Abstract
De novo formation of testis tissue from single cell suspensions allows manipulation of different testicular compartments before grafting to study testicular development and the spermatogonial stem cell niche. However, the low percentages of newly formed seminiferous tubules supporting complete spermatogenesis and lack of a defined protocol have limited use of this bioassay. Low spermatogenic efficiency in de novo formed tissue could result from the scarcity of germ cells in the donor cell suspension, cell damage caused by handling or from hypoxia during tissue formation in the host environment. Here, we compared different proportions of spermatogonia in the donor cell suspension and the use of Matrigel as scaffold to support de novo tissue formation and spermatogenesis. Then, we utilized the system to investigate the role of Vascular Endothelial Growth Factor-165 during testicular morphogenesis on blood vessel and seminiferous tubule formation, and on presence of germ cells in the de novo developed tubules. Our results show that donor cell pellets with 10×106 porcine neonatal testicular cells in Matrigel efficiently formed testis tissue de novo. Contrary to what was expected, the enrichment of the cell suspension with germ cells did not result in higher numbers of tubules supporting spermatogenesis. The addition of VEGF-165 did not improve blood vessel or tubule formation but it enhanced the number of tubules containing spermatogonia. These results indicate that spermatogenic efficiency was improved by the addition of Matrigel, and that VEGF-165 may have a protective role supporting germ cell establishment in their niche.
Introduction
The formation of the male gonad begins in the early stages of embryogenesis and results in an environment capable to sustain spermatogenesis at puberty (Chen et al. 2005). This supportive and instructive environment is constituted of Sertoli, peritubular myoid and Leydig cells together with the basement membrane and the vasculature compartment and it forms a niche for spermatogonial stem cells (SSC) to sustain a lifelong supply of gametes. Sertoli cells are major components of this environment, as they are in intimate contact with germ cells inside seminiferous tubules, being homogenously distributed and providing growth factors such as GDNF, bFGF and adhesion molecules maintaining SSC self renewal (Kanatsu-Shinohara et al. 2008) (Naughton et al. 2006). In contrast, undifferentiated spermatogonia are asymmetrically distributed, clustered adjacent to the vascular branch points and interstitial cells (Chiarini-Garcia et al. 2001, Yoshida et al. 2007) indicating a role for the blood supply guiding niche orientation in the testis.
The proximity of SSCs to the interstitial vasculature indicated a role for the family of vascular endothelial growth (VEGF) factors, more specifically VEGFA, which is the major promoter of physiological and pathological angiogenesis (Carmeliet 2003, Byrne et al. 2005) and protects extra vascular cells from deleterious effects induced by hypoxia (Lambrechts et al. 2003) .
In the testis, VEGFA is important during early stages of development promoting formation of sex cords (Cool et al. 2011). At post natal stages there is no active angiogenesis under physiological conditions. Nonetheless, Sertoli, Leydig and peritubular myoid cells release VEGFA and display its receptors in different stages of testis development (Bott et al. 2006).
The VEGFA synthesis by niche cells and the location of SSC closer to the vasculature make this factor worth further investigation for understanding the SSC niche. However, the study of SSCs and their niche in vivo is challenged by limitations in the available existing methods, especially in large mammalian species. To date, most of the knowledge was attained in rodents; and more data from other species is required to enable the accurate extrapolation of findings to higher mammals.
Testis tissue xenografting has been used to study male fertility in different mammalian species. It was the first method described to achieve full spermatogenesis from prepubertal donors after ectopic transplantation into immunocompromised mice (Honaramooz et al. 2002) and since then it has been used to study or preserve male fertility (Honaramooz et al. 2004, Ohta & Wakayama 2005, Arregui et al. 2008).
When testis tissue was treated with VEGF164 prior to tissue xenografting or added during tissue culture for 7 days before xenografting, the addition of VEGF164 resulted in a higher percentage of seminiferous tubules supporting spermatogenesis (Schmidt et al. 2006). This report was followed up by another manuscript investigating the role of VEGFA in testis development (Caires et al. 2009), where the addition of VEGF164 in tissue explants cultured in vitro increased the ratio of selected anti apoptotic genes versus pro apoptotic genes in the tissue.
In testis tissue xenografting, the architecture of the tissue is preserved and cell associations are maintained, therefore the ability to study individual cell types and their interaction within the SSC niche is limited.
We previously reported de novo morphogenesis of functional testis tissue form isolated testicular somatic and germ cells. Cells obtained by enzymatic digestion from neonatal porcine testes, when transplanted under the dorsal skin of immunocompromised mice, were able to rearrange into a functional endocrine and spermatogenic unit, supporting complete maturation and development of haploid male gametes (Honaramooz et al. 2007). Further reports described this morphogenic ability of isolated testis cells in different species such as rodents (Kita et al. 2007), ovine (Arregui et al. 2008) and bovine (Zhang et al. 2008) donors and also in species used as model organisms such as zebrafish (Kawasaki et al. 2010) and Xenopus (Kawasaki et al. 2006).
There are many differences between xenografts of testicular tissue and de novo morphogenesis of testis tissue after grafting of isolated cells. Grafting of cells more likely subjects all testicular cells to the same exposure to growth factors, such as VEGFA, while the existing structure and cell associations present in tissue fragments may limit growth factor uptake to deeper areas of the tissue.
De novo morphogenesis of testis tissue allows for manipulation of different compartments of the testicular niche prior to tissue reassembly, giving this technique potential to be used to study signaling, orientation and guidance of the cells when forming the testis and to elucidate factors controlling spermatogenesis. However, most of the de novo formed tubules contain only solely Sertoli cells leading to low spermatogenic efficiency, with the presence of elongated spermatids ranging from 10 to 20% in de novo formed seminiferous tubules (Honaramooz et al. 2007, Kita et al. 2007).
In this study our goals were to overcome the low spermatogenic efficiency of the system by testing different conditions when transplanting porcine testicular cells and to test the de novo morphogenesis as a functional assay to study aspects of testis function. We evaluated development of grafts with different cell numbers, percentage of germ cells and the use of Matrigel as a scaffold to maintain cells closer together. The improved method was then tested as a functional assay to study the effect of VEGF-165 on blood supply and reorganization of the testis tissue.
