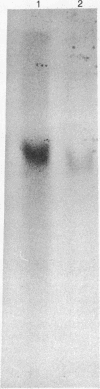
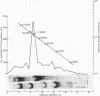
Abstract
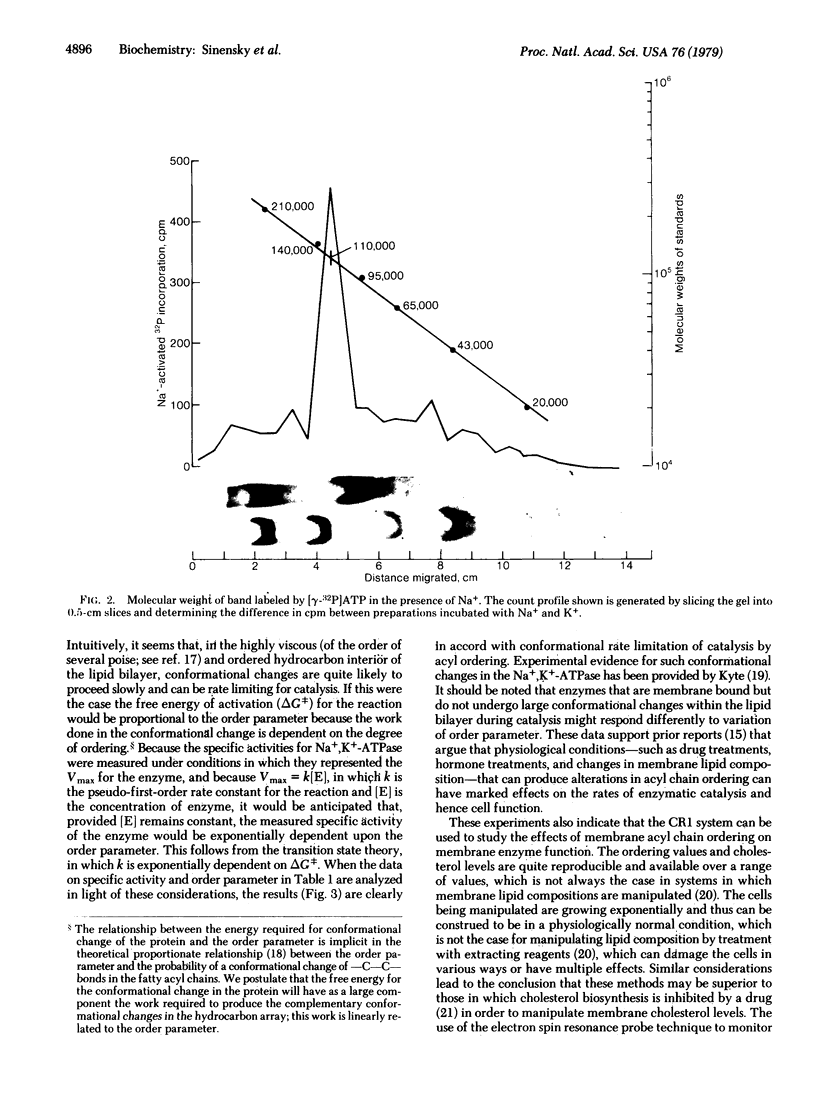
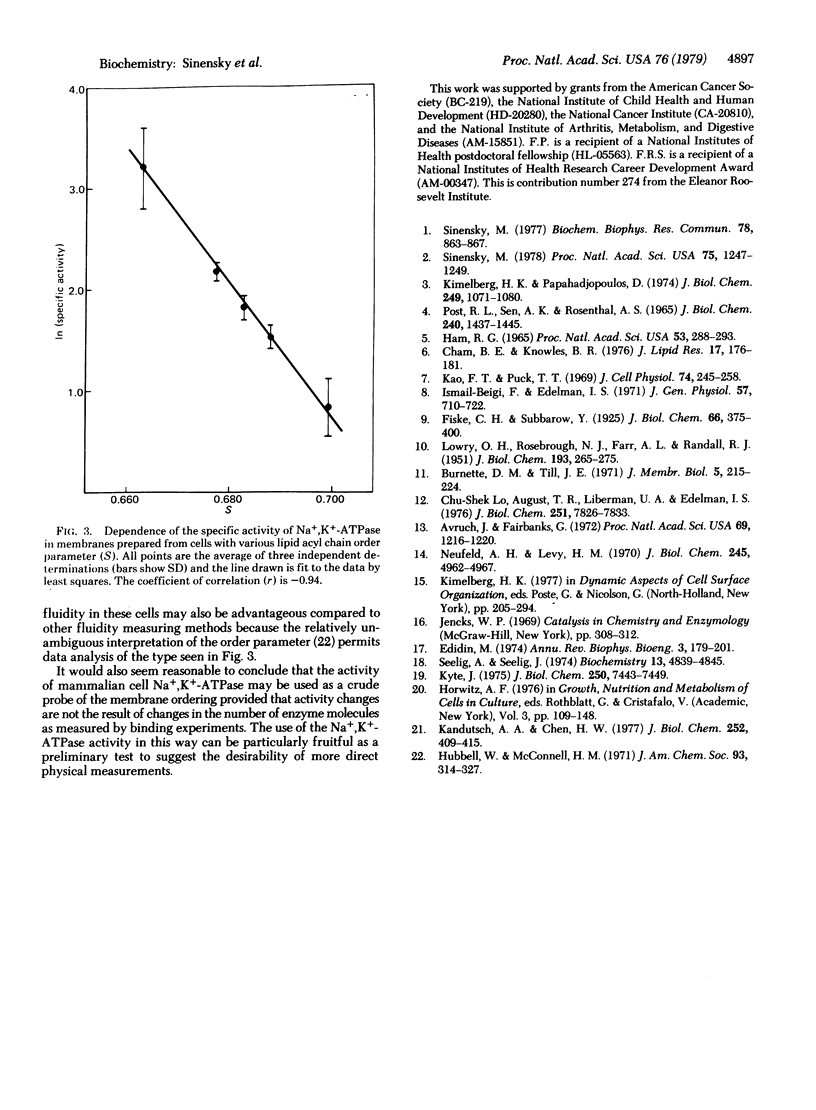
A somatic cell mutant (CR1) of the Chinese hamster ovary cell line (CHO-K1) that is defective in the regulation of cholesterol biosynthesis can be grown under conditions in which plasma membranes from these cells display various cholesterol contents and acyl chain order parameters. The (Na+ + K+)-stimulated adenosinetriphosphatase (ATP phosphohydrolase, EC 3.6.1.3) from these cells was shown to vary in activity by a factor of 10 as the order parameter was varied, and the activity exhibited an exponential dependence on this parameter. Under these conditions the number of Na+,K+-ATPase molecules was shown to remain constant by affinity labeling with [gamma-32P]ATP in the absence of Na+. Control experiments showed that alteration in cholesterol content without change in order parameter did not result in altered enzyme activity. It is concluded that, under our conditions, the rate of catalysis by the Na+,K+-ATPase is determined by the order parameter. These studies suggest a physical mechanism by which variation of membrane lipid composition or other factors that determine membrane lipid acyl chain order parameter can result in variation in membrane enzyme activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Fairbanks G. Demonstration of a phosphopeptide intermediate in the Mg ++ -dependent, Na + - and K + -stimulated adenosine triphosphatase reaction of the erythrocyte membrane. Proc Natl Acad Sci U S A. 1972 May;69(5):1216–1220. doi: 10.1073/pnas.69.5.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cham B. E., Knowles B. R. A solvent system for delipidation of plasma or serum without protein precipitation. J Lipid Res. 1976 Mar;17(2):176–181. [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F., Edelman I. S. The mechanism of the calorigenic action of thyroid hormone. Stimulation of Na plus + K plus-activated adenosinetriphosphatase activity. J Gen Physiol. 1971 Jun;57(6):710–722. doi: 10.1085/jgp.57.