Abstract
Gene exchange between species occurs in areas of secondary contact, where two species have the opportunity to hybridize. If heterospecific males are more common than conspecific males, females will experience more encounters with males of other species. These encounters might increase the likelihood of heterospecific matings, and lead to the production of hybrid progeny. I studied the mating behavior of two pairs of sibling species endemic to Africa: Drosophila yakuba/Drosophila santomea and Drosophila simulans/Drosophila sechellia. Drosophila yakuba and D. simulans are cosmopolitan species widely distributed in the African continent, while D. santomea and D. sechellia are island endemics. These pairs of species hybridize in nature and have the potential to exchange genes in natural conditions. I used these two pairs of Drosophila species, and constructed mating communities of different size and different heterospecific:conspecific composition. I found that both the total number of potential mates and the relative frequency of conspecific versus heterospecific males affect female mating decisions in the cosmopolitan species but not in the island endemics. These results suggest that the population characteristics, in which mating occurs, may affect the magnitude of premating isolation. Community composition might thus facilitate, or impair, gene flow between species.
Keywords: Behavioral isolation, Drosophila, hybridization
Introduction
When different animal species come together in the same geographical location and share at least part of their habitat, biological traits associated with mate choice can prevent interbreeding (Coyne and Orr 2004; Price 2007). One of the most effective mechanisms of reproductive isolation results from mate preferences that differ between species (Mayr 1942; Kirkpatrick and Ravigné 2002; Coyne and Orr 2004; Ritchie 2007). Premating behavioral isolation occurs when one or both partners discriminate against the other, thus precluding mating and gene flow (Kaneshiro 1980; Safran et al. 2013). Because of their greater investments of resources during and after mating, females usually are the ones that exert the choice and discriminate against heterospecific males. Mate choice can vary in response to factors intrinsic to the potential partners (i.e., age, health, reproductive fitness), or to extrinsic factors in the environment in which potential mates encounter each other (i.e., habitat and phenology differences; Rice and Hostert 1993; Rolán-Alvarez and Caballero 2000; Knowles et al. 2004; Coyne et al. 2005).
When heterospecific matings occur, and there is not complete intrinsic reproductive isolation between the species, interspecific hybrids are produced. The consequences of hybridization can vary. One potential outcome is that the production of unfit hybrids might impose selection to make premating isolation stronger. In this process, termed reinforcement, the enhancement of premating isolation occurs as a byproduct of selection against maladaptive hybridization (reviewed in Servedio and Noor 2003). A second outcome is that gene flow between species might lead to introgression of foreign genes into the genome; gene flow can have deleterious consequences (e.g., Fang et al. 2012), but also may become raw material for the origin of new adaptations (reviewed in Hedrick 2013). Finally, in rare instances, the production of hybrids can lead to the origin of a new lineage that shows reproductive isolation toward the parentals and thus constitutes a new hybrid species (Arnold and references therein, Schumer et al. 2014).
Even though there is consensus about the evolutionary importance of hybridization in nature, there is not a comprehensive understanding of what biotic or abiotic factors might facilitate hybridization between species. Several arguments posit that if heterospecific males are much more common than conspecific males, then females will experience more encounters with heterospecifics, and produce more hybrid progeny than females in areas where conspecifics are common and heterospecifics are rare (Volpe 1955; Waage 1979; Peterson et al. 2005; Nosil 2012). Nonetheless, the assumption that differential relative frequency of conspecific and heterospecific males can lead to different levels of hybridization has remained untested. Here, I used two pairs of Drosophila species to assess whether different ratios of conspecifics to heterospecifics can lead to increased hybridization.
Drosophila yakuba and D. santomea are two sister species of the melanogaster subgroup of species (Lachaise et al. 2000). Drosophila yakuba is a human-commensal species that is widespread throughout sub-Saharan Africa (Burla 1954, Lachaise et al. 1988). Drosophila santomea, the sister species of D. yakuba, is an endemic species to the highlands of São Tomé, a volcanic island off the coast of Cameroon (Lachaise et al. 2000). On Pico de São Tomé, D. yakuba inhabits low elevations (below 1450 m), and is found in open habitats (Llopart et al. 2005a). In contrast, D. santomea inhabits the mist forests of the island at elevations between 1153 m and 1800 m (Llopart et al. 2005a, Llopart et al. 2005b). Even though these two species diverged around million years ago, they occasionally hybridize in the midlands of the mountain Pico de São Tomé in a recent area of secondary contact (Llopart et al. 2005a, Llopart et al. 2005b). Hybrids between these two species show reduced fitness as a result of intrinsic postzygotic isolation: hybrids of both sexes show mildly reduced viability when compared to pure species. Hybrid females are usually fertile but F1 hybrid males, and a large proportion of the males from advanced intercrosses, are sterile and show gross defects in spermatogenesis (Moehring et al. 2006, Matute et al. 2009; Matute and Coyne 2010).
Drosophila simulans is a human commensal that is thought to have originated in Madagascar and currently has a cosmopolitan geographic range, including the Seychelles archipelago in the Indian Ocean. Drosophila sechellia (Tsacas and Bächli 1981; David et al. 2007), an endemic species to the Seychelles, is closely related to D. simulans (Amlou et al. 1998; Kliman et al. 2000; McDermott and Kliman 2008). The two species diverged approximately 0.2 million years ago (Kliman et al. 2000; McDermott and Kliman 2008). When the two species are crossed, they produce fertile hybrid females but sterile F1 males (Coyne and Kreitman 1986; Coyne et al. 1991; Cabot et al. 1994; Hollocher and Wu 1996). In a similar manner to the D. yakuba/D. santomea species pair, the majority of males from advanced intercrosses are sterile as well (Moehring et al. 2005). Drosophila simulans and D. sechellia currently hybridize in the central islands of the Seychelles archipelago (Matute and Ayroles 2014) and are thought to have experienced gene flow at some point (Solignac and Monnerot 1986; Garrigan et al. 2012), indicating that hybridization has played a role in their evolutionary history.
In both species pairs, and both directions of the cross, heterospecific matings occur at a much lower frequency than conspecific matings. In nonchoice experiments (i.e., one female and one male in a vial), females from both D. yakuba/D. santomea and D. simulans/D. sechellia species pairs show a strong tendency to mate with conspecific males and rarely accept heterospecific males (Coyne 1992; Coyne et al. 2002).
In Drosophila, attributes of individuals and their environments may affect the frequency of hybridization but they remain largely unstudied in experimental settings. Coyne et al. (2005) explored the effects of three factors – individual female health, the effect of female having more space to escape from unwanted mates, and the effect of having a fruit present in the mating arena – and found that none of them had an effect on female mating decisions. Many other attributes of the mating community remain to be tested.
Population composition may affect female mate choice and the frequency at which hybridization occurs. Here I show that the two population factors, the relative ratio of heterospecific versus conspecific males, and the total number of potential mates, affect female mating decisions in the aforementioned Drosophila species pairs. Gene flow between species may therefore be contingent upon the communities in which members of those species encounter one another.