Material and Methods
Tissue enzymatic digestion
Testes from one week old piglets were donated by a commercial farm in Strathmore, Alberta, Canada. Cells were harvested by a two step enzymatic protocol previously described (Honaramooz et al. 2002). The final cell population was dived in two groups. One was kept refrigerated at 4°C for 72 hours until grafting surgery and the other one was submitted to differential plating for enrichment of germ cells.
Enrichment of germ cells
50×106 cells were plated on 100 mm tissue culture dishes (BD Bioscience, Mississauga, Canada) in 15 ml of Dulbecco Modified Eagle Medium (DMEM) (Life technologies, Burlington, Canada) with 5% fetal bovine serum (FBS) (Thermo Scientific, Rochester, USA) and incubated at 37°C in 5% CO2 in air. After 18 hours, cells remaining in suspension and those slightly attached were removed by trypsinization for 30 seconds with 1:10 dilution of 0.25% trypsin-EDTA (Gibco, Life technologies), and plated in new flasks. This was repeated at 48 and 72 hours after the initial plating. Cell recovery and viability were recorded and immunocytochemistry using antibodies against UCHL1, an established marker to identify undifferentiated spermatogonia (Luo et al. 2009), was used to identify germ cells (UCHL1+, vimentin-) and somatic cells (UCHL1-, vimentin+; see below).
Cell characterization
Cells kept refrigerated and the enriched cell suspension used in all five replicates were characterized by immunocytochemistry with the following antibodies: mouse monoclonal anti vimentin Cy3 (1:500,Sigma –Aldrich), mouse monoclonal anti alpha-Smooth Muscle Actin FITC conjugated (1:500, Sigma –Aldrich), mouse monoclonal anti Gata4 (1:40;Santa Cruz Biotechnology, Dallas, USA), rabbit anti human protein gene product 9.5 (UCHL1) (1:500, AbDSerotec, Raleigh, Canada), mouse monoclonal anti P450c17 (1:500, kindly donated by Dr. Alan Conley, University of California at Davis), primary antibodies incubations were followed by secondary antibodies: goat anti rabbit Alexafluor 488 (1:400, Sigma –Aldrich) or donkey anti mouse Alexafluor 555 (1:400, Sigma –Aldrich) to identify spermatogonia (UCHL1+, Vimentin), Sertoli (Gata4+, UCHL1−), Leydig (P450c17+, Vimentin+) and myoid cells (alpha smooth muscle Actin+, Vimentin+).
After characterization of both cell populations, 4 different groups were created.
Effect of germ cell number and extracellular matrix on spermatogenic efficiency in de novo formed testis tissue
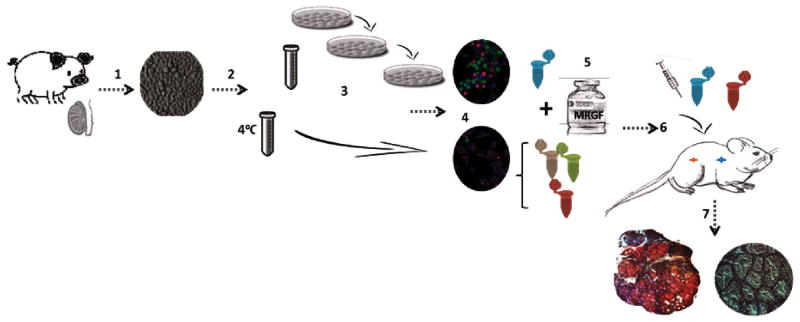
The experimental design is outlined schematically in Fig. 1. Four groups with different concentrations of spermatogonia and the scaffold Matrigel with reduced growth factors (MRGF) (BD Sciences, Bedford, United States) were created:
Figure 1.
Experimental design. 1 One week old piglet testes were enzymatically digested into a single cell suspension. 2 Cell suspension was split into two fractions: one was kept at 4°C until characterization and the other was used for enrichment. 3 Enrichment for germ cells by 3 sequential platings. 4 Both cell fractions were characterized by immunocytochemistry. 5 Cell pellets with the defined concentrations of germ cells were created and Matrigel was added (groups 2, 3 and 4). 6 Surgical grafting was performed. 7 After 24 and 40 weeks, the de novo formed tissue was harvested from host mice and cross sections were analyzed for the quantification of de novo formed tubules, detection of UCHL-1+ cells and development of spermatogenesis.
Group 1 (Control): 50×106 cells not enriched for spermatogonia (containing 3 to 4% spermatogonia) without MRGF; group 2: 50×106 cells not enriched for spermatogonia in 100μl of MRGF; group 3: 10×106 cells containing 25% spermatogonia in 100μl of MRGF, group 4: 10×106 cells not enriched for spermatogonia in 100μl of MRGF.
Immunodeficient recipient mice (26 NU/NCr, 6 SCID, Charles River, Wilmington, United States) were castrated and groups were randomly assigned to one of four injection sites located under the dorsal skin, spaced between the shoulder and the rump. Our previous studies demonstrated that the immunocompromised strains of the recipients do not influence the development of de novo formed tissue (Honaramooz et al. 2007, Arregui et al. 2008)
The experiment was performed in five replicates with different pools of testicular cells, to minimize any influence from the cell suspension, surgical procedure or batch of mice used on the observed results.
The care and use of research animals were approved by the Institutional Animal Care and Use Committee of the University of Calgary.
Sample Recovery and Processing
Mice from different surgery dates were randomly assigned to analysis at 24 and 40 weeks, data from both time points was combined to analyze each group. Mice were sacrificed by CO2 inhalation; grafts were recovered, fixed in Bouin's solution, and stored in 70% ethanol until processing for histology. Grafts were embedded in paraffin and cut into 5μm sections.
The reconstitution of functional spermatogenic tissue was assessed in cross sections from the central area of formed grafts by immunohistochemistry. UCHL1 was used to identify gonocytes and spermatogonia (Luo et al. 2009). Paraffin sections were dewaxed and dehydrated through xylene followed by a graded series of graded ethanol washes (100%, 95% and 70% ethanol, 5 minutes each) and treated with 3% hydrogen peroxide in distilled H2O to block endogenous peroxidase activity and were incubated at room temperature in CAS-Block (Invitrogen) for 20 minutes to block nonspecific antibody binding. Primary antibody incubation was performed overnight at 4°C using anti UCHL1 (1:500, AbDSerotec), followed by peroxidase-conjugated donkey anti-rabbit IgG (1:400,Sigma - Aldrich) as the secondary antibody for one hour at room temperature. Peroxidase activity was detected using Vector NovaRED (Vector Laboratories, Burlington, Canada) and tissue was counterstained with hematoxylin (Vector Laboratories).
