Abstract
The non-specific host response to implanted biomaterials is often a key challenge of medical device design. To evaluate biocompatibility, measuring the release of reactive oxygen species (ROS) produced by inflammatory cells in response to biomaterial surfaces is a well-established method. However, the detection of ROS in response to materials implanted in vivo has not yet been demonstrated. Here, we develop a bioluminescence whole animal imaging approach to observe ROS released in response to subcutaneously implanted materials in live animals. We compared the real-time generation of ROS in response to two representative materials, polystyrene and alginate, over the course of 28 days. High levels of ROS were observed near polystyrene, but not alginate implants, and persisted throughout the course of 28 days. Histological analysis revealed that high levels of ROS correlated not only with the presence of phagocytic cells at early timepoints, but also fibrosis at later timepoints, suggesting that ROS may be involved in both the acute and chronic phase of the foreign body response. These data are the first in vivo demonstration of ROS generation in response to implanted materials, and describe a novel technique to evaluate the host response.
Keywords: biocompatibility, immune response, foreign body response, free radical
Introduction
The foreign body response to biomaterials is a cascade of events triggered by implantation, followed by protein adsorption, adhesion and activation of immune cells, and ultimately recruitment of fibroblasts and formation of a fibrous capsule [1,2]. During this process, it is thought that reactive oxygen species (ROS) are released by activated phagocytes, and contribute to oxidative degradation of materials [3]. While ROS generation has been used to characterize inflammatory cell response to biomaterials in numerous in vitro studies [4-7], the detection of biomaterial-induced ROS has yet to be demonstrated in vivo.
ROS including superoxide, hypochlorite, hydroxyl anions, and hydrogen peroxide, are produced as a result of normal cellular respiration, as well as in response to environmental factors such as UV radiation, cigarette smoke, and under conditions of inflammation [8]. Excess ROS is scavenged by enzymes such as superoxide dismutase, but overproduction of ROS leads to oxidative stress. Chronically high levels of ROS causes damage to lipids, proteins, and DNA, and are involved in a number of pathological conditions including cancer, and neurodegenerative and cardiovascular diseases [9-12]. Mechanistically, ROS are generated by activated phagocytes not only to directly combat pathogens, but also to further recruit and activate inflammatory cells [13]. Furthermore, mounting evidence suggests that ROS are important regulators of cellular processes in non-phagocytes including fibroblasts, where they are generated in response to growth factor stimulation and extracellular matrix presentation, and contribute to signaling processes involved in myofibrogenesis [14-16]. Thus, we hypothesized that ROS generation could serve as a practical indicator of phagocytic cell recruitment and fibrosis resulting from the host response to biomaterial implantation.
The time evolution of the foreign body response has been determined largely based on histological evidence or analysis of cellular exudate collected from many animals sacrificed at various timepoints [17-19]. Few studies have examined the time-dependent local response to an implant within a single animal, in part because of the lack of appropriate imaging modalities. In one example, experiments were performed using caged implants, which allow the sampling of inflammatory cytokines and cells in the exudate surrounding the material in vivo [20,21]. While this method has provided valuable information, it requires a specialized implant and invasive sampling techniques that may potentially alter the host response. Recent advances in biological imaging tools have revolutionized the ability to non-invasively monitor the progression of diseases such as cancer, arthritis, and cardiovascular disease [22-24]. Moreover, it was recently demonstrated that fluorescent agents can be used to examine protease activity and phagocytic cell presence in response to implanted materials [25]. Here, we develop a complementary approach using a luminescent probe to detect ROS within live mice. ROS can be detected in numerous ways including electron spin resonance to measure unpaired electrons or trapping of reactive radicals to form a more stable product [26]. The latter method includes probes that luminesce or fluoresce upon reaction with oxidative species, and has been widely used to measure ROS in isolated cells and whole blood [27,28]. Recently, 5-amino-2,3-dihydro-1,4-phthalazinedione (luminol) and its analog, L-012, have been used to detect ROS in animals following lipopolysaccharide injection [29,30]. Luminol is non-toxic, small in size, and therefore rapidly distributed and cleared, making it an ideal probe for use in vivo.
