Abstract
Background
There is convincing evidence that circadian disruption mediated by exposure to light at night promotes mammary carcinogenesis in rodents. The role that light at night plays in human breast cancer etiology remains unknown. We evaluated the relationship between estimates of indoor and outdoor light at night and the risk of breast cancer among members of the California Teachers Study.
Methods
Indoor light-at-night estimates were based on questionnaire data regarding sleep habits and use of night time lighting while sleeping. Estimates of outdoor light at night were derived from imagery data obtained from the U.S. Defense Meteorological Satellite Program assigned to geocoded addresses of study participants. Analyses were conducted among 106,731 California Teachers Study members who lived in California, had no prior history of breast cancer, and provided information on lighting while sleeping. 5,095 cases of invasive breast cancer diagnosed 1995–2010 were identified via linkage to the California Cancer Registry. We used age-stratified Cox proportional hazard models to calculate hazard ratios (HRs) with 95% confidence intervals (CIs), adjusting for breast cancer risk factors and neighborhood urbanization and socioeconomic class.
Results
An increased risk was found for women living in areas with the highest quintile of outdoor light at night exposure estimates (HR=1.12 [95% CI=1.00 – 1.26], test for trend, P=0.06). While more pronounced among premenopausal women (HR=1.34 [95% CI=1.07 – 1.69], test for trend, P=0.04), the associations did not differ statistically by menopausal status (test for interaction, P=0.34).
Conclusions
Women living in areas with high levels of ambient light at night may be at an increased risk of breast cancer. Future studies that integrate quantitative measurements of indoor and outdoor light at night are warranted.
In 2010, the International Agency for Research on Cancer (IARC) classified “shiftwork that involves circadian disruption” as a probable human carcinogen (Group 2A).1 This classification was based largely on strong laboratory evidence for carcinogenic effects of light at night exposures in animals; it was supported by limited, although convincing, evidence from epidemiologic studies of increased cancer risks – particularly breast cancer risk – associated with shiftwork that involves night work. The degree to which light at night is the exposure underpinning the increased risks associated with night shift work remains unclear. To date, only a handful of epidemiologic studies have directly evaluated cancer risks associated with light at night exposures.2–8 These studies generally fall into one of two categories: ecologic studies correlating cancer incidence rates with estimates of outdoor ambient light at night, all of which have reported elevations in breast cancer2, 5, 6; and case-control studies examining risks associated with self-reported measures of indoor lighting, which have yielded more mixed results.3, 4, 7
We conducted an exploratory analysis of breast cancer risk associated with estimates of outdoor ambient light at night and self-reported indoor light at night among a large prospective cohort study of women living in California.
METHODS
Study Population
The study population for these analyses was drawn from the California Teachers Study, a large prospective cohort study of female California professional school employees. Participants are 133,479 women who responded to a 1995–1996 mailing to 329,000 active and retired female enrollees in the State Teachers Retirement System. A full description of the California Teachers Study is available elsewhere.9
For the present analyses, participants were excluded (in sequence) for the following reasons: living outside California at baseline (n=8,867); unknown history of prior cancer (n=139); prior history of invasive or in situ breast cancer (n=6,211); voluntary withdrawal from the study (n=1); missing or unknown information about indoor light at night (n=5,953); address that could not be geocoded (n= 5,565), or once geocoded, had missing census information on sociodemographics (n=12). The resulting study population comprised 106,731 women. All participants provided informed consent upon entry into the California Teachers Study, and the protocol was approved by the Institutional Review Boards at all participating institutions.
Outcome Assessment
The California Teachers Study cohort is followed annually for cancer diagnosis, death, and change of address. State and national mortality files, as well as reports from relatives, are used to ascertain date and cause of death. Address changes for continued follow-up are obtained on an on-going basis via several methods, including annual mailings, notifications of moves received from participants, and linkages to nationwide consumer reporting companies and the U.S. Postal Service National Change of Address database. Cancer outcomes are identified through linkages with the California Cancer Registry, a legally mandated statewide population-based cancer reporting system.10 For the purposes of the present analysis, the case group comprised eligible study participants diagnosed with incident invasive carcinoma of the breast (SEER site code=26000) after joining the cohort and before 1 January 2011 (n=5,095).
Exposure Assessment
Indicators of indoor light at night were derived from the California Teachers Study baseline questionnaire, a mailed survey, completed in 1995–1996 (http://www.calteachersstudy.org/surveys/BaselineL.pdf). Indicators were based on responses to the questions: “during the past year, have you used a bright light at night while sleeping at home?” Among those who responded “yes,” respondents were asked to further specify for how many months (0–3,4–6, 7–9, 10+), and, on average, for how many days per week (1–3, 4–5, 6+) and hours per night (1–2, 3–4, 5–6, 7+) they used a light. A summary metric was created that categorized women into four groups: non-users; heavy users (reported 10+ months of use for 5+ days per week and 7+ hours per night); light users (reported 0–3 months, 1–3 days per week and 1–2 hours per night); and medium users (all other combinations of duration and frequency).
Indicators of outdoor light at night were derived from satellite imagery data obtained from the archive of the U.S. Defense Meteorological Satellite Program’s Operational Linescan System, maintained by NOAA’s Earth Observation Group.11 This database contains annual composites made after excluding the outer quarters of the satellite swath; sun and moon luminance; glare; clouds; and atmospheric lightning. Ephemeral events such as fires are also removed. While these images capture only a fraction of the light originating from the earth’s surface, they accurately represent the relative levels of nighttime illumination at ground level.11 The processed imagery data is georectified to a 30 arc-second grid (equivalent to approximately one square kilometer). Two types of data are available. The first uses adaptive gain settings to create data with a dynamic range of six bits and contains unit-less luminance values scaled to range from 0 (background noise) to 63. The second type is created by combining the information from three fixed-gain sensors. This type of data has a much larger dynamic range and can be transformed into units of radiance (nanowatts/cm2/sterradian(sr)).11–13 Because our initial assessment of the low-dynamic range 6-bit data indicated that there was insufficient variability in light at night levels in urban areas (nearly every residence in an urban or suburban area was assigned the maximum value of 63),14 we used the high-dynamic range data (currently available only for 2006), for the present analysis. We used ArcGIS (ESRI, Redlands, CA, USA) to geocode the participants’ home address at baseline (1995–1996) to a latitude/longitude and to a Census 2000 block group. Residences of study participants were assigned the average annual 2006 night-time radiance value for the grid cell in which they were located (based on their latitude and longitude) using spatial analysis tools available in the raster, rgdal, and sp packages within R.15
Covariate Information
Data on the following personal breast-cancer risk factors were gathered from the baseline questionnaire: age at baseline, race/birthplace, family history of breast cancer, age at menarche, age at first full-term pregnancy, breast feeding history, average lifetime strenuous physical activity, body mass index (BMI), alcohol consumption, menopausal status and hormone therapy use at baseline, smoking status, and pack-years of smoking. These variables were selected a priori based on previous analyses within the California Teachers Study that found these to be important predictors of breast cancer risk.