Effect of VEGF-165 on formation of testis tissue
One week old piglet testicular cells were obtained as described above and the cell suspension obtained was immediately divided in pellets of 10×106 cells mixed in 100μl MRGF and assigned to two groups: VEGF-165 treated: 10×106 cells mixed in 100μl MRGF with 100ng/ml of recombinant human VEGF165 (R&D Systems,) and control: 10×106 cells mixed in 100μl MRGF. Four cell pellets per animal (3 treated, 1 control) were randomly assigned to injection sites and ectopically grafted under the dorsal skin of 12 castrated immunocompromised mice (NU/NCr, Charles Rivers) in three sets of replicates.
Sample recovery and analysis
Cell aggregates were recovered after 12 weeks of transplantation; at this time point seminiferous cords are organized and germ cells are located on the basement membrane. Recovered grafts were fixed and processed as described above.
An antibody to alpha smooth muscle actin was used to label blood vessels after comparison with other markers such as von Willerbrand Factor (vWf), VE cadherin and Cd31. VE cadherin is expressed in germ cells (Aivatiadou et al. 2007) and adhesion molecules from the cadherin family play a role in interaction amongst Leydig, Sertoli and germ cells (Byers et al. 1994), Cd31 is expressed in endothelial cells and leucocytes as well as murine primordial germ cells. Therefore, the use of these markers could lead to overestimate the number and location of blood vessel in xenografts recovered. vWf expression in porcine tissue is not consistent in all tissues and blood vessels (Rand et al. 1987,Giddings et al. 1997).
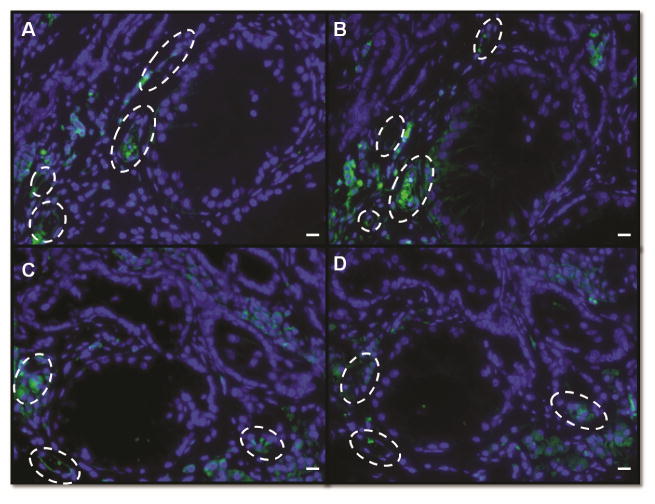
The reliability of alpha smooth muscle actin to identify blood vessels was verified by immunohistochemistry in sequential cross sections from grafts recovered after 12 weeks comparing CD31 and alpha smooth muscle actin. Paraffin sections from grafts recovered after 12 weeks were dewaxed and dehydrated as described above, incubated with CAS Block (Invitrogen) at room temperature for 20 minutes, followed by incubation overnight at 4°C with mouse monoclonal anti alpha smooth muscle actin, FITC conjugated (1:500, Sigma –Aldrich) or mouse anti porcine CD31, FITC conjugated (1:400,AbD Serotec). Both antibodies labeled the same structures; however, staining for alpha smooth muscle actin provided a clearer staining pattern making blood vessel counts easier and more reliable (Fig 2). Alpha smooth muscle actin is also present in peritubular myoid cells of the seminiferous tubule, therefore staining for alpha smooth muscle actin facilitated identification and measurement of newly formed seminiferous tubules as well as blood vessels.
Figure 2.
Validation of alpha smooth muscle actin as a marker to identify blood vessels. Immunofluorescence from sequential cross sections from grafts recovered after 12 weeks labeled against alpha smooth muscle actin and CD31, punctuated white lines indicates blood vessels. A, C Grafts stained against alpha smooth muscle actin. B, D Grafts stained against CD31. Bar=50μm.
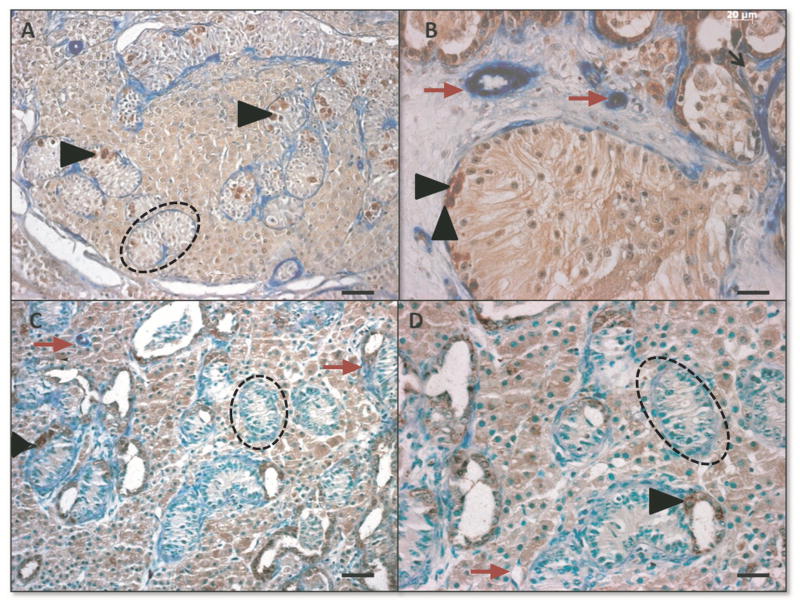
Cross sections from the middle area of recovered grafts were analyzed by double staining immunohistochemistry for the detection of UCHL1 cells and alpha smooth muscle actin to identify undifferentiated spermatogonia and blood vessels, respectively (Fig 3). Slides were treated with Bloxall (Vector laboratories, Burlingame, USA) to block the endogenous peroxidase and alkaline phosphatase activity, then CAS-Block (Invitrogen) was added for 20 minutes to block nonspecific antibody binding. Samples were incubated with primary antibody UCHL1 (1:500, AbDSerotec) and anti-alpha smooth muscle actin (1:400, Sigma-Aldrich) overnight at 4°C, followed by peroxidase-conjugated donkey anti-rabbit IgG (1:400, Sigma-Aldrich) as the secondary antibody. Peroxidase activity was detected using Vector NovaRED (Vector Laboratories), samples were then incubated with secondary alkaline-phosphatase conjugated goat anti mouse IgA (1:400, AbDSerotec). Alkaline phosphatase activity was detected using Vector Blue Alkaline Phosphatase kit (Vector Laboratories), followed by counterstaining with VECTOR Methyl Green (Vector Laboratories).
