Abstract
BACKGROUND & AIMS
Chronic kidney disease (CKD) can have a negative impact on the natural history of hepatitis C virus infection (HCV) infection; patients with HCV and CKD often have adverse outcomes. We evaluated a large and geographically diverse group of patients to determine whether HCV status has an independent effect on the risk of developing CKD.
METHODS
We conducted a cohort study of 167,569 patients included in a national health care claims database from January 1, 2003–December 31, 2006, with a mean follow-up of 25.3 months. We used multivariable logistic regression analyses to measure the independent effect of HCV status on the baseline prevalence of and progression to CKD (estimated glomerular filtration rate, <60 mL/min/1.73 m2).
RESULTS
The baseline prevalence of CKD was similar in patients with versus those without HCV (5.3% vs 5.1%, P = .3). Similarly, among patients with preserved renal function at baseline (n = 82,629), there was no difference in the overall progression to CKD in patients with versus those without HCV (3.8% vs 3.5%, P = .1). HCV status was not associated with progression to CKD, even after adjusting for patient demographics, comorbidities, and use of relevant medications (odds ratio, 0.92; 95% confidence interval, 0.79–1.08).
CONCLUSIONS
We found no association between HCV and risk of development of CKD. These data are relevant in counseling HCV patients regarding the impact of HCV on renal function.
Chronic hepatitis C virus infection (HCV) is a prevalent and expensive condition affecting more than 1.3% of the US population at an annual health care cost of more than 1 billion dollars.1,2 Although the burden of illness related to HCV is higher among patients with end-stage renal disease, it is not necessarily obvious that HCV would negatively affect renal function in the general population. HCV is associated with cryoglobulinemia and membranoproliferative glomerulonephritis, and these, in turn, can lead to chronic kidney disease (CKD).3 Patients with HCV might also be more likely to have diabetes, obesity, or human immunodeficiency virus (HIV); these conditions are independently associated with CKD.4–6 Collectively, these relationships provide the basis for a hypothesized negative impact of HCV on renal function. However, there are reasons to believe that HCV might not be associated with renal disease. For example, only a small proportion of patients with HCV develop membranoproliferative glomerulonephritis, and an even smaller subset might progress to symptomatic renal disease.7 It is also plausible that patients with known HCV might receive better quality care, such as regular clinical follow-up, compared with non-HCV patients.8 Receipt of better care might counterbalance the higher risk of renal disease associated with comorbid conditions.
The available data on the association between HCV and CKD are also conflicting. In a cohort study of patients seeking care in an urban inner city health care system, Moe et al9 found that patients with HCV were less likely to develop CKD compared with those without the infection. In contrast, another cohort study by Tsui et al10 found a positive association between HCV status and development of end-stage renal disease among older veterans. Given these disparate data, it is unclear whether and to what extent HCV affects renal function. Presence of CKD might not only adversely affect the course and management of HCV, but the coexistence of HCV and CKD might also have a negative synergistic impact on patient outcomes. Thus, the association between HCV and increased risk of CKD, if confirmed, might have important clinical implications for the care of HCV patients. In light of this, we sought to examine the independent effect of HCV status on the baseline prevalence of and progression to CKD in a large and geographically diverse sample of patients seeking health care in the US between 2003 and 2006.
Methods
Study Database
We analyzed data from a large conglomerate of health insurance carriers in the US. This database has previously been used to study delivery of care in patients with CKD.11 During the study period, the database included information on patient and health insurance characteristics for 6 million insured persons nationwide. Enrollees lived throughout the US, although the South was overrepresented. The database consisted of 4 files. The patient eligibility file included information on patient demographics (age, gender) and health insurance characteristics. Similar to other insurance databases, study data did not include information regarding patients’ race and socioeconomic status. The claims file documented all submitted claims for inpatient and outpatient services, including diagnoses, procedures, laboratory tests, and physician specialty associated with each claim. The pharmacy file documented all pharmacy claims, including the specific drug prescribed, the quantity, and the number of days for which drugs were supplied. Unlike other insurance databases, the insurance database has a laboratory results file that includes names (eg, HCV antibody tests, liver tests), dates, and results for 3.5 million persons in the cohort.