Neighborhood socioeconomic status (SES) and urbanization were based on 2000 U.S. census block group data. SES measures included the following: percentage of adults over age 25 years having completed a college degree or higher; percentage of adults without a high-school degree; median family income; percentage of adults employed in managerial/professional occupations; and percentage of population below the poverty line. To address the high degree of collinearity among these measures, we conducted a principal-components analysis to create a composite measure of SES based on the five individual variables described above. The loadings of the first principal component, categorized into quartiles, were then used in our Cox regression models. Neighborhood urbanization was defined as urban, suburban, non-metropolitan city, town, and rural, based on a previously developed algorithm using a combination of census block group characteristics, details of which can be found elsewhere.14, 16
Follow-up
Follow-up time was calculated as the number of days between the date the baseline questionnaire was completed and the earliest of four dates: date of invasive breast cancer diagnosis; date of first non-California residential address lasting 4 months or longer; date of death; or 31 December 2010. Women who were diagnosed with in situ breast cancer during the follow-up period were censored at the time of their diagnoses.
Statistical Analysis
Cox proportional hazards regression models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) associated with each indicator of light at night, using ages at the start and end of follow-up (in days) to define time on study. Indicators of indoor and outdoor light at night were considered in separate models, as were the individual components of indoor light-at-night exposures (i.e., month, days, hours, summary measure). Outdoor light at night was modeled continuously and as a categorical variable (using quintiles). All initial models were stratified by age at baseline (in single-year increments) and adjusted for race/birthplace. Subsequent multivariate models included adjustment for all personal risk factors and neighborhood characteristics, as described above. Since results from the age-stratified/race-adjusted models and multivariate models were not substantially different, we present the multivariate results only. Due to the sparseness of cell counts in the joint distribution of outdoor light at night and neighborhood urbanization, we collapsed the categories of urbanization from five to three categories (urban/suburban, non-metropolitan city, and town/rural) for the purposes of adjustment in multivariate models. Likelihood-ratio tests for trend across indoor and outdoor light at night categories were conducted using an ordinal variable coded as the median value of each category.
Regression models were also run with the data stratified on potential effect modifiers of interest, including menopausal status, body weight, and neighborhood urbanization and SES. Formal tests for interaction between light at night and each of these potential effect modifiers were performed by adding a multiplicative interaction term to the PHREG model in a forward fashion to a model with just the main effects and the P-values for the Wald X2 assessed for significance. Competing-risk models were run to evaluate risk estimates separately for hormonally responsive and non-responsive tumors. All models were run using the PHREG procedure in SAS Version 9.3 (SAS Institute, Cary, NC).
RESULTS
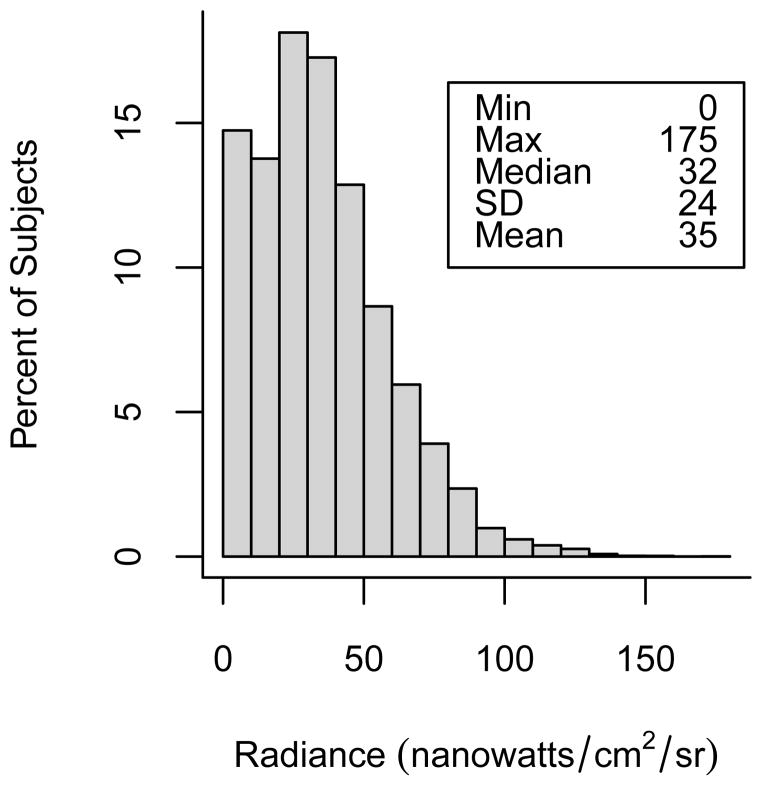
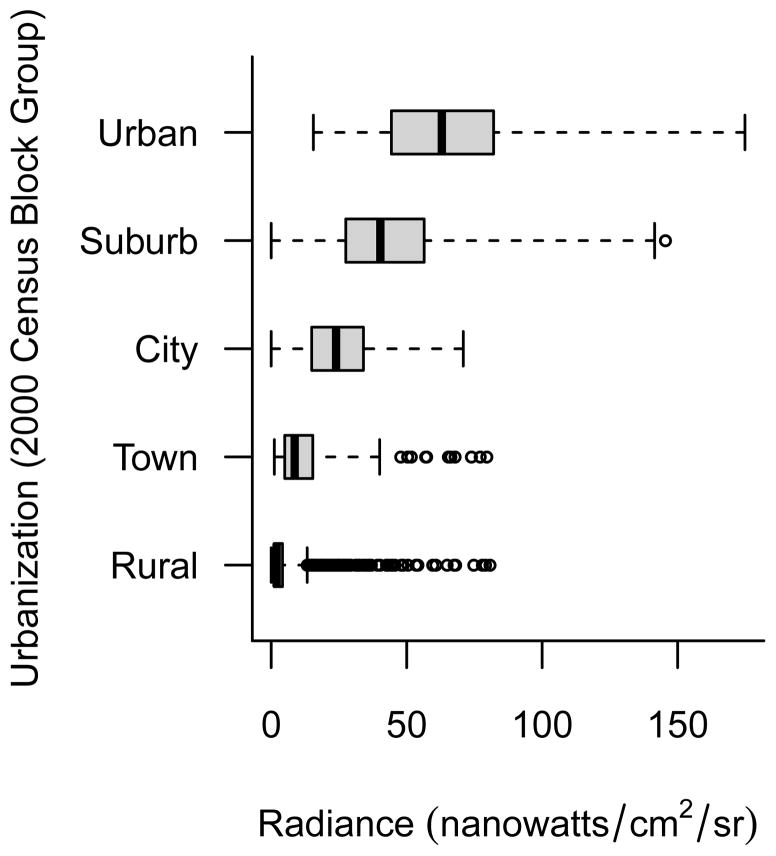