Figure 3.
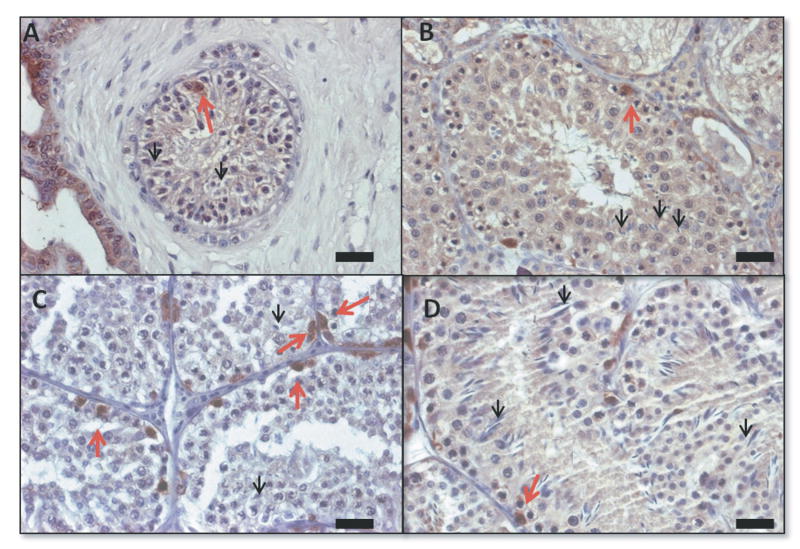
Morphology and immunohistochemistry to identify UCHL1+ (brown, black arrow heads) and alpha-smooth muscle actin + (blue, red arrows) cells in de novo formed testis tissue from VEGF experiment, punctuated black lines identify seminiferous tubules. A VEGF-165 treated tissue; bar=50μm, B: VEGF-165 treated tissue; bar=20μm, C Control tissue; bar=50μm; D Control tissue; bar=20μm.
Detection of apoptosis by TUNEL ( terminal deoxynucleotidyl transferase dUTP nick end label) assay
Paraffin embedded cross sections from de novo formed tissue recovered after 12 weeks and 10 week old testis tissue (positive control) were subjected to DeadEnd™ Fluorometric TUNEL System assay according to manufacturer's instructions (Promega, Wisconsin, USA) to identify apoptotic cells.
Cell proliferation assay
Cross sections from the middle area of recovered grafts were analyzed by immunohistochemistry for the detection of Ki67 to identify proliferative cells. Slides were subjected to heat induce epitope retrieval in citrate buffer for 20 minutes at 95°C, then endogenous peroxidase was quenched by incubation for 20 minutes in 3% H2O2 followed by CAS-Block (Invitrogen) for 20 minutes. Samples were incubated with Ki67 mouse monoclonal antibody (1:200, Dako, Glostrup,Denmark) overnight at 4°C, followed by peroxidase-conjugated donkey anti-mouse IgG (1:400,Sigma-Aldrich) as the secondary antibody. Peroxidase activity was detected using Vector NovaRED (Vector Laboratories), followed by counterstaining with Gill's hematoxylin (Vector Laboratories);
Statistical Analysis
Number of de novo formed tubules and percentage of tubules supporting spermatogenesis per group (mean ± SD) was compared using Kruskal-Wallis, P<0.05 was considered significant. Student t test with p<0.05 was used to test for statistical significance between groups from the VEGFA experiment.
Image Analysis
All sections were analyzed using a Zeiss microscopy AxioImager M2 equipped with a CCD camera and controlled with AxioVision 4.8 software.
Results
Effect of germ cell number and extracellular matrix
Based on immunocytochemistry for the detection of UCHL1, GATA 4, P450c17 and alpha smooth-muscle actin, the cell suspension used in Groups 1, 2 and 4 contained a mean ± SD of 3.1±1.1% undifferentiated spermatogonia, 72.8±14.6% Sertoli, 5±0.3% Leydig and 4.4±2.8% peritubular myoid cells, in Group 3 the germ cell population was adjusted to 25% undifferentiated spermatogonia, the other cell types were present in a concentration of 33.7±11.8% Sertoli, 10.7±2 Leydig and 3.3±2.2% peritubular myoid cells. The method used to attain enrichment of spermatogonia also provided a two fold increase in the Leydig cell population, probably due to the lower adherence of this cell type to plastic (Vernon et al. 1991).
From 32 recipients grafted, 3 did not survive up to the final end point of analysis at 40 weeks, thus grafts were not recovered. The percentage of grafts recovered from both time points that developed de novo formed tubules was 79% for Group 1 (control), 70% for group 2, 67% group 3 and 58% in group 4 (Table 1). Recovered grafts that did not develop into organized tubules were not included into further analysis.
Table 1. Summary of results for effects of cell number, germ cell concentration and Matrigel on de novo formed testis tissue.
| Group* | # of developed/ recovered grafts (%) | # of tubules/graft (x̄±SEM) | # of tubules with spermatogenesis/graft** (x̄±SEM) |
|---|---|---|---|
| Control | 23 /29 (79) | 142 ± 49a | 15±2%a |
| 50×106 M | 20/ 29 (70) | 267± 70b | 32±3%b |
| 10×106 ME | 19/ 29 (67) | 183±51a | 31±5%b |
| 10×106 M | 17/ 29 (58) | 67±16a | 39±7%b |
Cell #,M=Matrigel, E=enriched for germ cells
: values with different superscripts within columns are significantly different (p<0.05)
: values with different superscripts within columns are significantly different (p<0.05)
Presence of UCHL1+ spermatogonia at 24 weeks and spermatogonia and spermatids at 40 weeks.