Study Design
We conducted a cohort study of all adult (≥18 years) patients with available laboratory data who underwent ≥1 serum creatinine measurement and ≥1 HCV test between January 1, 2003, and December 31, 2006 (n = 167,569). We defined an HCV patient as a subject with at least 1 positive HCV test (ie, positive antibody, qualitative polymerase chain reaction, quantitative polymerase chain reaction, or genotype test). Patients who were tested for HCV but had a negative test result constituted the reference group for our analysis. The rationale behind our selection of reference group was that patients who receive a HCV test might be systematically different than those who did not receive the test, and that some of these differences might be prognostically important in terms of progression to CKD (such as illicit drug use, HIV, etc). Thus, identifying both study subjects and reference group from the same source population (ie, those who were tested for HCV) might reduce the selection bias that is otherwise inherent in observational studies.
We used the date of first creatinine measurement as the index date for the study cohort. We estimated patients’ glomerular filtration rate (eGFR) by using the Modification of Diet in Renal Disease (MDRD) equation and staged renal function into 5 categories according to the National Kidney Foundation K/DOQI criteria: 1, eGFR >90 mL per minute per 1.73 m2 (stage 1, corresponding to normal); 2, eGFR 60–90 mL/min/1.73 m2 (stage 2, corresponding to a mild reduction); 3, eGFR 30–59 mL/min/1.73 m2 (corresponding to stage 3 CKD); 4, eGFR 15–29 mL/min/1.73 m2 (corresponding to stage 4 CKD); and 5, eGFR <15 mL/min/1.73 m2 (corresponding to stage 5 CKD).12 Because race information was not available in our data, all participants were assigned a value of 1 instead of adjustment based on race (as shown below).11 The equation we used was the following: eGFR = 186.3 × (Creatinine−1.154) × (Age−0.203) × 0.742 (if female) × (1.21 if black).
We defined a patient as having CKD if the eGFR was lower than 60 mL/min/1.73 m2 (ie, those with stage 3 CKD or worse), as previously described.12 Because deterioration in renal function is a slow process and progression to CKD might develop during a longer period of time than described in our study, we examined an alternative definition of progression, defined as moderate (≥3.1 mL/min/1.73 m2) or severe (≥6.1 mL/min/1.73 m2) annual decline in eGFR. A cutoff of >3 mL/min/1.73 m2 has been shown in previous studies to describe clinically relevant decrease in renal function.13 Insurance coverage for hemodialysis is provided by Medicare. However, given that there is a lag of at least 90 days between initiation of dialysis and beginning of Medicare coverage, we were highly likely to capture the initiation of dialysis.
Statistical Methods
Study outcomes
We first examined the association of HCV and the baseline prevalence of CKD. We considered all patients, ie, those who underwent ≥1 serum creatinine measurement and ≥1 HCV test during the study period (prevalence cohort), for this outcome. Subsequently, we examined the association between HCV and moderate (≥3.1 mL/min/1.73 m2) or severe (≥6.1 mL/min/1.73 m2) annual decline in eGFR. For this analysis, we limited our cohort to those patients who had at least 2 serum creatinine measurements ≥6 months apart and who did not have any claims for renal transplantation (n < 293) or hemodialysis (n = 170) before or within 4 weeks of the index date (progression cohort). Finally, we evaluated the association between HCV and progression to CKD (defined as eGFR lower than 60 mL/min/1.73 m2 or stage 3) in patients without baseline CKD.