In the following study, we used luminol to examine ROS generation in response to implanted biomaterials within live mice. We compared the level of ROS associated with implantation of two materials known to invoke different host responses, polystyrene and alginate. In order to further evaluate the mechanism underlying ROS generation, we examined the tissue response by histology and immunohistochemical staining of myeloperoxidase, an enzyme involved in the production of ROS. Finally, we used luminol to examine phagocyte cell activation in response to biomaterials in vitro.
Materials and Methods
Bead Preparation
Polystyrene beads (106-125 μm) were purchased from Polysciences, Inc., sterilized by washing in 70% ethanol, and then washed and resuspended in PBS. Alginate beads were fabricated from a 3% (w/v) solution of alginate (Pronova UP VLVG, Novamatrix) in deionized water using the Inotech encapsulator (Inotech) set to a voltage of 1.1kV, vibration frequency of 2500 Hz and flow rate of 1.5 ml/minute. A 100 μm nozzle was used to generate beads approximately 175-225 μm in diameter. Beads were crosslinked with calcium chloride, washed extensively in distilled water, and then resuspended in PBS prior to implantation.
Animals
8-12 week old male SKH1 mice were obtained from Charles River Laboratories (Wilmington, MA). The mice were maintained at the animal facilities of Massachusetts Institute of Technology, accredited by the American Association of Laboratory Animal care, and were housed under standard conditions with a 12-hour light/dark cycle. Both water and food were provided ad libitum. The research protocol was approved by the local animal ethics committees at Massachusetts Institute of Technology (Committee on Animal Care) and Children’s Hospital Boston (Institutional Animal Care and Use Committee) prior to initiation of the study.
Biomaterial implantation and in vivo imaging
100 μl injections of a 50% slurry of alginate or polystyrene beads were made using an 18 gauge needle into the subcutaneous region on the dorsal side of SKH1 mice (Charles River). Mice were anesthetized by isoflurane inhalation and then injected with materials. For experiments using Prosense, 2 nmol in 150 μL Prosense solution was injected into the tail vein of mice one day prior to imaging. For experiments with luminol, mice were anesthetized with isoflurane and injected with 5 mg of luminol resuspended in 100 μl of PBS into the peritoneum (Sigma-Aldrich). Animals were then imaged using an IVIS Spectrum 200 (Caliper Lifesciences) with a 3 minute exposure (bioluminescence) or 1 second exposure (fluorescence) at the indicated times after luminol injection. Images were analyzed using LivingImage 3.0 Software.
Histology and Immunohistochemistry
Mice were sacrificed and implants were excised along with the surrounding skin tissue. Tissues were fixed by overnight incubation in 10% formalin and transferred to 70% ethanol for storage before preparation for histology. Tissues were then embedded within paraffin blocks and cut into 10 μm sections. Sections were then stained with hematoxylin and eosin (H&E, Sigma-Aldrich) or Masson’s trichrome (MT, Sigma-Aldrich), and evaluated for neutrophil, macrophage and fibroblast infiltration. For immunohistochemical evaluation of myeloperoxidase, tissue sections were stained with a rabbit polyclonal anti-myeloperoxidase antibody, followed by biotinylated goat anti-rabbit secondary antibody (both from Thermo Fisher Scientific), horseradish peroxidase-conjugated streptavidin (Dako), and 3,3-diaminobenzadinetetrahydrochloride (Sigma-Aldrich). Hematoxylin (Sigma-Aldrich) was used as a nuclear counterstain.
