. S., Sykes B. D. Calcium binding proteins. Elucidating the contributions to calcium affinity from an analysis of species variants and peptide fragments. Biochem Cell Biol. 1990 Mar;68(3):587–601. doi: 10.1139/o90-084. [DOI] [PubMed] [Google Scholar]
- Messerschmidt A. Structural studies on copper-containing plant oxidases. Biochem Soc Trans. 1992 May;20(2):364–368. doi: 10.1042/bst0200364. [DOI] [PubMed] [Google Scholar]
- Navab M., Hama S. Y., Nguyen T. B., Fogelman A. M. Monocyte adhesion and transmigration in atherosclerosis. Coron Artery Dis. 1994 Mar;5(3):198–204. doi: 10.1097/00019501-199403000-00003. [DOI] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. The structure of aconitase. Proteins. 1989;5(4):289–312. doi: 10.1002/prot.340050406. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Camp S., Maulet Y., Newton M., MacPhee-Quigley K., Taylor S. S., Friedmann T., Taylor P. Primary structure of Torpedo californica acetylcholinesterase deduced from its cDNA sequence. 1986 Jan 30-Feb 5Nature. 319(6052):407–409. doi: 10.1038/319407a0. [DOI] [PubMed] [Google Scholar]
- Scott D. L., White S. P., Otwinowski Z., Yuan W., Gelb M. H., Sigler P. B. Interfacial catalysis: the mechanism of phospholipase A2. Science. 1990 Dec 14;250(4987):1541–1546. doi: 10.1126/science.2274785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafferman A., Kronman C., Flashner Y., Leitner M., Grosfeld H., Ordentlich A., Gozes Y., Cohen S., Ariel N., Barak D. Mutagenesis of human acetylcholinesterase. Identification of residues involved in catalytic activity and in polypeptide folding. J Biol Chem. 1992 Sep 5;267(25):17640–17648. [PubMed] [Google Scholar]
- Smolen A., Eckerson H. W., Gan K. N., Hailat N., La Du B. N. Characteristics of the genetically determined allozymic forms of human serum paraoxonase/arylesterase. Drug Metab Dispos. 1991 Jan-Feb;19(1):107–112. [PubMed] [Google Scholar]
- Sultatos L. G., Murphy S. D. Hepatic microsomal detoxification of the organophosphates paraoxon and chlorpyrifos oxon in the mouse. Drug Metab Dispos. 1983 May-Jun;11(3):232–238. [PubMed] [Google Scholar]
- Taylor J. M., Jacob-Mosier G. G., Lawton R. G., Remmers A. E., Neubig R. R. Binding of an alpha 2 adrenergic receptor third intracellular loop peptide to G beta and the amino terminus of G alpha. J Biol Chem. 1994 Nov 4;269(44):27618–27624. [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. New perspective on zinc biochemistry: cocatalytic sites in multi-zinc enzymes. Biochemistry. 1993 Jul 6;32(26):6493–6500. doi: 10.1021/bi00077a001. [DOI] [PubMed] [Google Scholar]
- Vitarius J. A., Sultatos L. G. Kinetic mechanism of the detoxification of the organophosphate paraoxon by human serum A-esterase. Drug Metab Dispos. 1994 May-Jun;22(3):472–478. [PubMed] [Google Scholar]
- Vitarius J. A., Sultatos L. G. The role of calcium in the hydrolysis of the organophosphate paraoxon by human serum A-esterase. Life Sci. 1995;56(2):125–134. doi: 10.1016/0024-3205(94)00422-o. [DOI] [PubMed] [Google Scholar]
- Zech R., Röckseisen M., Kluge K., Dewald K., Armstrong V. W., Chemnitius J. M. Lipoproteins and hydrolysis of organophosphorus compounds. Chem Biol Interact. 1993 Jun;87(1-3):85–94. doi: 10.1016/0009-2797(93)90028-w. [DOI] [PubMed] [Google Scholar]