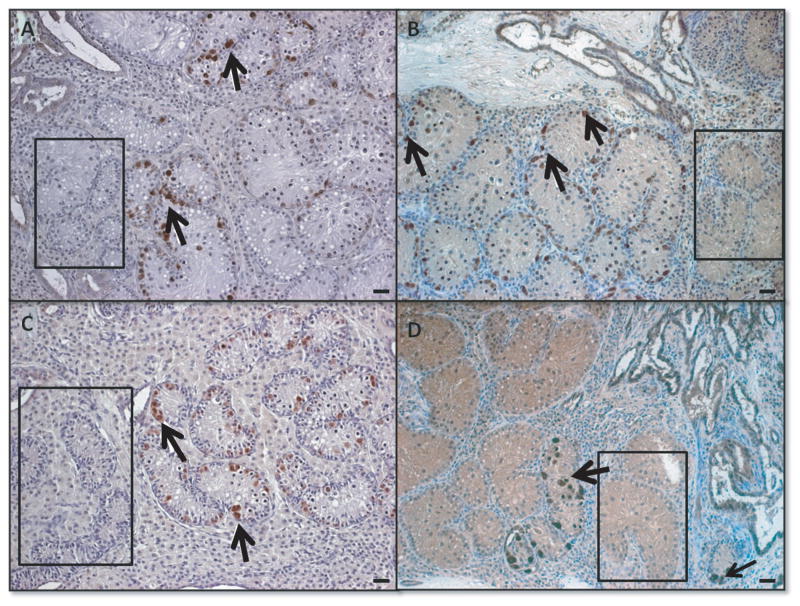
Immunohistochemical identification of undifferentiated spermatogonia, seminiferous tubules formed and the presence of a functional niche supporting proliferation and development of spermatogenesis was performed in cross sections at two time points of analysis. After 24 weeks of grafting, de novo formed seminiferous tubules resembled the morphology of prepubertal testis and the spermatogenic efficiency was determined as percentage of de novo formed tubules containing spermatogonia along the basement membrane per graft (Fig4). After 40 weeks of cell grafting, spermatogenic efficiency was determined by percentage of seminiferous tubules supporting germ cell differentiation (Fig5).
Figure 4.
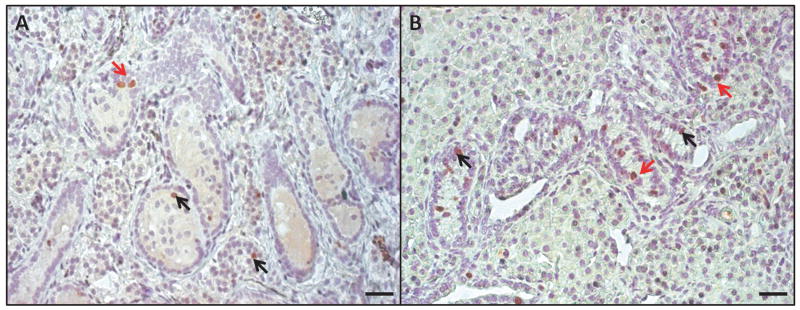
Morphology of de novo formed tubules and spermatogenesis 24 weeks after transplantation. A group1, control: 50×106 cells, B group 2: 50×106 cells in Matrigel, C group 3: 10×106 enriched (25% spermatogonia) in Matrigel, D group 4 10×106 in Matrigel. Spermatogenesis was detected by the presence of spermatogonia and UCHL1+ germ cells (brown), black arrows indicate examples of positive cells. Black rectangles demonstrate areas where tubules contain only Sertoli cells. Bar=20μm.
Figure 5.
Morphology of de novo formed tubules and spermatogenesis by immunohistochemistry for the detection of UCHL1 40 weeks after transplantation. A Control, group 1: 50×106 cells, B group 2: 50×106 cells in Matrigel, C group 3:10×106 enriched with spermatogonia in Matrigel, D group 4:10×106 cells in Matrigel. Red arrows indicate undifferentiated spermatogonia and black arrows point to elongated spermatids. Bars=20μm.
Grafts recovered from all groups displayed large variance within group. The mean ±SEM of de novo formed tubules per graft were group1 (control): 142 ± 49, group 2: 267± 70, group 3: 183±51 and group 4: 67±16. The number of de novo formed tubules was significantly lower (p<0.05) in group 1 (control; 50×106) than group 2 (50×106 in MRGF), when the same number of cells were grafted but without the addition of Matrigel as a supporting scaffold. Group 2 (50×106 in MRGF) and group 4 (10×106 in MRGF) were also significantly different, both groups had cells grafted under the same conditions mixed in Matrigel but they differed in number of cells injected, so we inferred that the amount of cells influenced number of tubules formed. No significant difference was detected between group 1 (control) and groups 3 and 4, and between group 3 (10×106 cells containing 25% spermatogonia) vs group 4 (10×106 in MRGF).
The mean ±SEM of spermatogenic efficiency measured in groups 1, 2, 3 and 4 was: 15±2%, 32±3%, 31±5% and 39±7% respectively. The control group was significantly less efficient (p<0.05) than all other groups when formation of a functional niche was measured, no significant difference was detected amongst the other groups. Based on our results, the enrichment of germ cells in the cell suspension grafted did not increase the number of tubules supporting spermatogenesis (Table 1).
Effect of VEGF-165
Based on the results of the first set of experiments we established Group 4 (10×106 cells in MRGF) to be used as the standard protocol to investigate the effect of VEGF-165 in de novo morphogenesis of the testis tissue.
35 out of 36 grafts were recovered from the VEGF-165 treated group and 12 out of 12 were recovered from control grafts. From all grafts recovered, 92% of grafts from the VEGF-165 treated group and 75% from the control group (p = 0.13) were organized as de novo formed testis tissue; grafts which did not develop into testis tissue were constituted mainly of Matrigel and were not analyzed.
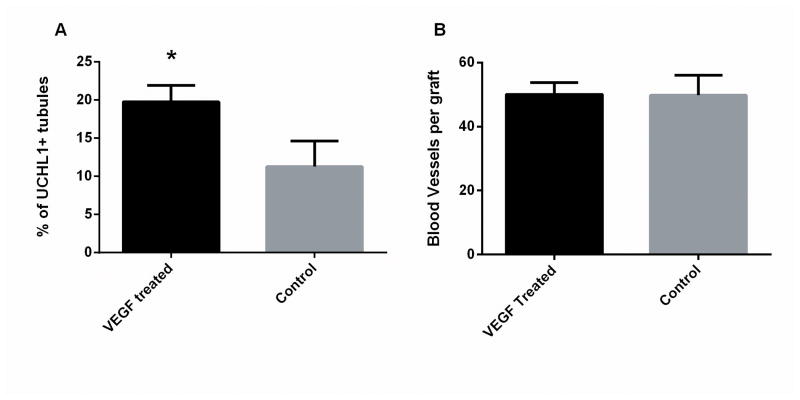
Immunohistochemistry for UCHL1 and alpha smooth muscle actin from all grafts recovered demonstrated that the number of blood vessels or seminiferous tubules formed did not differ significantly between treated and control groups: 50.0±3.7 vs 49.8±6.2 and 78.2±9.7 vs 100.8±23 (mean±SEM), respectively. However, when analyzing the spermatogenic efficiency between de novo formed tubules, VEGF-165 treated grafts displayed higher percentage of seminiferous tubules containing spermatogonia per graft than the control: 18.6±2.2% vs 11.3±3.3% (p<0.05) (Fig6). Table 2 summarizes the results.