Covariates
We adjusted for the presence of covariates that might be associated with progression to CKD. The ascertainment period for these diagnoses was presence of the covariates at any time before December 31, 2006. These included age and gender, patient’s baseline eGFR, medical comorbidity-related characteristics (cirrhosis, diabetes, hypertension, heart failure, peripheral vascular disease, coronary artery disease, chronic obstructive pulmonary disease, and HIV), drug or alcohol abuse, and psychiatric illness-related characteristics (eg, depression). To further strengthen the model, we examined the effect of selected medications that might impact renal function or eGFR estimation (diuretics and inhibitors of the reninangiotensin system). We used the Agency for Healthcare Research and Quality Clinical Classification System to classify all the patient International Classification of Diseases, 9th revision (ICD-9) codes into the relevant diagnosis included in Table 1.
Table 1.
Patient Characteristics by HCV Status
| Prevalence cohort N = 167,569 |
Progression cohort N = 88,822 |
|||
|---|---|---|---|---|
| HCV status | Negative N = 154,185 |
Positive N = 13,384 |
Negative N = 80,759 |
Positive N = 8063 |
| Female (%) | 56.2 | 39.9a | 59.2 | 40.8a |
| Age, mean (SD) (y) | 40.4 (11.8) | 47.8 (8.6) | 43.2 (11.8) | 48.7 (8.1) |
| Age distribution (%) | ||||
| 18–40 y | 52.9 | 16.2a | 42.6 | 13.4a |
| 41–50 y | 25.9 | 45.4 | 29.0 | 45.3 |
| 51–60 y | 16.2 | 33.4 | 21.3 | 35.6 |
| >60 y | 5.0 | 5.0 | 7.1 | 5.7 |
| Comorbidities (%) | ||||
| Cirrhosis | 2.2 | 16.5a | 3.2 | 20.5a |
| HIV | 3.2 | 4.0a | 4.4 | 5.2 |
| Diabetes | 6.7 | 9.6a | 10.3 | 12.4a |
| Hypertension | 7.6 | 9.7a | 11.1 | 12.3 |
| Coronary artery disease | 9.3 | 11.9a | 13.7 | 14.9 |
| Peripheral vascular disease | 0.8 | 1.2a | 1.2 | 1.6 |
| Cerebrovascular disease | 2.3 | 3.1a | 3.4 | 4.0 |
| Heart failure | 2.1 | 3.2a | 3.2 | 4.1a |
| Chronic obstructive pulmonary disease | 10.4 | 16.6a | 13.5 | 19.4a |
| Drug abuse | 8.5 | 21.1a | 9.6 | 21.6a |
| Alcohol abuse | 2.4 | 6.8a | 2.7 | 6.9a |
| Depression | 9.9 | 12.6a | 11.6 | 14.3a |
| Hepatitis B | 0.1 | 0.2 | 0.1 | 0.2 |
| Selected medicationsb (%) | 9.3 | 10.9a | 17.7 | 17.9 |
| Baseline creatinine, mean (SD) (mg/dL) | 1.02 (6.7) | 1.05 (4.5) | 1.02 (6.5) | 1.01 (4.0) |
| eGFR rate (%) | ||||
| ≥60 mL/min/1.73 m2 | 94.9 | 94.7 | ||
| CKD (GFR, <60 mL/min/1.73 m2) | 5.3 | 5.1 | 3.5 | 3.8 |
| 30–59 | 4.8 | 5.0 | 3.35 | 3.66 |
| 15–29 | 0.24 | 0.22 | 0.07 | 0.13 |
| <15 | 0.08 | 0.10 | 0.03 | 0.01 |
P < .0001.
Selected medications: diuretics or renin-angiotensin system inhibitors.
Statistical Analyses
We conducted between-group comparisons by using the independent Student t test for the continuous data and the χ2 tests of independence for categorical data. We created multivariable logistic regression models to identify the independent association of HCV with CKD.