The prevalence of indoor light at night use was very low. Only 5% of study respondents reported the use of a bright light while sleeping at home during the year prior to baseline. Of those who reported any use, most reported infrequent usage or usage of short durations (45% reported 0–3 months of use; 52% reported use of 1–3 days/week; 30% reported use of 1–2 hours/night). For our summary measure of indoor light at night, only 17% of users reported using a bright light at the highest levels of frequency and duration (data not shown). Outdoor light at night values ranged from 0 to 175 nanowatts/cm2/sr with a mean of 35 nanowatts/cm2/sr and a median of 32 nanowatts/cm2/sr (Figure 1). Outdoor light at night values were lowest among women living in rural neighborhoods and highest among women living in urban areas (Figure 2). There was, however, a fair degree of variability of light at night values within categories of neighborhood urbanization and some overlap of values between categories of urbanization.
Figure 1.
Distribution of 2006 average outdoor light at night satellite radiance values (nanowatts/cm2/sr) assigned to the baseline residences of 106,731 study participants.
Figure 2.
Distribution of 2006 average outdoor light at night satellite radiance values (nanowatts/cm2/sr) by level of residential neighborhood urbanization among 106,731 study participants.
The distribution of reported indoor light-at-night usage generally did not vary by personal risk factors or neighborhood characteristics (Table 1) except users were more likely to be nonwhite, obese, and live in urban areas than non-users. Women living in neighborhoods with the highest quintile of outdoor light at night were more likely to be nonwhite (22%) than those living in the the lowest quintile (7%). Furthermore, women in neighborhoods with the highest outdoor light at night estimates were more likely to have never had a live birth and were less likely to have breastfed for a year or more. In multivariate analyses, we observed no evidence of an increased risk of breast cancer with indicators of indoor light at night (Table 2). Women who reported using a bright light while sleeping at home over the prior year did not have an elevated risk of breast cancer (HR=1.03 [95% CI = 0.90 – 1.18]). More detailed analyses focusing on the individual measures of duration of use (months, days, hours) and combining those measures into a summary measure also provided no evidence of risk with all hazard ratios close to 1.0, 95% confidence intervals including 1.0, and no evidence of a linear trend.
Table 1.
Distribution of indoor and outdoor light-at-night estimates by selected characteristics of study population (n=106,731).
| Characteristics | Used a Bright Light While Sleeping | Outdoor Light-at-Night Quintiles | All | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No | Yes | Lowest | 2nd | 3rd | 4th | Highest | |||
|
| |||||||||
| (n=101,779) | (n=4,952) | (n=21,350) | (n=21,350) | (n=21,336) | (n=21,346) | (n=21,349) | (n=106,731) | ||
|
| |||||||||
| % | % | % | % | % | % | % | % | N | |
| Age at baseline (years) | |||||||||
| 20–39 | 19 | 22 | 15 | 18 | 20 | 20 | 21 | 19 | 19,910 |
| 40–49 | 27 | 28 | 30 | 29 | 27 | 26 | 24 | 27 | 29,223 |
| 50–59 | 25 | 24 | 25 | 25 | 24 | 25 | 24 | 25 | 26,325 |
| 60–69 | 16 | 14 | 16 | 15 | 16 | 16 | 17 | 16 | 16,968 |
| ≥70 | 13 | 12 | 14 | 13 | 13 | 13 | 14 | 13 | 14,305 |
| Race/Birthplace | |||||||||
| White | 87 | 74 | 92 | 89 | 88 | 86 | 77 | 87 | 92,339 |
| Non-white US-born | 10 | 21 | 6 | 8 | 9 | 10 | 17 | 10 | 10,871 |
| Non-white foreign-born | 2 | 4 | 1 | 2 | 2 | 3 | 5 | 2 | 2,684 |
| Other/Unknown | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 837 |
| Family history of breast cancer | |||||||||
| No | 84 | 85 | 85 | 85 | 85 | 84 | 85 | 85 | 90,369 |
| Yes | 12 | 11 | 12 | 11 | 11 | 12 | 11 | 12 | 12,461 |
| Unknown | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 3 | 3,901 |
| Age at menarche (years) | |||||||||
| ≤11 | 22 | 26 | 22 | 22 | 22 | 22 | 23 | 23 | 23,761 |
| 12–13 | 56 | 53 | 56 | 57 | 57 | 56 | 56 | 56 | 59,893 |