In vitro experiments
Thioglycollate (Sigma-Aldrich) was injected into the interperitoneal space of C57BL/6J mice (Jackson Labs). After 24h, animals were sacrificed, and peritoneal cells were isolated by lavage, and treated with red blood cell lysis buffer. Cells were washed in 1X HBSS and resuspended in DMEM supplemented with 10% bovine serum. Cells were seeded at 20,000 cells/cm2 on tissue culture surfaces or tissue culture surfaces covered with a 3% with a smooth alginate hydrogel. The smooth alginate hydrogel was made based on a previously published method [31]. Briefly, a 0.4% (w/v) sodium metaphosphate solution was used to make a 3% (w/v) alginate solution and a 0.4 g/ml CaSO4 slurry was added at a volume ratio of 1:50. The mixture was vortexed and dispensed into the plate for gelation. After seeding, cells were treated with phorbol myristate acetate (Sigma-Aldrich) for 10 minutes. Luminol (100 μm) was added and wells were imaged after 20 minutes using an Ivis Spectrum 200 with a 60 second exposure. Images were analyzed using LivingImage 3.0 software.
Statistical Analysis
All experiments performed in animals were conducted on at least 6 animals. Data presented are the average across all animals with one or two injections of each material per animal. For in vitro studies, experiments were performed at least three times with duplicate samples. Error bars show standard error of the mean.
Results
We first determined whether luminol could be used to detect reactive oxygen species (ROS) released in response to a biomaterial implanted in vivo. Polystyrene beads were injected into the subcutaneous region on the dorsal side of SKH1 mice, which are hairless and immunocompetent (Figure 1a). After 24 hours, luminol was administered and animals were imaged over the course of 1 hour after luminol injection (Figure 1b). We observed time-dependent luminescence localized to regions where polystyrene beads were implanted, peaking at 20 minutes and then decreasing over the course of the hour. Three-dimensional analysis of the luminescence signal revealed that the luminescent regions were in close proximity to the surface of the skin, precisely where the implant was placed (Supp. Figure 1). Quantification of total flux showed that the signal near the polystyrene implants was approximately three times higher at the peak of luminescence when compared to a background region adjacent to the site of material implantation (Figure 1c). Injection of a control, PBS solution using a needle of identical gauge induced only a small increase in luminescence relative to background levels. Background luminescence was observed throughout the mouse, and was more pronounced near lymph nodes. Interestingly, background luminescence was only observed in animals with materials implanted; animals that had received no implants but were injected with luminol did not exhibit any luminescence under the same acquisition conditions (data not shown). Together, these data demonstrate that luminol can detect ROS release in response to biomaterial implantation, and that the level of ROS released locally in response to the material is greater than that released by an injection wound alone.
Figure 1. Luminol bioluminescence image of polystyrene implants.
(a) Schematic of polystyrene beads and PBS (control) injections that were established in the dorsal subcutaneous region of SKH1 mouse. (b) Bioluminescence at indicated timepoints (minutes) after intraperitoneal injection of luminol. (c) Graph of quantification of luminol bioluminescence within the region of interest, including a background region obtained adjacent to the implant site. Error bars are standard error of the mean of n = 6 mice.
In order to examine ROS released in response to materials with varied degrees of biocompatibility, we compared the luminescence caused by polystyrene and alginate, materials that are known to cause high and low levels of phagocytic cell recruitment, respectively. We were also interested in the time-dependent ROS release over the course of one month after material implantation. We injected beads of polystyrene and alginate into the subcutaneous regions of mice in an array format as shown in Figure 2a, and examined luminol-induced luminescence at 1, 3, 7, 14 and 28 days after material implantation (Figure 2b). Quantification of luminescence revealed that polystyrene induced high levels of ROS at all of the timepoints examined over the course of the entire month. Alginate, on the other hand, exhibited very low luminescence, similar to levels observed at control, PBS injections (Figure 2c). We compared these findings to results using Prosense, a fluorescent probe that detects cathepsin activity, and has been used as a marker of phagocytic cell response to implanted biomaterials [25,32]. We found that polystyrene induced a high level of fluorescence at 1 and 3 days after material implantation, but this signal decreased over the course of one month (Figure 2d and e). Alginate beads induced a low level of fluorescence, which was comparable to control levels at early timepoints. At one month after material implantation, polystyrene and alginate elicited similar levels of fluorescence, and was slightly above the levels induced by the control PBS injection. The results obtained from both probes show that polystyrene induces a stronger host reaction when compared to alginate and a control, PBS injection. However, the differences in signals observed using the two probes at later timepoints also suggest that luminol and Prosense detect distinct tissue responses to implanted materials, and that ROS release and cathepsin activity in response to polystyrene are not directly correlated at two weeks to one month after material implantation.