Sensitivity Analyses
We conducted several sensitivity analyses to test the robustness of our findings. First, because the MDRD equation was developed and validated in patients with CKD, it might underestimate eGFR in patients with relatively well-preserved renal function.14 To address this limitation of MDRD equation, we examined the association between HCV and progression of renal disease in patients with baseline stage 3 CKD (eGFR, <60 mL/min/1.73 m2). Second, cirrhosis diagnosis might be disproportionately associated with HCV and also associated with progression to CKD. Therefore, we conducted separate regression models with or without cirrhosis diagnosis as well as tested for any potential interaction between HCV and cirrhosis diagnoses. Third, older HCV patients and those with diabetes might be more likely to experience adverse renal outcomes than younger patients without diabetes. Therefore, we tested for significant interactions between HCV status and age and diabetes.10
Results
Patient Characteristics
We identified 13,384 patients with positive antibody test (n = 7949), quantitative/qualitative polymerase chain reaction (n = 8092), or genotype (n = 4434) test. These patients constituted the HCV-positive group. The remaining patients (n = 154,185) constituted the reference group. Table 1 lists the characteristics of the study cohort. Compared with patients without HCV, those with HCV were more likely to be older (47.8 ± 8.6 vs 40.4 ± 11.8 years) and more likely to be men (60.1% versus 43.8%). Patients with HCV were also more likely to have a diagnosis of drug (21.1% vs 8.5%) or alcohol (6.8% vs 2.4%) abuse and have a diagnosis of cirrhosis (16.5% vs 2.2%). The prevalence of prespecified medical comorbidities was higher in the HCV-positive group (Table 1).
Baseline Prevalence of Chronic Kidney Disease
There was no difference in the baseline prevalence of CKD between patients with versus those without HCV (5.3% vs 5.1%, P = .3) by using the MDRD equation (Table 1). The extent and magnitude of this association did not change in a model adjusting for all prespecified covariates (odds ratio [OR], 0.90; 95% confidence interval [CI], 0.36 –2.27). The results were similar even after exclusion of all patients who underwent hemodialysis (n = 333) or renal transplantation (n = 293) at any time during the study. We also compared the baseline prevalence of CKD by ICD-9 diagnosis of stage 3, 4, or 5 CKD. Although fewer patients were diagnosed with CKD, the prevalence was similar in HCV-positive and HCV-negative patients (0.5% vs 0.5%). Last, the mean (standard deviation [SD]) serum creatinine was similar between the 2 study groups (Table 1).
Progression to Chronic Kidney Disease
Eight thousand sixty-three patients with HCV and 80,759 patients without HCV met the criteria to be included in the progression cohort (Table 1). There were no significant differences in the patients who met the inclusion criteria for the progression analysis compared with those who did not. The mean follow-up was 25.3 months (SD, 12.7 months), and all patients were followed for ≥6 months. The median number of creatinine measurements was similar regardless of HCV status (n = 5 in both groups). The mean duration between 2 creatinine measurements was 16.5 months and 17.4 months for HCV-positive and HCV-negative patients, respectively (P < .0001).