Figure 6.
Germ cells and number of blood vessels in VEGF-165 treated and control grafts after 12 weeks. A Percentage of tubules de novo formed containing UCHL1+germ cells; B Number of blood vessels formed in the recovered tissue. All data is presented as mean ±SD per graft, *p<0.05.
Table 2. Summary of results obtained from VEGF-165 treatment.
| Group (cell #) | # of developed/ recovered grafts (%) | # of blood vessels/graft (x̄±SEM) | # of tubules/graft (x̄±SEM) | # of tubules with spermatogenesis/graft* (x̄±SEM) |
|---|---|---|---|---|
| 10×106 | 32/35(92) | 49.8±6.2a | 100.8±23a | 11.3±3.3a |
| 10×106 VEGF-165 | 9/12(75) | 50.0±3.7a | 78.2±9.7a | 18.6±2.2b |
: values with different superscripts within columns are significantly different (p<0.05)
: values with different superscripts within columns are significantly different (p<0.05)
Presence of UCHL1+ spermatogonia at 12 weeks
To investigate how the addition of VEGF-165 benefited the spermatogenic efficiency in the treated group, apoptosis and proliferation assays were performed. TUNEL assay labeled very few apoptotic cells in recovered cell aggregates and in 10 week old testis tissue used as a positive control, and there was no difference in the percentage of de novo tubules with apoptotic cells between the Control and VEGF-165 treated groups.
To analyze proliferation in VEGF-165 treated versus Control groups we measured percentage of de novo formed tubules supporting proliferation (51.6±7.9 vs 39.5±12.7), and ratio of proliferating spermatogonia to Sertoli cells (0.40±0.11 vs 0.12±0.06). Both parameters did not differ significantly; however, there was a trend with the VEGF-165 treated group having a higher ratio of proliferating spermatogonia to proliferating Sertoli cell (p =0.09).There was a significantly higher number of proliferating cells per tubule (2.3±0.2 vs 1.8±0.1; p<0.05) in the VEGF-165 treated group compared to the control group (Fig7).
Figure 7.
Immunohistochemistry against Ki67 in de novo formed testis tissue from VEGF-165 experiment. A Control tissue, B VEGF-165 treated graft recovered after 12 weeks.Ki67+ cells are labeled in brown (arrows), bar=50μm.
Discussion
De novo morphogenesis of testis tissue is a unique assay which allows manipulation of cells prior to grafting and enables the recovery of cells in further phases of spermatogenesis. This bioassay can overcome existing challenges to study testicular morphogenesis and the SSC niche such as the difficult access to the testis during different stages of development and to maintain and study larger animal models. However, the majority of existing reports are descriptive and protocols used differ in number of cells grafted, recovery times and the use of cell pellets or combination with extracellular matrix.
Despite the disparity amongst protocols, all groups described low numbers of tubules with spermatogenesis in the de novo formed tissue. Most of the tubules formed were constituted only by Sertoli cells. The low efficiency observed can be attributed to several reasons: the testicular tissue contains low number of spermatogonia and SSC under physiological conditions (Toebosch et al. 1989) resulting in scarcity of spermatogonia in the transplanted pool of cells, the procedure is elaborate from the enzymatic digestion of the tissue to surgical grafting creating a harsh environment prone to cell damage and loss. Then, after transplantation, cells must adapt and reconstitute their previous microenvironment and reestablish germ cell development. Therefore, our first aim was to test different protocols and different conditions in a large number of samples to characterize and optimize this bioassay. Specifically, we evaluated the role of the extracellular matrix, number of cells grafted and the enrichment of undifferentiated spermatogonia in the development of de novo formed testicular tissue.
The comparison between control (established from our previous report by Honaramooz et al. 2007) and all other groups provided insight about the influence of Matrigel and the quantity of cells in the system. We concluded that addition of Matrigel to the mixture enhances tubule formation and decreases the requirement for large numbers of donor cells, which is beneficial for future studies in animal models where the availability of donor tissue may be limited. The positive influence of Matrigel on the development of tissue from a smaller number of testicular cells is in accordance to what has been reported (Kita et al. 2007, Watanabe et al. 2009). Similarly, this matrix has been used to support tumor growth or maintenance of pancreatic islets in xenogenic hosts (Ruggeri et al. 2002, Dufour et al. 2005).
The influence of number of cells grafted was determined comparing tubule formation when the same conditions were provided to different number of cells grafted; our results supported what has been previously hypothesized (Watanabe et al. 2009), that the number of cells grafted are correlated with tubule formation.
Different from what was anticipated, however, the fivefold enrichment of spermatogonia did not enhance number of tubules supporting spermatogenesis. We must consider that this bioassay faces a large variability in development of the recovered tissue and this might have hampered the detection of a significant difference. Nonetheless, we report that the limited availability of SSCs is not primarily responsible for the system's lower efficiency. However, addition of Matrigel to the cell pellet enhanced tubule formation and promoted the efficiency of de novo formed testis tissue, possibly by contributing to cell to cell contact while diminishing cell loss.
Optimization of the method was the first step to validate de novo morphogenesis as functional bioassay to study morphogenesis and spermatogenesis as demonstrated with the experiment investigating the effects of VEGF-165 on de novo formation of testis tissue.