Methods
Stocks
All collected stocks and populations were reared on standard cornmeal/Karo/agar medium at 24°C under a 12-h light/dark cycle. I used genetically heterogeneous strains of each species (i.e., synthetic lines) by combining virgin males and females from several isofemale lines collected in São Tomé outside the hybrid zone (i.e., allopatric lines). For all the experiments involving D. yakuba and D. santomea, I used the D. yakuba SYN2005 and D. santomea SYN2005 stocks, respectively (Matute et al. 2009; Matute and Coyne 2010). For the experiments involving D. simulans and D. sechellia, I used D. simulans Florida city (Coyne and Beecharn 1987) and D. sechellia SYNDenis (Matute and Ayroles 2014) synthetic stocks. All stocks were kept in large numbers after they were created.
Effect of relative frequency on mating behavior
I explored two components of mating behavior, conspecific copulation latency (i.e., how long does it take for a conspecific mating to take place) and conspecific copulation duration (i.e., how long did copulation last) when Drosophila females of four different species (D. yakuba, D. santomea, D. simulans, and D. sechellia) were exposed to mating situations in which conspecific males were present and males from a second hybridizing species were also present. I assayed all the possible combinations between the two factors: the relative frequency of heterospecific males (10 different frequencies from 10% to 99% in increments of 10%) and the total number of flies in the mating assay (10 different totals from 100 to 1000 in increments of 100). In total, I recorded both premating isolation behaviors in 100 combinations and assayed 15 females per combination.
All measurements of premating isolation were carried out using previously described methods (Coyne et al. 2002; Matute 2010); unlike previous experiments, females were allowed to choose their mate. Briefly, females and males were collected as virgins within 8 h of eclosion. All individuals were kept in vials of 22 flies of the same sex for 3 days. On Day 3, females were housed in red-colored food (which turned their abdomens red) for their easier identification in the experimental mating population. Red dye has a negligible effect on mating choice (Ting et al. 2001; Matute 2013). Four days after collection, a single virgin female and the virgin males dictated by each experimental combination, as described above, were combined. All flies were transferred without anesthesia to a bottle with cornmeal food. For each mated female, I recorded conspecific copulation latency (i.e., how long did it take for a D. yakuba female to find a conspecific mating partner and start copulating), and conspecific copulation duration (i.e., how long did conspecific females and males remained joint in mating). Flies were observed for 1 h. I did not observe any heterospecific matings using this approach.
In parallel, I set up mating trails in which the only males present were conspecific. I varied the number of flies in the mating assay (10 different totals from 100 to 1000 in increments of 100). I recorded both conspecific copulation latency and conspecific copulation duration in mating trials of ten different sizes and assayed 15 females per population size.
To study heterospecific mating frequency (i.e., how often females accepted heterospecific males even though conspecific males were present in the population), I measured premating isolation over 24 h for a single female that were housed with both conspecific and heterospecific males in the numbers and relative frequencies described above. I let trials proceed for 24 h and then anesthetized all the flies in the mating population with CO2, discarded the males and returned each female to the vial where the mating took place in order to observe the resulting F1 generation. I started 100 replicates per combination of variables (i.e., each combination of heterospecific relative frequency and total number of flies). I raised the progeny of each female by standard fly husbandry methods, and used the presence or absence of hybrid progeny as a conservative proxy of whether heterospecific matings occurred. The frequency of heterospecific matings for each treatment was calculated by counting how many of the vials within a block produced hybrid progeny. For D. yakuba and D. santomea experimental populations, I used abdominal pigmentation to identify whether vials produced hybrid progeny. I qualitatively scored the abdominal pigmentation of 100 males per vial. Drosophila yakuba males have a dark abdomen, while D. santomea males have a light abdomen. F1 males have an intermediate abdominal pigmentation (Llopart et al. 2002). For D. simulans and D. sechellia, experimental populations, I used male genital morphology to identify whether vials produced hybrid progeny. I qualitatively scored the morphology of the posterior genital arch of 100 males per vial. Drosophila simulans males have red spheroid large genital arches. Drosophila sechellia males have small and elongated genital arches. The hybrids show intermediate genital morphology and can be distinguished from the parental species (Coyne and Kreitman 1986; Coyne et al. 1991; MacDonald and Goldstein 1999; Matute and Ayroles 2014).
Statistical analyses
All statistical analyses were carried out using R (R Development Core Team 2005).
To determine the significance of the frequency of heterospecific males and the mating population size on mating behavior, I analyzed the conspecific copulation latency and conspecific copulation duration, I fitted a multiple regression for each of the two components of premating behavior. The premating behavior trait was the response, while the relative frequency of heterospecific males and the total number of flies were the continuous variables. Multiple regressions took the form:
where Yij is the response (behavioral trait), Fi is the frequency of heterospecific males, Sj is the mating population size (i.e., number of males in the mating assay), Fi × Sj is the interaction between the variables, and Eij is the error term. The significance of independent relationships in the multiple regressions was determined using a two-tailed t-test on the regression coefficients (degrees of freedom, df = 1496).
For the heterospecific mating frequency data (collected when females were housed with different combinations of males over 24 h), I used the “aod” package in R (Lesnoff and Lancelot 2012). I fitted generalized logistic models with binomial response distributions in which whether a vial produced hybrid progeny or not was the response. The total number of flies in the vial and the relative frequency of heterospecifics were considered continuous variables. I allowed for an interaction term between these two variables. Significance of each variable was assessed using a Wald text following a χ2 distribution (df = 1). Linear models followed the form:
P values under 0.05 were considered significant.
I assessed whether total number of flies and the ratio of heterospecifics were multicollinear variables (i.e., the two predictor variables were correlated). The reason for this concern was that the ratio of heterospecifics was calculated using the total number of flies as the denominator. This can lead to autocorrelation of the two variables. I calculated the variance inflation factor (VIF) between the two regression coefficients using the R package HH (Heiberger 2009). The VIF for predictor i equals:
where 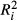 is the coefficient of determination from a regression of predictor i against the remaining predictors. In the case of the eight multiple regressions shown in this study, we calculated the VIF values for the two predictor variables in models that had no interaction term. Because all the multiple regressions had the same two predictor variables (and the same values), I only needed to calculate one VIF. The VIF for the model
is the coefficient of determination from a regression of predictor i against the remaining predictors. In the case of the eight multiple regressions shown in this study, we calculated the VIF values for the two predictor variables in models that had no interaction term. Because all the multiple regressions had the same two predictor variables (and the same values), I only needed to calculate one VIF. The VIF for the model
where Rij is either latency or duration, Fi is the ratio of heterospecifics and Sj is the mating population size, equaled 1. Values of VIF larger than 5 are considered evidence of collinearity between predictor variables (Stine 1995; O'Brien 2007). This result indicates that the two predictor variables are not multicollinear.
To plot heterospecific mating frequency data, I used Akima interpolation splines (Akima 1970) between sampling intervals, and plotted them using contour maps with the “akima” R package (Akima et al. 2006).