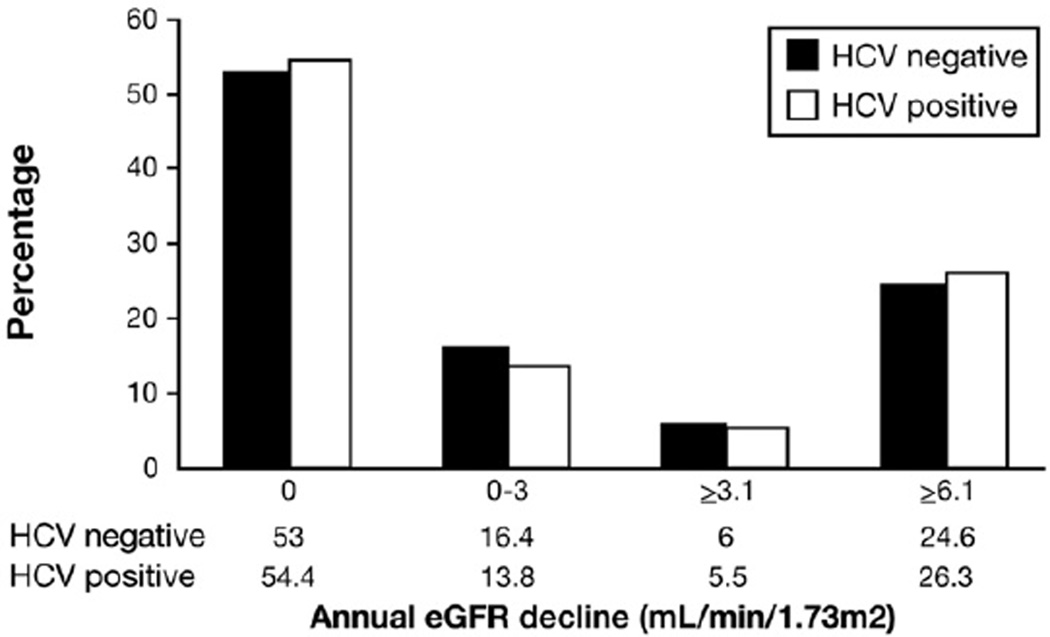
Figure 1 displays the annual decline in eGFR among all patients included in the progression cohort regardless of the baseline renal function. Compared with HCV-negative patients, fewer HCV patients had a decline in eGFR (45.6% vs 47.0%, P = .02). However, more HCV patients had severe annual decline (≥6.1 mL/min/1.73 m2) in the progression cohort than those without HCV (26.3% vs 24.6%, P < .0001).
Figure 1.
Annual eGFR decline in HCV progression cohort. Compared with HCV-negative patients, fewer HCV patients had a decline in eGFR (45.6% vs 47.0%, P = .02). However, more HCV patients had severe annual decline (≥6.1 mL/min/1.73 m2) in the progression cohort than those without HCV (26.3% vs 24.6%, P < .0001).
Approximately 3.5% (2876 of 82,629) of patients without CKD at baseline developed CKD during follow-up. There was no difference in the overall progression to CKD in patients with versus those without HCV (3.8% vs 3.5%, P = .1).
Table 2 displays the results from a multivariable model predicting progression to CKD. After adjusting for prespecified covariates, there was no significant association between HCV status and development of CKD (OR, 0.92; 95% CI, 0.79 –1.08). The results did not change when we used alternative definitions of renal disease progression. Specifically, in the multivariable analyses, HCV was not associated with moderate (≥3.1 mL/min/1.73 m2) or severe (≥6.1 mL/min/1.73 m2) annual decline in renal function, and these results did not change when we limited our analyses to patients with stage 3 CKD at baseline (data not shown). There was no significant interaction between HCV status and cirrhosis, diabetes, or age. Similarly, the results did not change when cirrhosis was excluded from the model. After adjusting for prespecified covariates as above, HCV was not a significant predictor of CKD with increasing age (Table 3).
Table 2.
Predictors of Development of CKD (eGFR, <60 mL/min/1.73 m2)
| N = 82,629 OR (95% CI) |
P value | |
|---|---|---|
| HCV positive | 0.92 (0.79–1.08) | .31 |
| Female sex | 1.16 (1.07–1.26) | <.01 |
| Age | 1.04 (1.04–1.05) | <.001 |
| Initial GFR | 0.93 (0.92–0.93) | <.001 |
| Comorbidities | ||
| Cirrhosis | 1.57 (1.31–1.88) | <.001 |
| HIV | 1.99 (1.68–2.38) | <.001 |
| Diabetes | 1.43 (1.28–1.59) | <.001 |
| Hypertension | 1.44 (1.30–1.60) | <.001 |
| Coronary artery disease | 1.17 (1.06–1.29) | .003 |
| Peripheral vascular disease | 1.25 (0.98–1.60) | .08 |
| Cerebrovascular disease | 1.08 (0.91–1.28) | .40 |
| Heart failure | 2.36 (2.03–2.73) | <.001 |
| Chronic obstructive pulmonary disease | 1.01 (0.91–1.12) | .87 |
| Drug abuse | 1.09 (0.96–1.23) | .17 |
| Alcohol abuse | 1.23 (0.99–1.54) | .06 |
| Depression | 0.96 (0.85–1.09) | .55 |
| Selected medicationsa | 1.45 (1.33–1.58) | <.001 |
Selected medications: diuretics or renin-angiotensin system inhibitors.