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandutsch A. A., Chen H. W. Consequences of blocked sterol synthesis in cultured cells. DNA synthesis and membrane composition. J Biol Chem. 1977 Jan 25;252(2):409–415. [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IX. Quantitation of mutagenesis by physical and chemical agents. J Cell Physiol. 1969 Dec;74(3):245–258. doi: 10.1002/jcp.1040740305. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K., Papahadjopoulos D. Effects of phospholipid acyl chain fluidity, phase transitions, and cholesterol on (Na+ + K+)-stimulated adenosine triphosphatase. J Biol Chem. 1974 Feb 25;249(4):1071–1080. [PubMed] [Google Scholar]
- Kyte J. Structural studies of sodium and potassium ion-activated adenosine triphosphatase. The relationship between molecular structure and the mechanism of active transport. J Biol Chem. 1975 Sep 25;250(18):7443–7449. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lo S. C., August T. R., Liberman U. A., Edelman I. S. Dependence of renal (Na+ + k+)-adenosine triphosphatase activity on thyroid status. J Biol Chem. 1976 Dec 25;251(24):7826–7833. [PubMed] [Google Scholar]
- Neufeld A. H., Levy H. M. The steady state level of phosphorylated intermediate in relation to the two sodium-dependent adenosine triphosphatases of calf brain microsomes. J Biol Chem. 1970 Oct 10;245(19):4962–4967. [PubMed] [Google Scholar]
- POST R. L., SEN A. K., ROSENTHAL A. S. A PHOSPHORYLATED INTERMEDIATE IN ADENOSINE TRIPHOSPHATE-DEPENDENT SODIUM AND POTASSIUM TRANSPORT ACROSS KIDNEY MEMBRANES. J Biol Chem. 1965 Mar;240:1437–1445. [PubMed] [Google Scholar]
- Seelig A., Seelig J. The dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic resonance. Biochemistry. 1974 Nov 5;13(23):4839–4845. doi: 10.1021/bi00720a024. [DOI] [PubMed] [Google Scholar]
- Sinensky M. Defective regulation of cholesterol biosynthesis and plasma membrane fluidity in a Chinese hamster ovary cell mutant. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1247–1249. doi: 10.1073/pnas.75.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M. Isolation of a mammalian cell mutant resistant to 25-hydroxy cholesterol. Biochem Biophys Res Commun. 1977 Oct 10;78(3):863–867. doi: 10.1016/0006-291x(77)90502-2. [DOI] [PubMed] [Google Scholar]