Results
D. yakuba/D. santomea
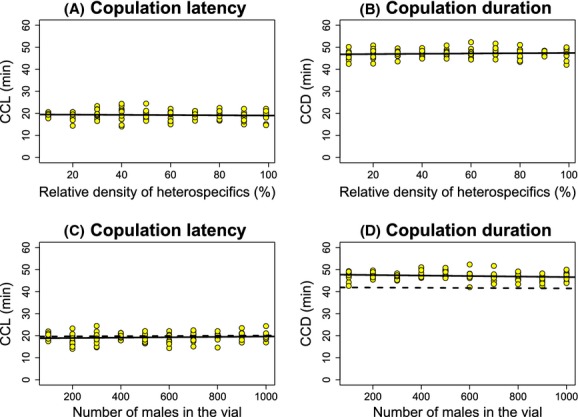
I studied the mating sexual behavior of D. yakuba females exposed to different relative frequencies of D. santomea and D. yakuba males in populations with different total numbers of males. The mating behaviors I studied were conspecific copulation latency (time until mating began) and duration. The average conspecific copulation latency and conspecific copulation duration per treatment are shown in Figure 1. Both the relative frequency of D. santomea males and population size affected the mating behavior of D. yakuba females toward conspecific males; in larger populations, the conspecific copulation latency was longer (Table 1, Fig. 1) and conspecific copulation duration was shorter (Table 2, Fig. 1). In the case of duration, but not of latency, the interaction between population size and composition was also significant, indicating a strong interplay between the size of the mating community and the ratio of heterospecific to conspecific males (Tables 1, 2).
Figure 1.
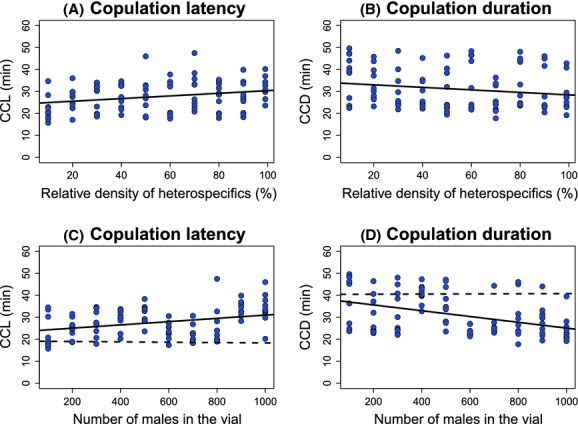
High ratios of Drosophila santomea males relative to Drosophila yakuba males affect two components of mating sexual behavior (conspecific copulation latency –CCL– and conspecific copulation duration –CCD–) in D. yakuba females. (A) The number of D. santomea males (%) significantly affected conspecific copulation latency in D. yakuba females. (B) The number of D. santomea males (%) significantly affected conspecific copulation duration in D. yakuba females. (C) The size of the mating population significantly affected conspecific copulation latency in D. yakuba females. (D) The size of the mating population significantly affected conspecific copulation duration in D. yakuba females. Each circle represents the average of 15 replicates. All significance values are shown in Table 1.
Table 1.
Multiple regressions for copulation latency for each of the four species
| Copulation latency | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Relative frequency of heterospecifics | Total number of flies | Interaction: Relative frequency of heterospecifics × Total number of flies | |||||||||
| Estimate | t value | P-value | Coefficient | t value | P-value | Coefficient | t value | P-value | Coefficient | t value | P-value | |
| Drosophila yakuba | 20.99 | 16.639 | <1 × 10−10 | 4.69 × 10−2 | 2.302 | 0.022 | 6.10 × 10−3 | 3.001 | 2.74 × 10−3 | 2.44 × 10−5 | 0.744 | 0.457 |
| Drosophila santomea | 18.75 | 17.404 | <1 × 10−10 | 9.38 × 10−4 | 0.054 | 0.957 | 1.39 × 10−3 | 0.802 | 0.423 | −1.03 × 10−5 | −0.367 | 0.714 |
| Drosophila simulans | 27.56 | 24.868 | <1 × 10−10 | 4.80 × 10−2 | 2.680 | 7.45 × 10−3 | −1.00 × 10−2 | −5.608 | 2.43 × 10−8 | 6.79 × 10−5 | 2.353 | 0.019 |
| Drosophila sechellia | 43.58 | 33.979 | <1 × 10−10 | 2.35 × 10−2 | 1.135 | 0.259 | 2.25 × 10−3 | 1.086 | 0.280 | −4.35 × 10−5 | −1.301 | 0.196 |
A multiple linear regression was fitted to study the effect of number of heterospecifics (Fi), the population size (Sj), and the interaction between these two variables (Fi × Sj) in the copulation latency of each of the four species in experimental cages (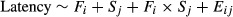 ). The two factors and the interaction were significant for D. yakuba and D. simulans but not for D. santomea or D. sechellia. Significant values (P < 0.05, df = 1496) are marked in bold.
). The two factors and the interaction were significant for D. yakuba and D. simulans but not for D. santomea or D. sechellia. Significant values (P < 0.05, df = 1496) are marked in bold.
Table 2.
Multiple regressions for copulation duration for each of the four species
| Copulation duration | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Relative frequency of heterospecifics | Total number of flies | Interaction: Relative frequency of heterospecifics × Total number of flies | |||||||||
| Estimate | t value | P-value | Coefficient | t value | P-value | Coefficient | t value | P-value | Coefficient | t value | P-value | |
| Drosophila yakuba | 45.41 | 30.847 | <1 × 10−10 | 0.13 | −5.469 | 5.30 × 10−8 | −2.05 × 10−2 | −8.625 | 1 × 10−10 | 1.33 × 10−4 | 3.458 | 5.58 × 10−4 |
| Drosophila santomea | 46.62 | 47.808 | <1 × 10−10 | 2.07 × 10−2 | 1.312 | 0.190 | 2.87 × 10−4 | 0.182 | 0.855 | −2.60 × 10−5 | −1.022 | 0.307 |
| Drosophila simulans | 41.18 | 27.189 | <1 × 10−10 | −0.12 | −4.694 | 2.92 × 10−6 | −9.78 × 10−3 | −4.005 | 6.50 × 10−5 | 8.01 × 10−5 | 2.030 | 0.043 |
| Drosophila sechellia | 15.36 | 19.299 | <1 × 10−10 | 8.56 × 10−4 | 0.067 | 0.947 | −5.27 × 10−4 | −0.411 | 0.681 | −6.26 × 10−6 | −0.302 | 0.763 |
A multiple linear regression was fitted to study the effect of number of heterospecifics (Fi), the population size (Sj), and the interaction between these two variables (Fi × Sj) in the copulation duration of each of the four species in experimental cages (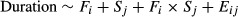 ). The two factors and the interaction were significant for D. yakuba and D. simulans but not for D. santomea or D. sechellia. Significant values (P < 0.05 df = 1496) are marked in bold.