Table 3.
Effect of HCV Infection on Progression to CKD Stratified by Age at Entry
| Age (y) | ORadj | 95% CI |
|---|---|---|
| 18–40 | 1.45 | 0.86–2.43 |
| 40.1–50 | 1.03 | 0.81–1.30 |
| 50.1–60 | 0.81 | 0.63–1.04 |
| >60 | 0.99 | 0.65–1.50 |
Reference = HCV negative. Adjusted for sex, first eGFR, medical comorbidities (cirrhosis, HIV, diabetes, hypertension, coronary artery disease, peripheral vascular disease, cerebrovascular disease, heart failure, chronic obstructive pulmonary disease), drug abuse, alcohol abuse, depression, and use of diuretics or renin-angiotensin system inhibitors.
Discussion
We did not find a significant association between HCV and CKD in a large and demographically diverse group of patients. In our study, the baseline prevalence of CKD was similar in patients with versus those without HCV. Compared with non-HCV patients, our HCV patients were older and were more likely to have cirrhosis, diabetes, hypertension, and diagnosis of drug abuse, all conditions that were independently associated with renal disease in our analysis. However, after adjusting for these factors, patients with HCV had similar, if not slightly lower, risk of developing CKD compared with patients without HCV. Furthermore, these results did not change after using alternative definitions of renal disease progression in prespecified sensitivity analyses. Our results are similar to the data reported by Moe et al.9 In this recent analysis of a predominantly younger inner city population, the hazard ratio for the development of CKD was not significant in patients with versus those without HCV. Although these latter data are limited by the lack of generalizability to patients seen in regular outpatient clinics in the US, the fact that they did not observe any association between HCV and CKD provides convergent validity to our findings. Our results are also consistent with numerous studies in medicine demonstrating that patients with HIV, cirrhosis, hypertension, and diabetes have an increased risk of CKD compared with patients without these chronic medical conditions in both HCV-positive and HCVnegative patients.
In a recent analysis of predominantly older veteran patients, Tsui et al10 found that HCV seropositivity was associated with a greater than 2-fold risk for developing end-stage renal disease (adjusted hazard rate, 2.8; 95% CI, 2.43–3.23). There might be several competing explanations for this disconnect between our findings and those reported by Tsui et al. The patients in the study by Tsui et al were significantly older and had a higher prevalence of comorbid conditions than the patients included in our analysis. We considered the possibility that an effect of HCV might be evident only in older patients and constructed models stratified by decade of age and models limited to patients older than 60 years (n = 1402). However, once again, we did not observe any positive association between HCV and progression to CKD in these analyses. The follow-up in our study was shorter (2.1 years vs 3.4 years), possibly accounting for the negative association. However, a recent cohort study with similar median follow-up (3.5 years) also failed to identify an association.9 It is plausible that differences in several unmeasurable factors such as clinical interactions in the diagnostic-treatment pathway and patients’ health-related knowledge and behaviors between the veteran and non-veteran populations might explain some of the differences observed in the 2 studies.