Figure 2. Comparison of polystyrene and alginate implants with luminol and Prosense over the course of 28 days.
(a) Schematic of polystyrene beads, alginate beads and PBS (control) injections that were established in the dorsal subcutaneous region of SKH1 mice. (b) Images of luminol bioluminescence at indicated timepoints (days) after material implantation. (c) Normalized material-induced luminol bioluminescence within the region of interest. (d) Images of Prosense fluorescence at indicated timepoints (days) after material implantation. (e) Quantification of material-induced Prosense fluorescence within the region of interest. Error bars are standard error of the mean of at least n = 6 mice for each time point.
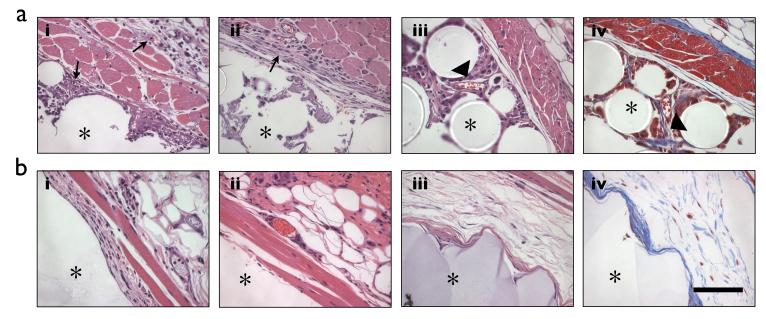
We used standard histological techniques to examine the tissue response to the implanted materials, and to gain further insight about which cell types might be responsible for releasing the ROS that we observed. Animals were sacrificed at 1, 7 and 28 days after material implantation and the tissue surrounding the implant was excised, fixed, and stained with H&E and Masson’s Trichrome. At 1 day after material implantation, we observed many neutrophils in the skin tissue surrounding the polystyrene implant, and cells beginning to infiltrate the space between the beads (Figure 3a). At 7 and 28 days, we observed a mixture of neutrophils, monocyte/macrophages, and fibroblasts, and increasing amounts of cell infiltration. In contrast, few inflammatory cells were present and almost no cell infiltration was observed near alginate beads throughout the 28 days (Figure 3b). Masson’s trichrome staining revealed the presence of collagen fibrils in the regions surrounding polystyrene beads at 28 days, suggesting the onset of fibrosis (Figure 3a). Significantly less fibrosis was observed surrounding alginate beads (Figure 3b). Together, these data suggest that neutrophils are primarily responsible for the generation of ROS at early timepoints. However, at longer timepoints neutrophils, along with monocyte/macrophages and fibroblasts may all contribute to the generation of ROS in response to the implantation of polystyrene beads, since all cell types were observed adjacent to the material implant.
Figure 3. Characterization of tissue response by histology.
(a) Representative sections of subcutaneous tissue implanted with polystyrene beads for 1 (i), 7(ii) or 28 (iii and iv) days and stained with H&E (i-iii) or Masson’s Trichrome (iv). Arrows indicate the presence of phagocytic cells (i and ii) and arrowheads indicate the presence of fibroblasts (iii) or collagen fibrils (iv). (b) Representative sections of subcutaneous tissue implanted with alginate beads for 1 (i), 7 (ii) or 28 (iii) days and stained with H&E (i-iii) or Masson’s Trichrome (iv). Asterick (*) denotes the region with the material. Scale bar, 100 μm.