). The two factors and the interaction were significant for D. yakuba and D. simulans but not for D. santomea or D. sechellia. Significant values (P < 0.05 df = 1496) are marked in bold.
Notably when a single D. yakuba female was housed with only conspecific males in populations of different sizes, both copulation latency and duration remained constant (Table 3). These results indicate that a simple increase in the population size (and consequently the sex ratio) does not lead to changes in mating behavior. Such changes are only observed when there are heterospecific males present in the assay.
Table 3.
Mating assays with only conspecific males and different population sizes
| Copulation latency | Copulation duration | |||||
|---|---|---|---|---|---|---|
| Species | Coefficient | t value | P-value | Coefficient | t value | P-value |
| Drosophila yakuba | −8.009 × 10−4 | −0.537 | 0.592 | 3.25 × 10−4 | 0.144 | 0.885 |
| Drosophila santomea | 4.746 × 10−4 | 0.381 | 0.704 | −4.875 × 10−4 | −0.318 | 0.751 |
| Drosophila simulans | 2.142 × 10−3 | 1.30 | 0.196 | −1.289 × 10−3 | −0.734 | 0.464 |
| Drosophila sechellia | 9.093 × 10−4 | 0.758 | 0.449 | −1.776 × 10−3 | −1.201 | 0.232 |
No heterogeneity was detected in any of the four species at the two mating behaviors. I found no significant values (P < 0.05, df = 1496).
In addition to conspecific copulation latency and duration, I investigated the effect of community composition on reproductive isolation in D. yakuba. There were no heterospecific matings observed in the 1-h trials, so I next looked at heterospecific mating frequencies in 24-h trials when D. yakuba females were housed with the same ratios of conspecific:heterospecific males and total numbers of males as in the 1-h observations. In this experiment, large populations that had a high ratio of heterospecific to conspecific males were more prone to produce hybrid progeny (binomial logistic regression, Wald test, χ2 = 384.9, df = 3, P < 1 × 10−10, Fig. 2). I found that the ratio of heterospecific to conspecific males had a significant effect on the frequency of heterospecific matings (Wald test, χ2 = 59.1, df = 1, P < 1.1 × 10−10). The total number of flies also had a significant effect on the frequency of heterospecific matings (χ2 = 35.4, df = 1, P = 2.9 × 10−9). The interaction between these two variables was also significant (χ2 = 11.9, df = 1, P = 5.60 × 10−4). In general, heterospecific matings were observed only in populations in which more than 70% of the males were D. santomea.
Figure 2.
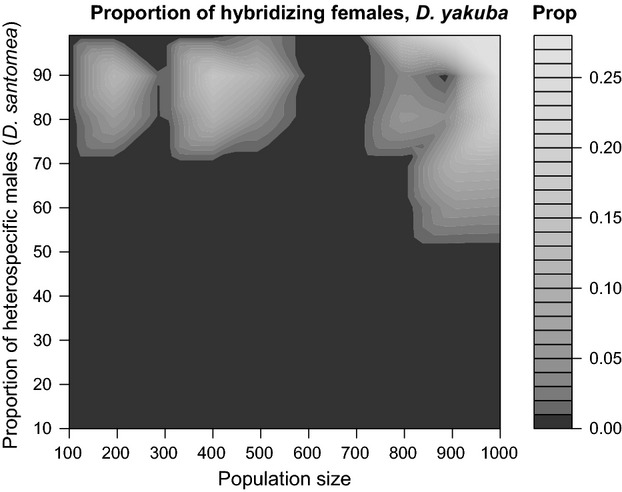
The ratio of Drosophila santomea males relative to that of Drosophila yakuba males affects the strength of premating isolation in D. yakuba females. In conditions where D. santomea males are overwhelmingly more common than D. yakuba males, D. yakuba females accept D. santomea males. Light gray indicates conditions where heterospecific matings were observed.
I next looked at the mating behavior of females from the other species of this pair. Using the same procedures but with the species reversed, I investigated whether conspecific mating behavior or levels of reproductive isolation in D. santomea females were also affected by the ratio of heterospecific versus conspecific males and the size of the mating population. Neither of the two factors that I manipulated (as above, ratio of heterospecific to conspecific males and the mating population size) led to changes of the magnitude of reproductive isolation in D. santomea females toward D. yakuba males, nor to changes in the conspecific copulation latency (Table 1) or duration of D. santomea females (Table 2). In controls that only included different numbers of D. santomea males I observed no differences in copulation latency or copulation duration among treatments (Fig. 3, Table 3). These results indicate that the mating behavior of D. santomea females is not affected by the presence of heterospecific males regardless of the frequency at which they are present. Furthermore, in populations where both D. yakuba and D. santomea males were present, I observed no heterospecific copulations, neither in the 1-h measurements (n = 1500 observations and 100 different treatments) nor in the 24-h trials (n = 10,000 observations and 100 different treatments). Although they will occasionally mate with D. yakuba males in no-choice matings (Coyne et al. 2002), D. santomea females will always choose D. santomea males when given the chance.
Figure 3.
The ratio of Drosophila yakuba males relative to Drosophila santomea males had no effect on two components of mating sexual behavior (conspecific copulation latency –CCL– and conspecific copulation duration –CCD–) in D. santomea females. (A) The number of D. yakuba males (%) did not affect conspecific copulation latency in D. santomea females. (B) The number of D. yakuba males (%) did not affect conspecific copulation duration in Drosophila santomea females. (C) The size of the mating population did not affect conspecific copulation latency in D. santomea females. (D) The size of the mating population did not affect conspecific copulation duration in D. santomea females. Each circle represents the average of 15 replicates. All significance values are shown in Table 1.
D. simulans/D. sechellia
In order to assess whether the results observed for D. yakuba/D. santomea were specific to that species pair or whether they also applied to other species; I followed the same experimental procedures in a second species pair: D. simulans and D. sechellia. I measured the magnitude of the two conspecific mating behavior traits in D. simulans females exposed to different ratios of D. sechellia to D. simulans males. For 1-h experiments, multiple regressions showed that the frequency of the heterospecific D. sechellia, and the total number of flies affected both components of mating behavior in each mating trial for D. simulans females (see Methods, Table 1 and 2, Fig. 4). The interaction between these two variables was marginally significant for conspecific latency (Table 1) and conspecific duration (Table 2). I also assayed mating behavior in cages that contained only D. simulans males and observed no variation in latency or duration (Table 3). These results, similar to the ones from D. yakuba, suggest that a simple increase in population size is not enough to modify the mating behavior of D. simulans females and that changes in mating behavior only occur when there are heterospecific males present in the assay (Table 3, Fig. 4).
Figure 4.