It has already been demonstrated that during morphogenesis, VEGFA amongst other growth factors orchestrates cord and vasculature formation by promoting migration of cells including pre endothelial cells from the mesonephros to the developing gonads (Bott et al. 2006, Bott et al. 2010, Cool et al. 2011); in our results we did not detect any improvement in neovascularization or number of tubules formed by exogenous supplementation of VEGF-165; we noticed a tendency in overall development and organization as de novo formed testis tissue from recovered grafts in the treated group but it was not significantly different. However, the higher percentage of tubules with spermatogonia in the VEGF-165 treated grafts suggested a role for VEGF-165 in testicular development and a protective effect against the transient hypoxia and/or excessive handling by which cells are exposed during the grafting process.
The protective mechanism of VEGFA has been described in the central nervous system and retina when cells face ischemic environments (Wick et al. 2002, Saint-Geniez et al. 2008). The ability to improve the adaptation of cells to a new environment by over expression of VEGFA has been reported in xenotransplantation of pancreatic islet cells and when testis tissue was treated prior to xenografting (Caires et al. 2009).
Analysis of apoptotic (TUNEL) and proliferative cells (Ki67) was used to detect a potential mechanism underlying the observed differences in tubules with germ cells formed with or without VEGF-165 treatment. Only few cells were labeled by TUNEL and there was no difference in the percentage of tubules with apoptotic cells between groups. It is possible that after 12 weeks, when grafts were recovered, cells that were damaged by the procedure may be no longer detectable. Also, sensitivity of TUNEL analysis in paraffin embedded tissue may be limited (Labat-Moleur et al. 1998).
On the other hand, the significant increase in numbers of proliferative (Ki67 positive) cells inside de novo formed tubules from VEGF-165 treated grafts indicated that VEGF-165 protected cells against negative effects associated with transplantation; the ratio between proliferative spermatogonia and Sertoli cells indicates that this benefited all cell types and not exclusively spermatogonia.
Recently, a study using Sertoli-germ cell specific VEGFA null transgenic mice reported similar findings to those reported here (Lu et al. 2013). The knockout mouse testis had morphologic impairment, seminiferous tubules were disorganized and contained less undifferentiated spermatogonia, yet impairment or decrease in number of blood vessels was not detected. The knockout animals showed different expression levels of genes responsible for cell survival (Bcl2) as reported by Caires et al. 2009 for bovine testis xenografts.
The similarity of finding by Lu et al. 2013 supports our conclusion that de novo morphogenesis can be used as a functional bioassay to study gonadal development, the spermatogonial cell and its niche. However, this bioassay displays large variability in the recovered grafts, therefore larger number of samples should be used to obtained meaningful results. The low spermatogenic efficiency initially reported can be improved by the addition of Matrigel and VEGF-165 to the grafted cell suspension.
Acknowledgments
We would like to thank Alberta Innovates - Health Solutions for a graduate scholarship and ACHRI/CIHR training program in Genetics, Child Development and Health. We also would like to acknowledge the staff of the HSARC Biocontainment Level 2 Unit from the Faculty of Medicine of the University of Calgary and Sunterra farms.
Funding: This work was supported by NIH/ORIP RR 17359-12
Footnotes
Declaration of interest: The authors declare there is no conflict of interests that could prejudice the impartiality of the manuscript.
References
- Aivatiadou E, Mattei E, Ceriani M, Tilia L, Berruti G. Impaired fertility and spermiogenetic disorders with loss of cell adhesion in male mice expressing an interfering Rap1 mutant. Molecular biology of the cell. 2007;18:1530–1542. doi: 10.1091/mbc.E06-10-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arregui L, Rathi R, Megee SO, Honaramooz A, Gomendio M, Roldan ERS, Dobrinski I. Xenografting of sheep testis tissue and isolated cells as a model for preservation of genetic material from endangered ungulates. Reproduction. 2008;136:85–93. doi: 10.1530/REP-07-0433. [DOI] [PubMed] [Google Scholar]
- Bott RC, McFee RM, Clopton DT, Toombs C, Cupp AS. Vascular endothelial growth factor and kinase domain region receptor are involved in both seminiferous cord formation and vascular development during testis morphogenesis in the rat. Biology of Reproduction. 2006;75:56–67. doi: 10.1095/biolreprod.105.047225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott R, Clopton DT, Cupp AS. A Proposed Role for VEGF Isoforms in Sex Specific Vasculature Development in the Gonad. Reproduction in Domestic Animals. 2008;43:310–316. doi: 10.1111/j.1439-0531.2008.01179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott RC, Clopton DT, Fuller AM, McFee RM, Lu N, McFee RM, Cupp AS. KDR-LacZ-expressing cells are involved in ovarian and testis-specific vascular development, suggesting a role for VEGFA in the regulation of this vasculature. Cell and tissue research. 2010;342:117–130. doi: 10.1007/s00441-010-1038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers SW, Sujarit S, Jegou B, Butz S, Hoschutzky H, Herrenknecht K, MacCalman C, Blaschuk OW. Cadherins and cadherin-associated molecules in the developing and maturing rat testis. Endocrinology. 1994;134:630–639. doi: 10.1210/endo.134.2.7507830. [DOI] [PubMed] [Google Scholar]
- Byrne AM, Bouchier Hayes D, Harmey J. Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF) Journal of cellular and molecular medicine. 2005;9:777–794. doi: 10.1111/j.1582-4934.2005.tb00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction. 2009;138:667–677. doi: 10.1530/REP-09-0020. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in health and disease. Nature medicine. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–1034. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarini-Garcia H, Hornick JR, Griswold MD, Russell LD. Distribution of type A spermatogonia in the mouse is not random. Biology of Reproduction. 2001;65:1179–1185. doi: 10.1095/biolreprod65.4.1179. [DOI] [PubMed] [Google Scholar]