We examined the expression of myeloperoxidase, an enzyme produced by neutrophils that releases ROS during inflammation, in tissues excised near polystyrene and alginate implants at 1, 7, and 28 days after implantation. We found positively stained cells at 1 day after implantation in the tissue surrounding and infiltrating the polystyrene beads. At 7 and 28 days after implantation, myeloperoxidase staining was primarily observed in the regions adjacent to the beads, and limited amount of staining was observed in the surrounding tissue. Moreover, the staining appeared diffuse and was not localized to neutrophils. In tissue surrounding alginate implants, there was little or no myeloperoxidase staining at all timepoints examined. The presence of positive myeloperoxidase staining near polystyrene but not alginate implants at all timepoints, suggest a correlation between myeloperoxidase expression and ROS generation as observed with luminol.
Finally, we confirmed that luminol could be used to detect ROS released by phagocytic cells in response to polystyrene and alginate surfaces in vitro. Peritoneal cells were extracted from thioglycollate-injected C57BL/6J mice, and seeded onto polystyrene wells with and without a coating of alginate. Cells were treated with phorbol myrystate-acetate (PMA) for 10 minutes before the addition of luminol, and then luminescence was imaged at 20 minutes after luminol addition (Figure 5a). We observed two-fold higher luminescence in neutrophils seeded on polystyrene when compared to neutrophils on alginate (Figure 5b). These data correlate with the trends observed with these biomaterials and luminol-induced luminescence in vivo.
Figure 5. Generation of ROS by phagocytes in vitro.
(a) Image of luminol bioluminescence of peritoneal cells seeded on polystyrene and alginate surfaces, and stimulated with PMA for 10 minutes. (b) Quantification of luminol bioluminescence. Error bar indicates standard error of the mean for three separate experiments.
Discussion
Numerous studies have examined the release of ROS by inflammatory cells in response to biomaterials in vitro as a method to evaluate the host response [4-7]. Moreover, investigators have developed materials that release ROS scavengers as a method to mitigate the foreign body response [33]. These studies have been performed based on the assumption that ROS are generated in response to materials in vivo, and that high levels of ROS are associated with the subsequent foreign body response. Here, we provide the first evidence of ROS released in response to implanted materials in live animals. Using luminol, we detected ROS generation localized to sites of subcutaneously-implanted materials. We found that polystyrene beads, which are known to cause significant foreign body response, induced higher levels of ROS when compared to alginate beads, which elicit an appreciably decreased response. Notably, we revealed that ROS are present for at least 28 days after material implantation, and may have an important role in the chronic as well as the acute response to implanted biomaterials.
Histological evaluation of the tissue surrounding the polystyrene implants showed a cascade of events similar to what others have observed, including the infiltration of neutrophils at early timepoints, followed by monocyte/macrophages and then fibroblasts at longer timepoints [1]. At 28 days after material implantation, collagen fibrils and fibroblasts were present surrounding polystyrene beads indicating the onset of fibrosis, whereas alginate beads invoked little or no tissue response. These data suggest that inflammatory cells and fibroblasts infiltrating the site of polystyrene bead implantation may all contribute to the release of ROS. Phagocytes such as neutrophils and macrophages have been known to release ROS when activated. In addition, several studies have demonstrated that ROS are also released by nonphagocytic cells such as fibroblasts [15]. These authors demonstrated that TGF-β1-stimulated fibroblasts exhibit a myofibroblast phenotype and release ROS [14]. Hence, the detection of ROS at these later timepoints in our studies may indicate the presence of transformed fibroblasts that are involved in the formation of the fibrous capsule surrounding biomaterial implants. Our data show that myeloperoxidase is one specific enzyme that is involved in ROS generation in response to materials, but do not preclude the possibility that other ROS releasing enzymes including NADPH oxidases and xanthine oxidase may also contribute to the inflammatory response to biomaterials and the ROS signal detected by luminol. These detailed studies are a subject for future investigation.