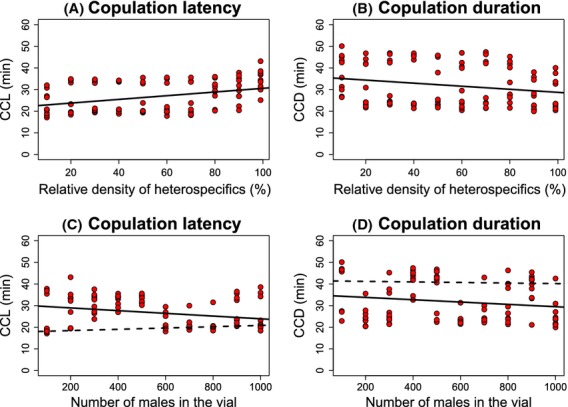
High ratios of Drosophila sechellia males relative to Drosophila simulans males affect two components of mating sexual behavior (conspecific copulation latency –CCL– and conspecific copulation duration –CCD–) in D. simulans females. (A) The number of D. sechellia males (%) significantly affected conspecific copulation latency in D. simulans females. (B) The number of D. sechellia males (%) significantly affected conspecific copulation duration in D. simulans females. (C) The size of the mating population significantly affected conspecific copulation latency in D. simulans females. (D) The size of the mating population significantly affected conspecific copulation duration in D. simulans females. Each circle represents the average of 15 replicates. All statistics and significance values are shown in Table 1.
While both latency and duration of conspecific matings were affected by the presence and number of heterospecifics, no heterospecific matings were observed in these 1-h trials either. Next I examined heterospecific matings when I housed D. simulans females with different combinations of D. simulans and D. sechellia males for 24 h. Similar to the observations of D. yakuba, large populations with few conspecific males were more likely to produce hybrid progeny (binomial logistic regression, Wald test, χ2 = 361.1, df = 3, P < 1.4 × 10−13, Fig. 5). I found there was a strong effect on the number of heterospecific matings of the relative frequency of conspecific to heterospecific males (χ2 = 54.7, df = 1, P = 1.9 × 10−6), the total number of flies in the cage (χ2 = 22.7, df = 1, P = 1.9 × 10−6), and the interaction between these two variables (χ2 = 6.20, df = 1, P = 0.013).
Figure 5.
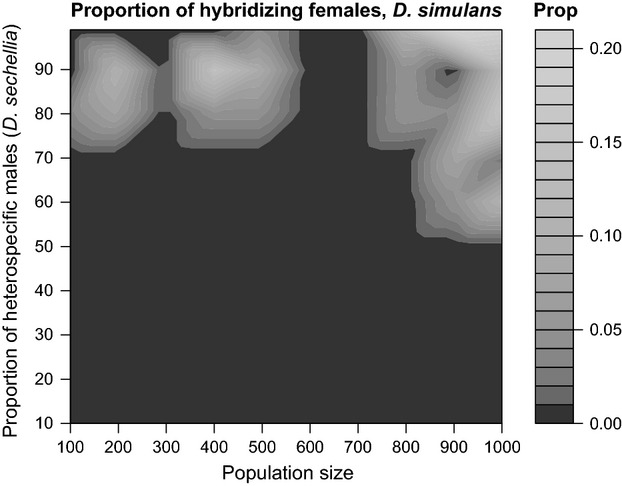
The ratio of Drosophila sechellia males relative to that of Drosophila simulans males affects the strength of premating isolation in D. simulans females. In conditions where Drosophila sechellia males are overwhelmingly more common than D. simulans males, D. simulans females accept D. sechellia males.
Finally, I studied the mating behavior of females from the second species of this pair, D. sechellia, in communities of different sizes and compositions. Neither of the two experimental variables (population size nor relative frequency of heterospecifics) affected the mating behavior (conspecific mating latency or duration) of D. sechellia females (Tables 1, 2, Fig. 6). I observed no changes in copulation latency or copulation duration when the assays only included D. sechellia males (Table 3).
Figure 6.
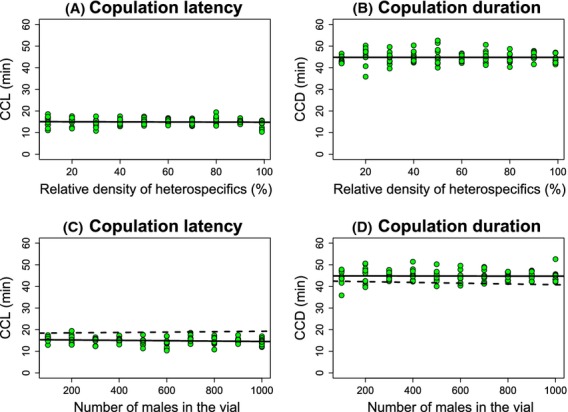
The ratio of Drosophila simulans males relative to Drosophila sechellia males had no effect on two components of premating sexual behavior (conspecific copulation latency –CCL– and conspecific copulation duration –CCD–) in D. sechellia females. (A) The number of D. simulans males (%) did not affect conspecific copulation latency in D. sechellia females. (B) The number of D. simulans males (%) did not affect conspecific copulation duration in D. sechellia females. (C) The size of the mating population did not affect conspecific copulation latency in D. sechellia females. (D) The size of the mating population did not affect conspecific copulation duration in D. sechellia females. Each circle represents the average of 15 replicates. All statistics and significance values are shown in Table 1.
No heterospecific matings were observed with D. sechellia for either 1-h or 24-h trials. These results indicate that, as was the case for D. santomea, the mating behavior of D. sechellia females is not affected by the presence of heterospecific males regardless of the ratio of heterospecific to conspecific males and the mating population size.
Discussion
This study explores how relative frequency and population size, individually and interacting, influence mating behavior in at least two species of Drosophila. The results shown here have two implications for our understanding of how premating isolation is involved in the persistence of potentially hybridizing species. First, they confirm that, in some animal species, hybridization is more likely if heterospecific males are disproportionally more abundant than conspecifics males. The relative frequency of males has been proposed as a factor influencing the magnitude of reproductive isolation in natural populations (Harper and Paulson 1994; Berglund 1995; Sprenger et al. 2011; Willis et al. 2011, 2012; Verzijden et al. 2012). Under this regime, females will accept males from other species if the environment in which mating takes place is not conducive to finding a conspecific partner – for instance, if females cannot detect the few conspecific males in the community, or if they encounter many heterospecific males before a conspecific (Wilson and Hedrick 1982; Willis et al. 2012). These results are of particular importance for the study of reinforcing selection, the enhancement of prezygotic isolation as a byproduct of maladaptive hybridization (reviewed in Servedio and Noor 2003; Coyne and Orr 2004; Pfennig and Pfennig 2009). It is commonly argued that if reinforcing selection affects only one species in a hybrid zone, it should be the rarest one (Coyne and Orr 2004; Price 2007; Nosil 2012). The basic premise of this assertion is that the less abundant species will be more at risk of hybridizing because they will face more encounters with heterospecific mates. This claim makes intuitive sense but has remained largely untested. My results provide strong evidence that for some Drosophila species, the less abundant species in a community is more at risk of mating heterospecifically.