- Cool J, DeFalco TJ, Capel B. Vascular-mesenchymal cross-talk through Vegf and Pdgf drives organ patterning. Proceedings of the National Academy of Sciences. 2011;108:167–172. doi: 10.1073/pnas.1010299108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour JM, Rajotte RV, Zimmerman M, Rezania A, Kin T, Dixon DE, Korbutt GS. Development of an ectopic site for islet transplantation, using biodegradable scaffolds. Tissue engineering. 2005;11:1323–1331. doi: 10.1089/ten.2005.11.1323. [DOI] [PubMed] [Google Scholar]
- Giddings J, Banning A, Ralis H, Lewis M. Redistribution of von Willebrand factor in porcine carotid arteries after balloon angioplasty. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17:1872–1878. doi: 10.1161/01.atv.17.10.1872. [DOI] [PubMed] [Google Scholar]
- Hess RA, Cooke PS, Hofmann MC, Murphy KM. Mechanistic insights into the regulation of the spermatogonial stem cell niche. Cell cycle. 2006;5:1164–1170. doi: 10.4161/cc.5.11.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Dobrinski I. Germ cell transplantation in pigs. Biology of Reproduction. 2002;66:21–28. doi: 10.1095/biolreprod66.1.21. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Snedaker A, Boiani M, Schler H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;418:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Megee SO, Rathi R, Dobrinski I. Building a testis: formation of functional testis tissue after transplantation of isolated porcine (Sus scrofa) testis cells. Biology of Reproduction. 2007;76:43–47. doi: 10.1095/biolreprod.106.054999. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, Shinohara T. Homing of Mouse Spermatogonial Stem Cells to Germline Niche Depends on [beta] 1-Integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Imura F, Nakada A, Kubota H, Sakamaki K, Abe SI, Takamune K. Functional demonstration of the ability of a primary spermatogonium as a stem cell by tracing a single cell destiny in Xenopus laevis. Development, growth & differentiation. 2006;48:525–535. doi: 10.1111/j.1440-169X.2006.00890.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Saito K, Shinya M, Olsen LC, Sakai N. Regeneration of spermatogenesis and production of functional sperm by grafting of testicular cell aggregates in zebrafish. Biology of Reproduction. 2010;4:533–539. doi: 10.1095/biolreprod.110.085159. [DOI] [PubMed] [Google Scholar]
- Kita K, Watanabe T, Ohsaka K, Hayashi H, Kubota Y, Nagashima Y, Aoki I, Taniguchi H, Noce T, Inoue K. Production of functional spermatids from mouse germline stem cells in ectopically reconstituted seminiferous tubules. Biology of Reproduction. 2007;76:211. doi: 10.1095/biolreprod.106.056895. [DOI] [PubMed] [Google Scholar]
- Labat-Moleur F, Guillermet C, Lorimier P, Robert C, Lantuejoul S, Brambilla E, Negoescu A. TUNEL apoptotic cell detection in tissue sections: critical evaluation and improvement. Journal of Histochemistry & Cytochemistry. 1998;46:327–334. doi: 10.1177/002215549804600306. [DOI] [PubMed] [Google Scholar]
- Lambrechts D, et al. VEGF is a modifier of amyotrophic lateral sclerosis in mice and humans and protects motoneurons against ischemic death. Nature genetics. 2003;34:383–394. doi: 10.1038/ng1211. [DOI] [PubMed] [Google Scholar]
- Lu N, Sargent KM, Clopton DT, Pohlmeier WE, Brauer VM, McFee RM, Weber JS, Ferrara N, Silversides DW, Cupp AS. Loss of vascular endothelial growth factor A (VEGFA) isoforms in the testes of male mice causes subfertility, reduces sperm numbers and alters expression of genes that regulate undifferentiated spermatogonia. Endocrinology. 2013;154(12):4790–802. doi: 10.1210/en.2013-1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Megee S, Dobrinski I. Asymmetric Distribution of UCH L1 in Spermatogonia Is Associated With Maintenance and Differentiation of Spermatogonial Stem Cells. Journal of Cellular Physiology. 2009;220:460–468. doi: 10.1002/jcp.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, Jain S, Strickland AM, Gupta A, Milbrandt J. Glial cell-line derived neurotrophic factor-mediated RET signaling regulates spermatogonial stem cell fate. Biology of Reproduction. 2006;74:314–321. doi: 10.1095/biolreprod.105.047365. [DOI] [PubMed] [Google Scholar]
- Ohta H, Wakayama T. Generation of normal progeny by intracytoplasmic sperm injection following grafting of testicular tissue from cloned mice that died postnatally. Biology of Reproduction. 2005;73:390–395. doi: 10.1095/biolreprod.105.041673. [DOI] [PubMed] [Google Scholar]
- Ruggeri BA, Robinson C, Angeles T, Wilkinson J, Clapper ML. The Chemopreventive Agent Oltipraz Possesses Potent Antiangiogenic Activity in Vitro, ex Vivo, and in Vivo and Inhibits Tumor Xenograft Growth. Clinical Cancer Research. 2002;8:267–274. [PubMed] [Google Scholar]
- Saint-Geniez M, Maharaj AS, Walshe TE, Tucker BA, Sekiyama E, Kurihara T, Darland DC, Young MJ, D'Amore PA. Endogenous VEGF is required for visual function: evidence for a survival role on Müller cells and photoreceptors. PLoS One. 2008;3:e3554. doi: 10.1371/journal.pone.0003554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JA, de Avila JM, McLean DJ. Effect of vascular endothelial growth factor and testis tissue culture on spermatogenesis in bovine ectopic testis tissue xenografts. Biology of Reproduction. 2006;75:167–175. doi: 10.1095/biolreprod.105.049817. [DOI] [PubMed] [Google Scholar]
- Toebosch A, Brussée R, Verkerk A, Grootegoed J. Quantitative evaluation of the maintenance and development of spermatocytes and round spermatids in cultured tubule fragments from immature rat testis. International journal of andrology. 1989;12:360–374. doi: 10.1111/j.1365-2605.1989.tb01325.x. [DOI] [PubMed] [Google Scholar]
- Vernon RB, Lane TF, Angello JC, Sage H. Adhesion, shape, proliferation, and gene expression of mouse Leydig cells are influenced by extracellular matrix in vitro. Biology of Reproduction. 1991;44:157–170. doi: 10.1095/biolreprod44.1.157. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Hayashi H, Kita K, Kubota Y, Ogawa T. Ectopic porcine spermatogenesis in murine subcutis: tissue grafting versus cell-injection methods. Asian Journal of Andrology. 2009;11:317–323. doi: 10.1038/aja.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick A, Wick W, Waltenberger J, Weller M, Dichgans J, Schulz JB. Neuroprotection by hypoxic preconditioning requires sequential activation of vascular endothelial growth factor receptor and Akt. The Journal of neuroscience. 2002;22:6401–6407. doi: 10.1523/JNEUROSCI.22-15-06401.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hill J, Holland M, Kurihara Y, Loveland KL. Bovine Sertoli Cells Colonize and Form Tubules in Murine Hosts Following Transplantation and Grafting Procedures. J Androl. 2008;29:418–430. doi: 10.2164/jandrol.107.004465. [DOI] [PubMed] [Google Scholar]