Luminol was chosen because of its proven efficacy in vivo [29], however many additional chemiluminescent and fluorescent probes exist, including dihydroethidium, dihydrorhodamine, and dichlorofluorescein diacetate. Moreover, a luminol analogue L-012 has been reported to have increased detection sensitivity and has also been used in vivo [30,34]. The use of more specific and sensitive probes in future studies may be useful for the detection of different levels of specific ROS, as well as the presence of small amounts of ROS in response to more compatible materials such as alginate. Another whole animal imaging method for studying inflammation involves the use of the imaging agent, Prosense [25]. Prosense fluoresces upon cleavage by cathepsin and is thought to detect the presence of activated neutrophils and monocyte/macrophages [32]. Although polystyrene beads elicited a high signal with both Prosense and luminol, we found the signals were not directly correlated at longer timepoints: cathepsin activity decreased at 14-28 days, while ROS persisted. These data suggest that cathepsin activity is only involved in the acute or early phase, while ROS contributes to both the acute and chronic phase of the host response to biomaterials. Alternatively, the transport of Prosense into the implant site may be limited at longer timepoints due to the formation of the fibrous capsule, as has been observed in tumor models [35]. The combination of these two reagents may be used to distinguish between early and late response, and together may provide valuable information about the biocompatibility of new materials in vivo. Since both probes can be injected into the same mouse and it is not necessary to sacrifice animals to visualize the results, these tools can be used to examine the real-time host response to materials in a single mouse over a long time period.
Conclusion
A better understanding of the mechanisms underlying the foreign body response may help to develop new biomaterials that invoke a controlled foreign body response. In this study we learned that ROS are involved in the short- and long-term response to biomaterials. While we only tested two materials here, we can use the same techniques to test the in vivo biocompatibility of many new materials in parallel. As we learn more about the role of ROS in biomaterial-induced inflammation, we can also design novel materials that prevent or actively combat their formation. Such materials may be useful for implanted devices including cell encapsulation therapies, cardiovascular devices such as stents and vessel grafts, artificial tissues, and biosensors.
Supplementary Material
Supplemental Figure 1. Three-dimensional rendering of luminol bioluminescence from polystyrene bead implantation. (a) Image of whole mouse with sagittal (blue), coronal (red) and transverse (green) planes indicated. (b) Cross-sectional views of the coronal (i), sagittal (ii) and transverse (iii) planes. Polystyrene beads were implanted in the region where all three planes intersect.
Figure 4. Characterization of myeloperoxidase expression.
(a) Representative sections of subcutaneous tissue implanted with polystyrene beads for 1 (i), 7(ii) or 28 (iii and iv) days and stained for myeloperoxidase. Arrows indicate the presence of punctate neutrophil cell staining (i) and arrowheads indicate the presence of diffuse staining (iii). (b) Representative sections of subcutaneous tissue implanted with alginate beads for 1 (i), 7 (ii) or 28 (iii) days and stained for myeloperoxidase. Asterick (*) denotes the location of the material. Scale bar, 100 μm.
Acknowledgements
We thank Mr. Chakib Boussahmain for preparation of histological samples, Dr. Rod Bronson and Dr. Scott Malstrom for assistance with data analysis, and Mr. Phillip Kim for technical assistance. This research was supported by the Juvenile Diabetes Research Foundation under grant 17-2007-1063. T.D. is grateful for support from the Agency for Science, Technology and Research of Singapore for the A*STAR National Science Graduate Fellowship. K.B. is grateful for the support from the National Institutes of Health Postdoctoral Fellowship F32 EB011580-01.