| ≥14 | 20 | 19 | 21 | 20 | 20 | 20 | 20 | 20 | 21,535 |
| Unknown/Never | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1,542 |
| Age at first full-term pregnancy (years) | |||||||||
| Nulliparous or Nulligravid | 26 | 32 | 21 | 24 | 25 | 29 | 34 | 27 | 28,261 |
| ≤24 | 25 | 26 | 29 | 26 | 26 | 24 | 22 | 25 | 27,117 |
| 25–29 | 30 | 25 | 31 | 31 | 30 | 28 | 25 | 29 | 31,223 |
| ≥30 | 17 | 15 | 17 | 17 | 17 | 17 | 17 | 17 | 18,144 |
| Unknown | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1,986 |
| Breastfeeding history (months) | |||||||||
| Nulliparous | 20 | 24 | 16 | 19 | 20 | 22 | 26 | 21 | 21,912 |
| Nulligravid | 6 | 8 | 5 | 5 | 6 | 6 | 8 | 6 | 6,223 |
| Never breastfed | 16 | 14 | 15 | 16 | 16 | 16 | 16 | 16 | 16,851 |
| <6 | 17 | 16 | 18 | 18 | 17 | 18 | 16 | 17 | 18,598 |
| 6–11 | 14 | 12 | 14 | 14 | 14 | 13 | 12 | 13 | 14,425 |
| ≥12 | 25 | 23 | 30 | 26 | 25 | 22 | 20 | 25 | 26,245 |
| Unknown | 2 | 3 | 2 | 2 | 2 | 3 | 2 | 2 | 2,477 |
| Strenuous Physical Activity (hours per week) | |||||||||
| 0–0.50 | 29 | 29 | 28 | 29 | 29 | 30 | 32 | 29 | 31,570 |
| 0.51–2.00 | 32 | 32 | 32 | 33 | 32 | 31 | 31 | 32 | 34,167 |
| 2.01–3.50 | 18 | 17 | 18 | 18 | 17 | 18 | 17 | 18 | 18,768 |
| 3.51–5.00 | 10 | 9 | 10 | 10 | 10 | 9 | 9 | 10 | 10,235 |
| >5.00 | 10 | 12 | 11 | 10 | 11 | 11 | 10 | 10 | 11,252 |
| Unknown | 1 | 1 | 1 | <1 | 1 | 1 | 1 | 1 | 739 |
| Body mass index (kg/m2) | |||||||||
| 16.0–24.9 | 59 | 49 | 59 | 60 | 59 | 59 | 57 | 59 | 62,765 |
| 25.0–29.9 | 24 | 26 | 24 | 23 | 24 | 23 | 24 | 24 | 25,273 |
| 30.0–54.8 | 13 | 21 | 13 | 13 | 13 | 14 | 14 | 13 | 14,395 |
| Unknown | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 4 | 4,298 |
| Alcohol Consumption (g/day) | |||||||||
| None | 32 | 38 | 30 | 31 | 31 | 32 | 36 | 32 | 34,113 |
| <20 | 55 | 50 | 56 | 56 | 56 | 56 | 52 | 55 | 58,808 |
| ≥20 | 8 | 6 | 9 | 8 | 8 | 7 | 6 | 8 | 8,251 |
| Unknown | 5 | 6 | 5 | 5 | 5 | 5 | 6 | 5 | 5,559 |
| Menopausal Status/Hormone Therapy Use | |||||||||
| Premenopausal | 41 | 44 | 40 | 42 | 42 | 42 | 42 | 41 | 44,264 |
| Peri/post-menopausal | |||||||||
| never used hormone therapy | 12 | 13 | 11 | 11 | 11 | 11 | 14 | 12 | 12,293 |
| former hormone therapy use | 7 | 7 | 7 | 6 | 7 | 6 | 7 | 7 | 7,038 |
| current estrogen use | 13 | 11 | 14 | 13 | 13 | 13 | 12 | 13 | 13,842 |
| current estrogen/progestin use | 14 | 11 | 14 | 15 | 14 | 15 | 13 | 14 | 15,144 |
| Other/unknown | 13 | 14 | 14 | 13 | 13 | 13 | 12 | 13 | 14,150 |
| Smoking Status | |||||||||
| Never | 66 | 62 | 66 | 66 | 66 | 66 | 66 | 66 | 70,758 |
| Former | 28 | 30 | 29 | 28 | 28 | 28 | 27 | 28 | 29,922 |
| Current | 5 | 7 | 4 | 5 | 5 | 5 | 6 | 5 | 5,390 |
| Unknown | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 661 |
| Smoking pack-years | |||||||||
| Never smoker | 67 | 62 | 66 | 66 | 66 | 66 | 66 | 66 | 70,758 |
| ≤10 | 16 | 16 | 17 | 16 | 16 | 16 | 16 | 16 | 17,378 |
| 11–20 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6,036 |
| 21–30 | 3 | 4 | 3 | 3 | 3 | 3 | 4 | 3 | 3,573 |
| ≥31 | 5 | 8 | 5 | 5 | 5 | 5 | 5 | 5 | 5,244 |
| Unknown | 3 | 4 | 3 | 4 | 4 | 4 | 3 | 4 | 3,742 |
| Neighborhood Socioeconomic Status | |||||||||
| Lowest quartile | 25 | 29 | 33 | 19 | 21 | 23 | 29 | 25 | 26,684 |
| 2nd quartile | 25 | 24 | 27 | 24 | 24 | 23 | 26 | 25 | 26,688 |
| 3rd quartile | 25 | 25 | 21 | 25 | 26 | 27 | 26 | 25 | 26,677 |
| Highest quartile | 25 | 22 | 19 | 32 | 29 | 27 | 19 | 25 | 26,682 |
| Urban/Rural (2000 block group baseline residence) | |||||||||
| Rural | 8 | 5 | 36 | 1 | <1 | <1 | <1 | 7 | 7,904 |
| Town | 4 | 3 | 14 | 5 | 1 | <1 | <1 | 4 | 4,346 |
| Non-metropolitan City | 29 | 24 | 33 | 48 | 36 | 23 | 3 | 28 | 30,331 |
| Suburban | 54 | 59 | 17 | 45 | 60 | 71 | 79 | 55 | 58,272 |
| Urban | 5 | 9 | 0 | 1 | 3 | 6 | 18 | 6 | 5,878 |
| Residentially stable (non-movers) | 53 | 49 | 54 | 53 | 52 | 52 | 53 | 53 | 56,112 |
Table 2.