Not only are D. yakuba and D. simulans less abundant in their respective hybrid zones, but they are more widespread outside of the hybrid zone. Drosophila santomea and D. sechellia, however, are endemic to their island habitats. Behavioral isolation is much stronger when the matings involve females from the island endemics than when they involve females from the widespread species, regardless of the experimental design used to quantify the reproductive isolation (Coyne 1992; Coyne et al. 2002). The results from this report are in the same vein of previous studies and show that even in environments in which heterospecific males are overwhelmingly more common than conspecific males, D. santomea and D. sechellia females strongly discriminate against heterospecific males (Coyne 1996; Tomaru et al. 2004).
Finally, I also demonstrate that the size of the mating population has an effect on the likelihood of the occurrence of heterospecific matings. Because each assay was composed with a single female, when the population size of the male conspecific varies, the sex ratio also varies. This means I cannot fully disentangle the effects of population size and sex ratio with the current set of data, and direct observations of multifemale populations would pose distinct challenges. The results from the mating assays with only conspecific males do allow me to rule out that a simple increase in the population size (and consequently the sex ratio) causes changes in copulation latency and copulation duration. Instead, changes in these behaviors are only observed when heterospecific males are present in the assay. Regardless, these results indicate that the characteristics of the community in which mating takes place can affect the magnitude of reproductive isolation between potentially hybridizing species.
The effects of biotic factors on hybridization are largely unexplored in animals but not in plants. Pollen dispersal, a major factor leading to gene flow in plants, is heavily influenced by heterospecific relative frequency in nature (Campbell and Halama 1993; Bosch and Waser 1999, 2001). Studies on pollinator competition have revealed that the presence of heterospecifics at different densities can affect the relative rates of interspecific pollen transfer (Price and Waser 1982; Kohn and Waser 1985; Campbell and Waser 1989; Campbell and Halama 1993; Bosch and Waser 2001; reviewed in Mitchell et al. 2009). The nature of reproductive isolation differs dramatically between plants and animals with internal fertilization; while the results in plants are pollinator-mediated and thus extrinsic, the results shown here are intrinsic to the decision-making process of females choosing whether to accept a potential mate.
Even though there is little empirical evidence for potential effects of the relative frequency of heterospecific to conspecific males on the magnitude of reproductive isolation in animals, there is no reason why heterospecific males cannot be seen as low-quality males and conspecifics as high-quality males (Penteriani 2003; Kokko and Rankin 2006). This is bound to be especially true in those cases in which postzygotic isolation is already in place, as happens in the two studied species pairs (i.e., hybrid males from the crosses D. yakuba × D. santomea, and from the crosses D. simulans × D. sechellia are completely sterile). Many mating systems have demonstrated that when males interact in nature, high-quality males usually win the competition for females (Howard et al. 1997, 1998; Correa and Thiel 2003; Thiel and Correa 2004). Under some circumstances, however, females may not be able to exert their preferences (Hirotani 1994). In dung flies and colonial blackbirds, females are only able to realize their preferences for high-quality males in low-density populations. High-quality males cannot exclude other males and low-quality males get access to females when population density is high (Borgia 1981; Webster and Robinson 1999; Wong and Candolin 2005). The results here shown indicate that high densities of heterospecific males (low quality) interfere with the decision process that Drosophila females must make in order to choose conspecific over heterospecific males.
This report demonstrates that at least for these two pairs of species of Drosophila, a high relative frequency of heterospecific males and a large mating population size can affect mating behaviors and lead to increased levels of hybridization even in situations in which females have access to conspecific males. This might recapitulate the situation at the edges of hybrid zones where the relative frequency of one of the species is low; in these cases, females might be exposed to high ratios of heterospecific to conspecific males, which in turn might lead to interspecific matings and increase the likelihood of admixture and gene flow.
Acknowledgments
I would like to thank J. A. Coyne, K. L. M. Gordon, J. Gavin-Smyth, T. D. Price, M. F. Przeworski, for scientific discussions and comments at every stage of the manuscript. I would also like to thank the Bioko Biodiversity Protection Program, the National University of Equatorial Guinea, and the Ministry of Environment, Republic of São Tomé and Príncipe for permission to collect and export specimens for study. D.R.M. is funded by a Chicago Fellowship.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Data S1. Raw data and analytical code used in this report. Dryad link: doi: 10.5061/dryad.fp861.
References
- Akima H. A new method of interpolation and smooth curve fitting based on local procedures. J. Assoc. Comput. Mach. 1970;17:589–602. [Google Scholar]
- Akima H, Gebhardt A, Petzoldt T, Maechler M. 2006. akima: Interpolation of irregularly spaced data. R package version 0.5-1. Available via http://CRAN. R-project. org/package= akima.
- Amlou M, Moreteau B, David JR. Genetic analysis of Drosophila sechellia specialization: oviposition behavior toward the major aliphatic acids of its host plant. Behav. Genet. 1998;28:455–464. doi: 10.1023/a:1021689312582. [DOI] [PubMed] [Google Scholar]
- Arnold ML. Evolution through genetic exchange. Oxford, U.K: Oxford Univ. Press; 2006. [Google Scholar]
- Berglund A. Many mates make male pipefish choosy. Behaviour. 1995;132:213–218. [Google Scholar]
- Borgia G. Mate selection in the fly Scatophaga stercoraria: female choice in a male-controlled system. Anim. Behav. 1981;29:71–80. [Google Scholar]
- Bosch M, Waser NM. Effects of local density on pollination and reproduction in Delphinium nuttallianum and Aconitum columbianum (Ranunculaceae) Am. J. Bot. 1999;86:871–879. [PubMed] [Google Scholar]