Our study has several strengths. First, our analysis relies on data from a large demographically diverse group of privately insured patients enrolled in a conglomerate of health care plans. Therefore, our study does not suffer from the potential selection bias associated with recruitment limited to tertiary care clinics or to one health care system. We believe that our sample is a closer reflection of the general patient population seen in the outpatient clinics in the US. The mean age for patients with HCV and the distribution of CKD in our cohort are similar to published analysis of National Health and Nutrition Examination Survey data.15–17 Second, we relied on a positive test to identify patients with HCV and did not include patients with only HCV-related ICD-9 codes, as done in previous studies. As a result, we minimized any ascertainment bias in classifying patients with HCV. Third, we had access to a broad array of data sources, allowing us to capture and adjust for the wide range of patient-level variables that might affect development of CKD. Fourth, we were able to conduct several sensitivity analyses to assess the effect of cirrhosis and HCV on progression to CKD as well as consider alternative definitions of progression of renal disease such as annual decline in eGFR. To increase the robustness of our estimates, we were able to adjust for use of selected medications (such as diuretics or reninangiotensin system inhibitors). The apparent association between these medications and progression to CKD in the analysis likely reflects the prescription of some of these medications to patients who are developing CKD or as a marker for a higher burden of comorbidities.
We are cognizant of several limitations in our analysis. First, we used the MDRD equation to estimate GFR. MDRD was developed and validated in patients with CKD, and it might have limited ability to estimate eGFR in patients without CKD.18 However, the MDRD equation has been successfully used in large nonselected patient populations and found to have high predictive validity (in terms of predicting an increased risk of death and cardiovascular events with worsening eGFR).19 Regardless of these data, to account for this possible limitation of the MDRD equation, we limited our analysis to patients with CKD at baseline and found similar results.20 Second, given that race information is unavailable in our database, we were unable to adjust for race in our MDRD calculations, which might have resulted in a lower estimate of eGFR for black patients. Thus, we might have misclassified certain black patients as having CKD. However, because a greater proportion of HCV-related outpatient visits occur in blacks compared with whites, this misclassification would bias the results away from the null. If this were to be true, then we could expect an even stronger protective effect of HCV on CKD than seen in the present analysis. Third, given the relatively short follow-up in our study, we could not evaluate clinically important outcomes such as progression to dialysis-dependent end-stage renal disease. To address this limitation, we modeled severe annual decline in eGFR as a surrogate of clinically important renal disease. Although the results of this analysis did not find any association between HCV and CKD, our findings will need to be confirmed by using a longer duration of patient follow-up. Fourth, our sample consisted of patients with stable health insurance coverage and access to health care. Therefore, our results might not generalize to patients without health insurance and with limited access to care. However, our data are consistent with results from a recent analysis that examined a primarily inner city population and found no association between HCV and decline in eGFR. Fifth, the use of creatinine as a surrogate marker for renal dysfunction might underestimate the severity of renal disease in the cohort.14 Proteinuria is a common finding in patients with renal insufficiency, and patients with HCV might have worse proteinuria compared with HCV-negative patients.15 Hence, the renal manifestations of HCV might be underdiagnosed, and subclinical renal involvement might be prevalent among patients with HCV, even in the setting of normal or slightly decreased eGFR.21 Regardless, serum creatinine and MDRD derived eGFR are currently used by the National Health and Nutrition Examination Survey as well as the National Kidney Foundation Kidney Early Evaluation Program as part of a community-based CKD screening program.22 Last, our analysis is based on administrative data. Because the administrative data are entered for record keeping reasons, absence of diagnostic codes might not mean that patients did not have the given diagnoses. However, the use of ICD-9 diagnoses for capturing patients with chronic diseases has been validated in previous studies.23
In conclusion, we did not find any association between HCV and CKD in this large and demographically diverse patient population. Our data are relevant in counseling HCV patients regarding the impact of HCV on renal function. Our study also underlines the importance of aggressive management of diabetes, hypertension, and other medical comorbidities to prevent a decline in renal function in all patients with these chronic medical conditions, regardless of their HCV status.