Risk of breast cancer associated with indicators of indoor light-at-night among 106,731 study participants: adjusted hazard ratios (HRs) and 95% confidence intervals (95% CI) estimated from Cox proportional hazard models.a
| Indoor light-at-night | Category | No. Casesb | HR (95% CI) | Test for (trend) |
|---|---|---|---|---|
| Uses light | Non-user | 4,869 | 1.0 | |
| Yes | 226 | 1.03 (0.90–1.18) | - | |
| Duration (months) | Non-user | 4,869 | 1.0 | |
| 0–3 | 97 | 1.09 (0.89–1.33) | ||
| 4–6 | 19 | 0.92 (0.59–1.45) | ||
| 7–9 | 11 | 0.79 (0.44–1.43) | ||
| 10+ | 80 | 1.08 (0.87–1.35) | P = 0.62 | |
| Days/week | Non-user | 4,869 | 1.0 | |
| 1–3 | 122 | 1.14 (0.95–1.37) | ||
| 4–5 | 25 | 0.87 (0.59–1.30) | ||
| 6+ | 60 | 0.97 (0.75–1.25) | P = 0.97 | |
| Hours/day | Non-user | 4,869 | 1.0 | |
| 1–2 | 66 | 1.01 (0.79–1.29) | ||
| 3–4 | 43 | 1.07 (0.80–1.45) | ||
| 5–6 | 43 | 1.03 (0.76 to 1.40) | ||
| 7+ | 56 | 1.06 (0.81–1.38) | P = 0.55 | |
| Summary c | Non-user | 4,869 | 1.0 | |
| Light | 45 | 1.17 (0.87–1.57) | ||
| Medium | 109 | 0.99 (0.82–1.20) | ||
| Heavy | 44 | 1.13 (0.84–1.52) | P = 0.53 |
adjusted for: age strata (1-year), race/birthplace, family history of breast cancer, age at menarche, pregnancy history, breast feeding history, physical activity, BMI, alcohol consumption, menopausal status and hormone therapyuse combination, smoking status, and smoking pack years, neighborhood SES and urbanization.
Number of cases does not always sum to total (n=5,095) due to missing/unknown values for light-at-night usage.
Summary : light = uses light 0–3 months and 1–3 days/week and 1–2 hours/night; heavy = uses light 10+ months and 5+ days/week and 7+ hours/day; medium = all other combinations of monthly, weekly, and hourly usage.
Risk estimates for indicators of outdoor light at night are presented in Table 3. Overall, a modest association was observed between outdoor light-at-night exposure and breast cancer risk (HR=1.12 [95% CI: 1.00 – 1.26] for the highest quintile of light at night; test for trend, P=0.06). When the data were stratified by menopausal status, the risk was evident only among women who were premenopausal at baseline (HR for highest light at night quintile= 1.34 [95% CI = 1.07–1.69]; test for trend, P = 0.04) and not among those who were post-menopausal at baseline (HR for highest light at night quintile= 1.04 [0.90 – 1.20]; test for trend, P = 0.44), although there was no statistical evidence for interaction (test for interaction, P = 0.34). Overall, there was no evidence of heterogeneity in risk across categories of body weight (test for interaction, P = 0.90). Examining risk among premenopausal and postmenopausal women by BMI (Table 4), it appears that the increased risk among premenopausal women was confined to women with BMI < 25 kg/m2 (HR=1.56 [1.16 – 2.08]) for the highest quintile; test for trend, P =0.02). No association was observed among women with BMI ≥ 25 (1.06 [0.72 – 1.56]); test for trend, P = 0.59), although there was no statistical evidence for interaction (test for interaction, P = 0.77). mong women post-menopausal at baseline, there was no evidence for heterogeneity in risk across categories of relative bodyweight (test for interaction, P = 0.88). While we did not observe an excess risk among post-menopausal women, when we stratified by hormone therapy use, there was some suggestion of an increased risk only among the never users. Competing-risk analyses to examine whether risks differed depending on tumor hormone responsiveness (estrogen receptor, ER; progesterone receptor, PR) were hampered by small numbers, although risk estimates were marginally higher among women with ER-negative and PR-negative tumors than those with tumors that were either ER-positive or PR-positive (data not shown).
Table 3.
Risk of invasive breast cancer associated with outdoor estimates of Light-at-Night among 106,731 study participants: adjusted hazard ratios (HRs) and 95% confidence intervals (95% CI) estimated from Cox proportional hazard models.a
| Outdoor Light-at-Night Estimates (nanowatts/cm2/sr) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lowest Quintileb | Quintile 2 | Quintile 3 | Quintile 4 | Highest Quintile | |||||||
| 0–14.2 | 14.3 – 26.4 | 26.5 to 37.4 | 37.5 to 53.3 | 53.4 to 175.2 | |||||||
|
| |||||||||||
| No. cases | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | Test for (trend) | |
| All | 1,006 | 1.0 | 1,029 | 1.05 (0.95 – 1.16) | 1,010 | 1.06 (0.95 – 1.17) | 1,009 | 1.05 (0.95 – 1.17) | 1,041 | 1.12, 1.00 – 1.26 | 0.06 |
| Menopausal Status | |||||||||||
| Premenopausal | 232 | 1.0 | 269 | 1.20 (0.98 – 1.48) | 255 | 1.20 (0.97 – 1.49) | 241 | 1.16 (0.93 – 1.46) | 270 | 1.34 (1.07 – 1.69) | 0.04 |
| Postmenopausal | 646 | 1.0 | 632 | 0.99 (0.88 – 1.13) | 630 | 0.99 (0.87–1.13) | 652 | 1.01 (0.88 – 1.15) | 663 | 1.04 (0.90 – 1.20) | 0.44 |
| Body Weight | |||||||||||
| BMI < 25 | 563 | 1.0 | 566 | 1.01 (0.89 – 1.16) | 560 | 1.04 (0.91 – 1.20) | 562 | 1.05 (0.91 – 1.21) | 551 | 1.09 (0.94 – 1.27) | 0.20 |
| BMI ≥ 25 | 403 | 1.0 | 416 | 1.10 (0.93 – 1.28) | 412 | 1.09 (0.92 – 1.29) | 408 | 1.08 (0.91 – 1.28) | 441 | 1.18( 0.98 – 1.41) | 0.12 |
adjusted for: age strata (1-year), race/birthplace, family history of breast cancer, age at menarche, pregnancy history, breast feeding history, physical activity, BMI, alcohol consumption, menopausal status and hormone therapy use combination, smoking status, and smoking pack years, neighborhood SES and urbanization.
Reference category
Table 4.