- Bosch M, Waser NM. Experimental manipulation of plant density and its effect on pollination and reproduction of two confamilial montane herbs. Oecologia. 2001;126:76–83. doi: 10.1007/s004420000488. [DOI] [PubMed] [Google Scholar]
- Burla H. Zur kenntnis der Drosophiliden der Elfenbeinkuste (Franzosisch West-Afrika) Rev. Suisse Zool. 1954;61:1–218. [Google Scholar]
- Cabot EL, Davis AW, Johnson NA, Wu C-I. Genetics of reproductive isolation in the Drosophila simulans clade: complex epistasis underlying hybrid male sterility. Genetics. 1994;137:175–189. doi: 10.1093/genetics/137.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DR, Halama KJ. Resource and pollen limitations to lifetime seed production in a natural plant population. Ecology. 1993;74:1043–1051. [Google Scholar]
- Campbell DR, Waser NM. Variation in pollen flow within and among populations of Ipomopsis aggregata. Evolution. 1989;43:1444–1455. doi: 10.1111/j.1558-5646.1989.tb02595.x. [DOI] [PubMed] [Google Scholar]
- Correa C, Thiel M. Population structure and operational sex ratio in the rock shrimp Rhynchocinetes typus (Decapoda: Caridea) J. Crustac. Biol. 2003;23:849–861. [Google Scholar]
- Coyne JA. Genetics of sexual isolation in females of the Drosophila simulans species complex. Genet. Res. 1992;60:25–31. doi: 10.1017/s0016672300030639. [DOI] [PubMed] [Google Scholar]
- Coyne JA. Genetics of a difference in male cuticular hydrocarbons between two sibling species, Drosophila simulans and D. sechellia. Genetics. 1996;143:1689–1698. doi: 10.1093/genetics/143.4.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Beecharn E. Heritability of two morphological characters within and among natural populations of Drosophila melanogaster. Genetics. 1987;117:727–737. doi: 10.1093/genetics/117.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Kreitman M. Evolutionary genetics of two sibling species, Drosophila simulans and D. sechellia. Evolution. 1986;40:673–691. doi: 10.1111/j.1558-5646.1986.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer Associates; 2004. p. 545. [Google Scholar]
- Coyne JA, Rux J, David JR. Genetics of morphological differences and hybrid sterility between Drosophila sechellia and its relatives. Genet. Res. 1991;57:113–122. doi: 10.1017/s0016672300029177. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Kim SY, Chang AS, Lachaise D, Elwyn S. Sexual isolation between two sibling species with overlapping ranges: Drosophila santomea and Drosophila yakuba. Evolution. 2002;56:2424–2434. doi: 10.1111/j.0014-3820.2002.tb00168.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Elwyn S, Rolán-Alvarez E. Impact of experimental design on Drosophila sexual isolation studies: direct effects and comparison to field hybridization data. Evolution. 2005;59:2588–2601. [PubMed] [Google Scholar]
- David JR, Lemeunier F, Tsacas L, Yassin A. The historical discovery of the nine species in the Drosophila melanogaster species subgroup. Genetics. 2007;177:1969–1973. doi: 10.1534/genetics.104.84756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang S, Yukilevich R, Chen Y, Turissini DA, Zeng K, Boussy IA, et al. Incompatibility and competitive exclusion of genomic segments between sibling Drosophila species. PLoS Genet. 2012;8:e1002795. doi: 10.1371/journal.pgen.1002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigan D, Kingan SB, Geneva AJ, Andolfatto P, Clark AG, Thornton K, et al. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 2012;22:1499–1511. doi: 10.1101/gr.130922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JP, Paulson SL. Reproductive isolation between Florida strains of Aedes aegypti and Aedes albopictus. J. Am. Mosq. Control Assoc. 1994;10:88–92. [PubMed] [Google Scholar]
- Hedrick PW. Adaptive introgression in animals: examples and comparison to new mutation and standing variation as sources of adaptive variation. Mol. Ecol. 2013;22:4606–4618. doi: 10.1111/mec.12415. [DOI] [PubMed] [Google Scholar]
- Heiberger RM. 2009. HH: statistical analysis and data display: Heiberger and Holland. R package version, 2-1. Available via http://CRAN.R-project.org/package=HH.
- Hirotani A. Dominance rank, copulatory behaviour and estimated reproductive success in male reindeer. Anim. Behav. 1994;48:929–936. [Google Scholar]
- Hollocher H, Wu C-I. The genetics of reproductive isolation in the Drosophila simulans clade: X vs. autosomal effects and male vs. female effects. Genetics. 1996;143:1243–1255. doi: 10.1093/genetics/143.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RD, Moorman RS, Whiteman HH. Differential effects of mate competition and mate choice on eastern tiger salamanders. Anim. Behav. 1997;53:1345–1356. doi: 10.1006/anbe.1996.0359. [DOI] [PubMed] [Google Scholar]
- Howard RD, Martens RS, Innis SA, Drnevich JM, Hale J. Mate choice and mate competition influence male body size in Japanese medaka. Anim. Behav. 1998;55:1151–1163. doi: 10.1006/anbe.1997.0682. [DOI] [PubMed] [Google Scholar]
- Kaneshiro KY. Sexual isolation, speciation and the direction of evolution. Evolution. 1980;34:437–444. doi: 10.1111/j.1558-5646.1980.tb04833.x. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick M, Ravigné V. Speciation by natural and sexual selection: models and experiments. Am. Nat. 2002;159:S22–S35. doi: 10.1086/338370. [DOI] [PubMed] [Google Scholar]
- Kliman RM, Andolfatto P, Coyne JA, Depaulis F, Kreitman M, Berry AJ, et al. The population genetics of the origin and divergence of the Drosophila simulans complex species. Genetics. 2000;156:1913–1931. doi: 10.1093/genetics/156.4.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles LL, Hernandez BB, Markow TA. Exploring the consequences of postmating-prezygotic interactions between the sexes. Proc. Biol. Sci. 2004;271:S357–S359. doi: 10.1098/rsbl.2004.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn JR, Waser NM. The effect of Delphinium nelsonii pollen on seed set in Ipomopsis aggregata, a competitor for hummingbird pollination. Am. J. Bot. 1985;72:1144–1148. [Google Scholar]
- Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise D, Harry M, Solignac M, Lemeunier F, Bénassi V, Cariou ML. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tomé. Proc. Biol. Sci. 2000;267:1487–1495. doi: 10.1098/rspb.2000.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise D, Cariou ML, David JR, Lemeunier F, Tsacas L, Ashburner M. Historical biogeography of the Drosophila melanogaster species subgroup. Evol. Ecol. 1988;22:159–225. [Google Scholar]
- Lesnoff M, Lancelot R. 2012. aod: Analysis of overdispersed data. R package version 1.3. Available via http://cran.r-project.org/package=aod.