Acknowledgments
Funding
This study was supported by an intramural award from the Saint Louis University Liver Center (F.K.) and a digestive diseases training grant 5-T32-DK07198 (S.A.).
Abbreviations used in this paper
- CI
confidence interval
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- HCV
hepatitis C virus infection
- HIV
human immunodeficiency virus
- ICD-9
International Classification of Diseases, ninth revision
- MDRD
Modification of Diet in Renal Disease
- OR
odds ratio
- SD
standard deviation.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 2.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36:S30–S34. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Hampel H, Yeh C, et al. Extrahepatic manifestations of hepatitis C among United States male veterans. Hepatology. 2002;36:1439–1445. doi: 10.1053/jhep.2002.37191. [DOI] [PubMed] [Google Scholar]
- 4.White DL, Ratziu V, El-Serag HB, et al. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. J Hepatol. 2008;49:831–844. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backus LI, Phillips BR, Boothroyd DB, et al. Effects of hepatitis C virus coinfection on survival in veterans with HIV treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2005;39:613–619. [PubMed] [Google Scholar]
- 6.Bjornsson E, Angulo P. Hepatitis C and steatosis. Arch Med Res. 2007;38:621–627. doi: 10.1016/j.arcmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Tarantino A, De Vecchi A, Montagnino G, et al. Renal disease in essential mixed cryoglobulinaemia: long-term follow-up of 44 patients. Q J Med. 1981;50:1–30. [PubMed] [Google Scholar]
- 8.Higashi T, Wenger NS, Adams JL, et al. Relationship between number of medical conditions and quality of care. N Engl J Med. 2007;356:2496–2504. doi: 10.1056/NEJMsa066253. [DOI] [PubMed] [Google Scholar]
- 9.Moe SM, Pampalone AJ, Ofner S, et al. Association of hepatitis C virus infection with prevalence and development of kidney disease. Am J Kidney Dis. 2008;51:885–592. doi: 10.1053/j.ajkd.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsui JI, Vittinghoff E, Shlipak MG, et al. Association of hepatitis C seropositivity with increased risk for developing end-stage renal disease. Arch Intern Med. 2007;167:1271–1276. doi: 10.1001/archinte.167.12.1271. [DOI] [PubMed] [Google Scholar]
- 11.Philipneri MD, Rocca Rey LA, Schnitzler MA, et al. Delivery patterns of recommended chronic kidney disease care in clinical practice: administrative claims-based analysis and systematic literature review. Clin Exp Nephrol. 2008;12:41–52. doi: 10.1007/s10157-007-0016-3. [DOI] [PubMed] [Google Scholar]
- 12.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Moss SE, et al. The 10-year incidence of renal insufficiency in people with type 1 diabetes. Diabetes Care. 1999;22:743–751. doi: 10.2337/diacare.22.5.743. [DOI] [PubMed] [Google Scholar]
- 14.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15:276–284. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 15.Liangpunsakul S, Chalasani N. Relationship between hepatitis C and microalbuminuria: results from the NHANES III. Kidney Int. 2005;67:285–290. doi: 10.1111/j.1523-1755.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 17.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation—Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 19.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 20.Tsui JI, Maselli J, Gonzales R. Sociodemographic trends in national ambulatory care visits for hepatitis C virus infection. Dig Dis Sci. 2008 doi: 10.1007/s10620-008-0659-2. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barsoum RS. Hepatitis C virus: from entry to renal injury: facts and potentials. Nephrol Dial Transplant. 2007;22:1840–1848. doi: 10.1093/ndt/gfm205. [DOI] [PubMed] [Google Scholar]
- 22.Vassalotti JA, Li S, Chen SC, et al. Screening populations at increased risk of CKD: the Kidney Early Evaluation Program (KEEP) and the public health problem. Am J Kidney Dis. 2009;53:S107–S114. doi: 10.1053/j.ajkd.2008.07.049. [DOI] [PubMed] [Google Scholar]
- 23.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]