Risk of invasive breast cancer associated with outdoor estimates of Light-at-Night among 106,731 study participants: adjusted hazard ratios (HRs) and 95% confidence intervals (95% CI) estimated from Cox proportional hazard models, stratified by menopausal status and relative body weight. a
| Outdoor Light-at-Night Estimates (nanowatts/cm2/sr) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lowest Quintilec | Quintile 2 | Quintile 3 | Quintile 4 | Highest Quintile | |||||||
| 0 – 14.2 | 14.3 – 26.4 | 26.5 – 37.4 | 37.5 – 53.3 | 53.4 – 175.2 | |||||||
|
| |||||||||||
| No. casesb | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | No. cases | HR (95% CI) | Test for (trend) | |
| PREMENOPAUSAL | |||||||||||
| Relative Body Weight | |||||||||||
| BMI < 25 | 142 | 1.0 | 175 | 1.33 (1.03 – 1.73) | 167 | 1.37 (1.05 – 1.80) | 151 | 1.30 (0.98 – 1.72) | 167 | 1.56 (1.16 – 2.08) | 0.02 |
| BMI ≥ 25 | 87 | 1.0 | 86 | 0.94 (0.67 – 1.33) | 83 | 0.92 (0.64 – 1.32) | 80 | 0.91 (0.62 – 1.32) | 98 | 1.06 (0.72 – 1.56) | 0.59 |
| POSTMENOPAUSAL | |||||||||||
| Relative Body Weight | |||||||||||
| BMI < 25 | 341 | 1.0 | 322 | 0.94 (0.79 – 1.12) | 324 | 0.95 (0.80 – 1.14) | 352 | 1.03 (0.86 – 1.24) | 326 | 0.98 (0.80 – 1.18) | 0.82 |
| BMI ≥ 25 | 271 | 1.0 | 273 | 1.06 (0.87 – 1.28) | 277 | 1.07 (0.87 – 1.31) | 272 | 1.02 (0.82 – 1.25) | 295 | 1.11 (0.89 – 1.39) | 0.44 |
adjusted for: age strata (1-year), race/birthplace, family history of breast cancer, age at menarche, pregnancy history, breast feeding history, physical activity, BMI, alcohol consumption, menopausal status and hormone therapy use combination, smoking status, and smoking pack years, neighborhood SES and urbanization.
number of pre-menopausal and post-menopausal cases do not sum to total number of cases because peri-menopausal women were included in total.
reference category
Because of the high degree of correlation between outdoor light at night indicators and neighborhood urbanization, we repeated our analysis stratified by two levels of urbanization (urban/suburban areas and rural/town/non-metropolitan city). In these analyses, the breast cancer risk associated with outdoor light at night persisted for women residing in both areas. While the point estimate for the highest quintile of outdoor light at night among women living in rural/town/non-metropolitan cities was somewhat higher (HR=1.33 [95% CI = 0.98 – 1.82]) than among the urban/suburban women (1.09 [0.93 – 1.28]), there was no statistical evidence for interaction (p=0.46). Analyses stratified by neighborhood SES yielded generally similar patterns of risk across all levels of SES (test for interaction, P = 0.54) (data not shown).
Finally, we repeated our analyses restricted to the residentially stable subset of the study population (n=56,112; 3,142 cases) who lived at their baseline addresses during the full follow-up period of our study. These analyses yielded point estimates of risk very similar to those for the full study population, albeit with marginally wider confidence intervals (HR = 1.08 [95% CI = 0.95 – 1.23] for Q2; 1.07 [0.93 – 1.22] for Q3; 1.11 [0.97 – 1.27] for Q4; 1.10 [0.96 – 1.27] for Q5; test for trend, P = 0.26)). Stratified analyses among this residentially-stable subset yielded patterns of risk estimates that did not substantially differ from those observed in the full study population (data not shown).
DISCUSSION
Our findings suggest a small increased risk of breast cancer among women who live in neighborhoods with high levels of outdoor light at night. While our results suggest this risk may be confined to premenopausal women who were not overweight or obese, we did not have the statistical power to state this with confidence. Future research is needed to determine if indeed this risk is restricted to this subgroup of women.
These analyses were initiated in response to the growing interest in light at night as a potential etiologic factor in carcinogenesis. Capitalizing on the use of existing data for a large, well-defined cohort, we sought to improve upon earlier ecologic analyses that have suggested an association between breast cancer risk and ambient outdoor light-at-night as estimated using satellite imagery data.2, 5, 6 Unlike prior studies, we were able both to adjust for established breast cancer risk factors and to take into account neighborhood SES and urbanization, which are known to be associated with breast cancer risk and are likely to be associated with outdoor light at night values.14 Our finding that the risk associated with outdoor light at night persisted after adjusting for neighborhood urbanization, and was evident even among women living in rural areas, provides some assurance that the observed risks do not merely reflect the higher rates of breast cancer in urban areas.17–19
There are a number of limitations to our ascertainment of outdoor light at night. While we used the best available satellite imagery data to estimate outdoor light at night independent of neighborhood urbanization, the light-at-night estimates were not temporally congruent with the 1995–1996 baseline addresses because the high-dynamic range data was available only for 2006. An examination of the low-dynamic-range data for the years spanning our follow-up time, however, suggested relative stability in the ranking of light at night values over this time for the study area. Furthermore, our analyses repeated among study participants who had not moved during the follow-up yielded essentially the same results.
Our study failed to find an association with reported use of indoor light at night. Our indoor light at night analyses, however, were based on fairly small numbers. This not only reduced our power to detect an independent effect of indoor light at night, but also precluded our ability to assess it jointly with outdoor estimates of light at night. Furthermore, the questions on indoor light at night were not originally designed to capture light at night exposures but rather to ascertain household exposures related to electromagnetic fields. The questions, therefore, did not capture other sources of indoor light such as TVs and other electronic devices, nor did they characterize attributes of light that are critical in influencing circadian disruption, such as intensity, wavelength, and timing.20–24 Given these limitations, the lack of an association with indoor light at night may be due to exposure misclassification rather than an absence of effect.
Three other epidemiologic studies have evaluated breast cancer risk associated with self-reported use of indoor night-time lighting.3, 4, 7 The most recent asked about light at night in the workplace setting (where high exposure was considered “having enough light to be able to read”), and found a small increase in breast cancer risk with this category (odds ratio (OR) = 1.25 [95% CI = 0.98 – 1.59]), although there was no evidence of increasing risk with increasing duration of such exposure.4 The two other studies focused on light at night exposures in the home. The first of these examined three measures of night-time lighting (number of times light was turned on at night; percentage of time the light was on; the reported ambient bedroom light level) and found none to be related to breast cancer risk.3 In contrast, O’Leary and colleagues7 reported an elevated risk of breast cancer among women who reported a high frequency of night-time light usage (i.e, those who woke up at least twice a night and at least twice a week and turned on the lights (OR=1.65 [95% CI=1.02 – 2.69]). The authors, however, were not able to ascertain if this increased risk was truly a light at night effect or a reflection of risk associated with sleep disruption.