- Llopart A, Lachaise D, Coyne JA. Multilocus analysis of introgression between two sympatric sister species of Drosophila: Drosophila yakuba and D. santomea. Genetics. 2005a;171:197–210. doi: 10.1534/genetics.104.033597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopart A, Lachaise D, Coyne JA. An anomalous hybrid zone in Drosophila. Evolution. 2005b;59:2602–2607. [PubMed] [Google Scholar]
- Llopart A, Elwyn S, Lachaise D, Coyne JA. Genetics of a difference in pigmentation between Drosophila yakuba and Drosophila santomea. Evolution. 2002;56:2262–2277. doi: 10.1111/j.0014-3820.2002.tb00150.x. [DOI] [PubMed] [Google Scholar]
- MacDonald SJ, Goldstein DB. A quantitative genetic analysis of male sexual traits distinguishing the sibling species Drosophila simulans and D. sechellia. Genetics. 1999;153:1683–1699. doi: 10.1093/genetics/153.4.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute DR. Reinforcement of Gametic Isolation in Drosophila. PLoS Biol. 2010;8:e1000341. doi: 10.1371/journal.pbio.1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute DR. The role of founder effects on the evolution of reproductive isolation. J. Evol. Biol. 2013;26:2299–2311. doi: 10.1111/jeb.12246. [DOI] [PubMed] [Google Scholar]
- Matute DR, Ayroles JF. Hybridization occurs between Drosophila simulans and D. sechellia in the Seychelles archipelago. J. Evol. Biol. 2014;27:1057–1068. doi: 10.1111/jeb.12391. [DOI] [PubMed] [Google Scholar]
- Matute DR, Coyne JA. Intrinsic reproductive isolation between two sister species of Drosophila. Evolution. 2010;64:903–920. doi: 10.1111/j.1558-5646.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- Matute DR, Novak CJ, Coyne JA. Thermal adaptation and extrinsic reproductive isolation in two species of Drosophila. Evolution. 2009;63:583–594. doi: 10.1111/j.1558-5646.2008.00588.x. [DOI] [PubMed] [Google Scholar]
- Mayr E. Systematics and the origin of species: from the viewpoint of a zoologist. New York, NY: Columbia Univ. Press; 1942. p. 334. [Google Scholar]
- McDermott SR, Kliman RM. Estimation of isolation times of the island species in the Drosophila simulans complex from multilocus DNA sequence data. PLoS ONE. 2008;3:e2442. doi: 10.1371/journal.pone.0002442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RJ, Flanagan RJ, Brown BJ, Waser NM, Karron JD. New frontiers in competition for pollination. Ann. Bot. 2009;103:1403–1413. doi: 10.1093/aob/mcp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring AJ, Llopart A, Elwyn S, Coyne JA, Mackay TF. The genetic basis of postzygotic reproductive isolation between Drosophila santomea and D. yakuba due to hybrid male sterility. Genetics. 2006;173:225–233. doi: 10.1534/genetics.105.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P. Degree of sympatry affects reinforcement in Drosophila. Evolution. 2012;67:868–872. doi: 10.1111/j.1558-5646.2012.01817.x. [DOI] [PubMed] [Google Scholar]
- O'Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual. Quant. 2007;41:673–690. [Google Scholar]
- Penteriani V. Breeding density affects the honesty of bird vocal displays as possible indicators of male/territory quality. Ibis. 2003;145:E127–E135. [Google Scholar]
- Peterson MA, Honchak BM, Locke SE, Beeman TE, Mendoza J, Green J, et al. Relative abundance and the species-specific reinforcement of male mating preference in the Chrysochus (Coleoptera: Chrysomelidae) hybrid zone. Evolution. 2005;59:2639–2655. [PubMed] [Google Scholar]
- Pfennig KS, Pfennig DW. Character displacement: ecological and reproductive responses to a common evolutionary problem. Q. Rev. Biol. 2009;84:253–276. doi: 10.1086/605079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TD. Speciation in birds. Woodbury, NY: Roberts and Company; 2007. [Google Scholar]
- Price MV, Waser NM. Experimental studies of pollen carryover: hummingbirds and Ipomopsis aggregata. Oecologia. 1982;54:353–358. doi: 10.1007/BF00380004. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: the R Foundation for Statistical Computing; 2005. ISBN: 3-900051-07-0. Available online at http://www.R-project.org/ [Google Scholar]
- Rice WR, Hostert EE. Laboratory experiments on speciation: what have we learned in 40 years? Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- Ritchie MG. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 2007;38:79–102. [Google Scholar]
- Rolán-Alvarez E, Caballero A. Estimating sexual selection and sexual isolation effects from mating frequencies. Evolution. 2000;54:30–36. doi: 10.1111/j.0014-3820.2000.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Safran RJ, Scordato ES, Symes LB, Rodríguez RL, Mendelson TC. Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 2013;28:643–650. doi: 10.1016/j.tree.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schumer M, Rosenthal GG, Andolfatto P. How common is homoploid hybrid speciation? Evolution. 2014;68:1553–1560. doi: 10.1111/evo.12399. doi: 10.1111/evo.12399. [DOI] [PubMed] [Google Scholar]
- Servedio MR, Noor MAF. The role of reinforcement in speciation: theory and data. Annu. Rev. Ecol. Evol. Syst. 2003;34:339–364. [Google Scholar]
- Solignac M, Monnerot M. Race formation, speciation, and introgression within Drosophila simulans D. mauritiana, and D. sechellia inferred from mitochondrial DNA analysis. Evolution. 1986;40:531–539. doi: 10.1111/j.1558-5646.1986.tb00505.x. [DOI] [PubMed] [Google Scholar]
- Sprenger D, Lange R, Anthes N. Population density and group size effects on reproductive behavior in a simultaneous hermaphrodite. BMC Evol. Biol. 2011;11:107. doi: 10.1186/1471-2148-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine RA. Graphical interpretation of variance inflation factors. Am. Stat. 1995;49:53–56. [Google Scholar]
- Thiel M, Correa C. Female rock shrimp Rhynchocinetes typus mate in rapid succession up a male dominance hierarchy. Behav. Ecol. Sociobiol. 2004;57:62–68. [Google Scholar]
- Ting CT, Takahashi A, Wu C-I. Incipient speciation by sexual isolation in Drosophila: concurrent evolution at multiple loci. Proc. Natl Acad. Sci. USA. 2001;98:6709–6713. doi: 10.1073/pnas.121418898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaru M, Yamada H, Oguma Y. Female mate recognition and sexual isolation depending on courtship song in Drosophila sechellia and its siblings. Genes Genet. Syst. 2004;79:145–150. doi: 10.1266/ggs.79.145. [DOI] [PubMed] [Google Scholar]
- Tsacas L, Bächli G. Drosophila sechellia, N. Sp., huitième espèce du sous-groupe melanogaster des iles Seychelles (Diptera, Drosophilidae) Rev. Fr. Entomol. 1981;3:146–150. [Google Scholar]
- Verzijden MN, Culumber ZW, Rosenthal GG. Opposite effects of learning cause asymmetric mate preferences in hybridizing species. Behav. Ecol. 2012;23:1133–1139. [Google Scholar]
- Volpe EP. Intensity of reproductive isolation between sympatric and allopatric populations of Bufo americanus and Bufo fowleri. Am. Nat. 1955;89:303–317. [Google Scholar]
- Waage JK. Dual function of the damselfly penis—sperm removal and transfer. Science. 1979;203:916–918. doi: 10.1126/science.203.4383.916. [DOI] [PubMed] [Google Scholar]
- Webster MS, Robinson SK. Courtship disruptions and male mating strategies: examples from female-defense mating systems. Am. Nat. 1999;154:717–729. doi: 10.1086/303267. [DOI] [PubMed] [Google Scholar]
- Willis PM, Ryan MJ, Rosenthal GG. Encounter rates with conspecific males influence female mate choice in a naturally hybridizing fish. Behav. Ecol. 2011;22:1234–1240. [Google Scholar]
- Willis PM, Rosenthal GG, Ryan MJ. An indirect cue of predation risk counteracts female preference for conspecifics in a naturally hybridizing fish Xiphophorus birchmanni. PLoS ONE. 2012;7:e34802. doi: 10.1371/journal.pone.0034802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Hedrick A. Speciation and the economics of mate choice. Evol. Theory. 1982;6:15–24. [Google Scholar]
- Wong B, Candolin U. How is female mate choice affected by male competition? Biol. Rev. 2005;80:559–571. doi: 10.1017/S1464793105006809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Raw data and analytical code used in this report. Dryad link: doi: 10.5061/dryad.fp861.