A comprehensive evaluation of light at night exposures and breast cancer risk would ideally capture total light exposures, emanating both from the indoor and outdoor environments, and integrate them into a single measure. Personal photometric monitors have been developed for this purpose.22, 25 Initial validation studies using such devices have indicated good agreement between estimates of ambient light at night from satellite imagery data and ground-level outdoor photometric readings,2 but poor agreement with indoor bedroom light levels.26 This latter finding underscores the importance of evaluating the degree to which outdoor light penetrates the indoor environment and identifying factors that modify its penetrance, such as the use of blinds/shades and the presence of outdoor vegetation, as well as climate and seasonality that are likely to influence the use or presence of these intervening factors in different geographic areas and at different times of the year. esearchers have developed a paper-based questionnaire (the Harvard Light Exposure Assessment Questionnaire (H-LEA)) for use in epidemiologic studies.27 A 7-day validation of this questionnaire with real-time photometric values from personal light monitors among a subset of the Nurses Health Study has indicated good agreement, suggesting it could be a useful method for ascertaining light at night exposures in large-scale epidemiologic studies. The questionnaire, however, consists of a highly-detailed 24-hour diary. This requires diligence from study subjects that could pose compliance issues in less-motivated study populations. Further evaluation of this questionnaire in other study populations and its adaptation to capture historical exposures over a longer time period are required.
Given the dearth of data on light at night exposures and breast cancer risk, there is little context in which to interpret our finding that the risk may be limited to premenopausal women who are not overweight. The bulk of evidence for circadian disruption and breast cancer risk emanates from studies of shiftwork that generally have not considered pre- and post-menopausal breast cancer risks separately.3, 7, 28–33 These studies, however, have reported risks across all age groups and in both the Nurses Health Study I (which is predominantly postmenopausal women)34 and the Nurses Health Study II (which is predominantly premenopausal women).35 If the risk of breast cancer associated with light at night is mediated by melatonin suppression, as is the prevailing hypothesis, our finding of an increased risk among pre- but not post-menopausal women may reflect a stronger biologic response to light at night exposures in young women. The suppressive effects of light at night on melatonin secretion have been reported to be stronger in young people, and to decline with age.36, 37
A variety of mechanisms have been postulated for melatonin’s anti-carcinogenic effects, including both direct oncostatic properties (anti-proliferative, antioxidant, suppression of DNA adducts, enhanced DNA repair), and anti-estrogenic effects mediated through interactions with estrogen-signaling pathways and down-regulation of the hypothalamic-pituitary-reproductive axis.36, 38 It is beyond the scope of the current study to evaluate potential etiologic pathways for light at night exposures. We did, however, conduct a number of stratified analyses in an attempt to elucidate whether the effects we observed might be operating via an estrogen-mediated pathway; no coherent pattern emerged. Our finding that among premenopausal women, outdoor light at night might increase risk among lean women but not overweight women warrants further investigation. The relationship between adiposity and breast cancer risk itself is complex, differs by menopausal status, and is likely mediated by adiposity-related differences in endogenous estrogen biosynthesis.39, 40 Heavier premenopausal women, who are more likely to have menstrual cycle irregularities, have lower breast cancer risks than their leaner counterparts.39, 40 Consequently, we might expect that the estrogenic effects of light at night (through suppression of melatonin) would be more readily observed in the overweight premenopausal women against the backdrop of lower background risk. Alternatively, it is possible that it is only in the context of higher estrogen levels among the thin, premenopausal women that the additional estrogenic effects of light at night are sufficient to result in disease.
In summary, our study provides evidence that women who live in areas with high levels of ambient light at night are at an increased risk of breast cancer not readily explained by other neighborhood characteristics or personal breast-cancer risk factors. These results, found in a well-specified cohort of women who do not typically work at night, add another important line of evidence to the circadian disruption hypothesis beyond the well-documented increased breast cancer risk among night shift workers, suggesting that light at night may be a contributing etiologic factor.
Acknowledgments
Sources of Financial Support: This research was supported by funds provided by The Regents of the University of California, California Breast Cancer Research Program (Grant number 16IB-0071) and National Cancer Institute grants R01 CA77398 and K05 CA136967.
The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000036C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred
We express our appreciation to all the participants in the California Teachers Study and to the researchers, analysts and staff who have contributed so much for the success of this research project. We also thank Minhthu Le for administrative support, and the California Teachers Study Steering Committee members who are responsible for the formation and maintenance of the cohort within which this study was conducted but who did not directly contribute to the current paper: Hoda Anton-Culver, Jessica Clague, Christina A. Clarke, Dennis Deapen, James V. Lacey Jr, Yani Lu, Huiyan Ma, Susan L. Neuhausen, Hannah Park, Rich Pinder, Fredrick Schumacher, Sophia S. Wang, and Argyrios Ziogas.
Footnotes
The opinions, findings, and conclusions herein are those of the author and not necessarily represent those of The Regents of the University of California, or any of its programs.
Conflicts of Interest: None of the authors have any potential conflicts of interest.
References
- 1.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–6. doi: 10.1016/S1470-2045(07)70373-X. [DOI] [PubMed] [Google Scholar]
- 2.Bauer SE, Wagner SE, Burch J, et al. A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr. 2013;12:23. doi: 10.1186/1476-072X-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–62. doi: 10.1093/jnci/93.20.1557. [DOI] [PubMed] [Google Scholar]
- 4.Fritschi L, Erren TC, Glass DC, et al. The association between different night shiftwork factors and breast cancer: a case-control study. Br J Cancer. 2013;109(9):2472–80. doi: 10.1038/bjc.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kloog I, Haim A, Stevens RG, et al. Light at night co-distributes with incident breast but not lung cancer in the female population of Israel. Chronobiol Int. 2008;25(1):65–81. doi: 10.1080/07420520801921572. [DOI] [PubMed] [Google Scholar]
- 6.Kloog I, Stevens RG, Haim A, et al. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. 2010;21(12):2059–68. doi: 10.1007/s10552-010-9624-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Leary ES, Schoenfeld ER, Stevens RG, et al. Shift work, light at night, and breast cancer on Long Island, New York. Am J Epidemiol. 2006;164(4):358–66. doi: 10.1093/aje/kwj211. [DOI] [PubMed] [Google Scholar]
- 8.Kloog I, Haim A, Stevens RG, et al. Global co-distribution of light at night (LAN) and cancers of prostate, colon, and lung in men. Chronobiol Int. 2009;26(1):108–25. doi: 10.1080/07420520802694020. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein L, Allen M, Anton-Culver H, et al. High breast cancer incidence rates among California teachers: results from the California Teachers Study (United States) Cancer Causes Control. 2002;13(7):625–35. doi: 10.1023/a:1019552126105. [DOI] [PubMed] [Google Scholar]
- 10.California Cancer Registry (CCR) Home Page. 2010 Nov; http://www.ccrcal.org/
- 11.Image and Data Processing by NOAA’s National Geophysical Data Center. DMSP data collected by the US Air Force Weather Agency. http://ngdc.noaa.gov/eog.
- 12.Elvidge C. Who’s in the Dark: Satellite Based Estimates of Electrification Rates. In: Yang X, editor. Urban Remote Sensing: Monintoring, Synthesis, and Modeling in the Urban Environment. Oxford, UK: Wiley-Blackwell; 2010. [Google Scholar]
- 13.Ziskin DBK, Hsu FC, Ghosh T, Elvidge C. Methods Used for the 2006 Radiance Lights. Proceedings of the Asia Pacific Advanced Network Meeting; 2010; 2010. [Google Scholar]
- 14.Hurley S, Nelson DO, Garcia E, et al. A cross-sectional analysis of light at night, neighborhood sociodemographics and urinary 6-sulfatoxymelatonin concentrations: implications for the conduct of health studies. Int J Health Geogr. 2013;12(1):39. doi: 10.1186/1476-072X-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- 16.Reynolds P, Hurley SE, Quach AT, et al. Regional variations in breast cancer incidence among California women, 1988–1997. Cancer Causes Control. 2005;16(2):139–50. doi: 10.1007/s10552-004-2616-5. [DOI] [PubMed] [Google Scholar]
- 17.Doll R. Urban and rural factors in the aetiology of cancer. Int J Cancer. 1991;47(6):803–10. doi: 10.1002/ijc.2910470602. [DOI] [PubMed] [Google Scholar]
- 18.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds P, Hurley S, Goldberg DE, et al. Regional variations in breast cancer among California teachers. Epidemiology. 2004;15(6):746–54. doi: 10.1097/01.ede.0000134863.45834.50. [DOI] [PubMed] [Google Scholar]
- 20.Bullough JD, Rea MS, Figueiro MG. Of mice and women: light as a circadian stimulus in breast cancer research. Cancer Causes Control. 2006;17(4):375–83. doi: 10.1007/s10552-005-0574-1. [DOI] [PubMed] [Google Scholar]
- 21.Figueiro MG, Rea MS, Bullough JD. Does architectural lighting contribute to breast cancer? J Carcinog. 2006;5:20. doi: 10.1186/1477-3163-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller D, Bierman A, Figueiro M, et al. Ecological measurements of light exposure, activity, and circadian disruption. Light Res Technol. 2010;42(3):271–84. doi: 10.1177/1477153510367977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevens RG, Brainard GC, Blask DE, et al. Adverse health effects of nighttime lighting: comments on american medical association policy statement. Am J Prev Med. 2013;45(3):343–6. doi: 10.1016/j.amepre.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Pauley SM. Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med Hypotheses. 2004;63(4):588–96. doi: 10.1016/j.mehy.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Figueiro MG, Hamner R, Higgins P, et al. Field measurements of light exposures and circadian disruption in two populations of older adults. J Alzheimers Dis. 2012;31(4):711–5. doi: 10.3233/JAD-2012-120484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rea MS, Brons JA, Figueiro MG. Measurements of light at night (LAN) for a sample of female school teachers. Chronobiol Int. 2011;28(8):673–80. doi: 10.3109/07420528.2011.602198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajaj A, Rosner B, Lockley SW, et al. Validation of a light questionnaire with real-life photopic illuminance measurements: the Harvard Light Exposure Assessment questionnaire. Cancer Epidemiol Biomarkers Prev. 2011;20(7):1341–9. doi: 10.1158/1055-9965.EPI-11-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grundy A, Richardson H, Burstyn I, et al. Increased risk of breast cancer associated with long-term shift work in Canada. Occup Environ Med. 2013 doi: 10.1136/oemed-2013-101482. [DOI] [PubMed] [Google Scholar]
- 29.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–7. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 30.Kojo K, Pukkala E, Auvinen A. Breast cancer risk among Finnish cabin attendants: a nested case-control study. Occup Environ Med. 2005;62(7):488–93. doi: 10.1136/oem.2004.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lie JA, Roessink J, Kjaerheim K. Breast cancer and night work among Norwegian nurses. Cancer Causes Control. 2006;17(1):39–44. doi: 10.1007/s10552-005-3639-2. [DOI] [PubMed] [Google Scholar]
- 32.Megdal SP, Kroenke CH, Laden F, et al. Night work and breast cancer risk: a systematic review and meta-analysis. Eur J Cancer. 2005;41(13):2023–32. doi: 10.1016/j.ejca.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Pesch B, Harth V, Rabstein S, et al. Night work and breast cancer - results from the German GENICA study. Scand J Work Environ Health. 2010;36(2):134–41. doi: 10.5271/sjweh.2890. [DOI] [PubMed] [Google Scholar]
- 34.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst. 2001;93(20):1563–8. doi: 10.1093/jnci/93.20.1563. [DOI] [PubMed] [Google Scholar]
- 35.Schernhammer ES, Kroenke CH, Laden F, et al. Night work and risk of breast cancer. Epidemiology. 2006;17(1):108–11. doi: 10.1097/01.ede.0000190539.03500.c1. [DOI] [PubMed] [Google Scholar]
- 36.Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17(4):273–84. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 37.Herljevic M, Middleton B, Thapan K, et al. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40(3):237–42. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Barcelo EJ, Cos S, Mediavilla D, et al. Melatonin-estrogen interactions in breast cancer. J Pineal Res. 2005;38(4):217–22. doi: 10.1111/j.1600-079X.2004.00207.x. [DOI] [PubMed] [Google Scholar]
- 39.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150(6):2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green LE, Dinh TA, Smith RA. An estrogen model: the relationship between body mass index, menopausal status, estrogen replacement therapy, and breast cancer risk. Comput Math Methods Med. 2012;2012:792375. doi: 10.1155/2012/792375. [DOI] [PMC free article] [PubMed] [Google Scholar]