References
- [1].Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Williams DF. On the mechanisms of biocompatibility. Biomaterials. 2008;29(20):2941–53. doi: 10.1016/j.biomaterials.2008.04.023. [DOI] [PubMed] [Google Scholar]
- [3].Sutherland K, Mahoney JRn, Coury AJ, Eaton JW. Degradation of biomaterials by phagocyte-derived oxidants. J Clin Invest. 1993;92(5):2360–7. doi: 10.1172/JCI116841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Henson PM. The immunologic release of constituents from neutrophil leukocytes. II. Mechanisms of release during phagocytosis, and adherence to nonphagocytosable surfaces. J Immunol. 1971;107(6):1547–57. [PubMed] [Google Scholar]
- [5].Ginis I, Tauber AI. Activation mechanisms of adherent human neutrophils. Blood. 1990;76(6):1233–9. [PubMed] [Google Scholar]
- [6].Kaplan SS, Basford RE, Mora E, Jeong MH, Simmons RL. Biomaterial-induced alterations of neutrophil superoxide production. J Biomed Mater Res. 1992;26(8):1039–51. doi: 10.1002/jbm.820260806. [DOI] [PubMed] [Google Scholar]
- [7].Jiang WW, Su SH, Eberhart RC, Tang L. Phagocyte responses to degradable polymers. J Biomed Mater Res A. 2007;82(2):492–7. doi: 10.1002/jbm.a.31175. [DOI] [PubMed] [Google Scholar]
- [8].Gilbert DL, Colton CA. Reactive oxygen species in biological systems: an interdisciplinary approach</div>. Plenum Pub Corp; 1999. [Google Scholar]
- [9].Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3(4):276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- [10].Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3(3):205–14. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- [12].Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid beta protein toxicity. Cell. 1994;77(6):817–27. doi: 10.1016/0092-8674(94)90131-7. [DOI] [PubMed] [Google Scholar]
- [13].Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42(2):153–64. doi: 10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- [14].Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270(51):30334–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- [15].Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- [16].Kheradmand F, Werner E, Tremble P, Symons M, Werb Z. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280(5365):898–902. doi: 10.1126/science.280.5365.898. [DOI] [PubMed] [Google Scholar]
- [17].Higgins DM, Basaraba RJ, Hohnbaum AC, Lee EJ, Grainger DW, Gonzalez-Juarrero M. Localized immunosuppressive environment in the foreign body response to implanted biomaterials. Am J Pathol. 2009;175(1):161–70. doi: 10.2353/ajpath.2009.080962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rhodes NP, Hunt JA, Williams DF. Macrophage subpopulation differentiation by stimulation with biomaterials. J Biomed Mater Res. 1997;37(4):481–8. doi: 10.1002/(sici)1097-4636(19971215)37:4<481::aid-jbm6>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [19].Hunt JA, Flanagan BF, McLaughlin PJ, Strickland I, Williams DF. Effect of biomaterial surface charge on the inflammatory response: evaluation of cellular infiltration and TNF alpha production. J Biomed Mater Res. 1996;31(1):139–44. doi: 10.1002/(SICI)1097-4636(199605)31:1<139::AID-JBM15>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- [20].Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM. In vivo leukocyte cytokine mRNA responses to biomaterials are dependent on surface chemistry. J Biomed Mater Res A. 2003;64(2):320–9. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- [21].Rodriguez A, Meyerson H, Anderson JM. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J Biomed Mater Res A. 2009;89(1):152–9. doi: 10.1002/jbm.a.31939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452(7187):580–9. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wunder A, Tung CH, Muller-Ladner U, Weissleder R, Mahmood U. In vivo imaging of protease activity in arthritis: a novel approach for monitoring treatment response. Arthritis Rheum. 2004;50(8):2459–65. doi: 10.1002/art.20379. [DOI] [PubMed] [Google Scholar]
- [24].Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116(24):2841–50. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- [25].Bratlie KM, Dang TT, Lyle S, Nahrendorf M, Weissleder R, Langer R, et al. Rapid biocompatibility analysis of materials via in vivo fluorescence imaging of mouse models. PLoS One. 2010;5(4):e10032. doi: 10.1371/journal.pone.0010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].DeChatelet LR, Long GD, Shirley PS, Bass DA, Thomas MJ, Henderson FW, et al. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982;129(4):1589–93. [PubMed] [Google Scholar]
- [28].Hallett MB, Cole C, Dewitt S. Detection and visualization of oxidase activity in phagocytes. Methods Mol Biol. 2003;225:61–7. doi: 10.1385/1-59259-374-7:61. [DOI] [PubMed] [Google Scholar]
- [29].Gross S, Gammon ST, Moss BL, Rauch D, Harding J, Heinecke JW, et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat Med. 2009;15(4):455–61. doi: 10.1038/nm.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kielland A, Blom T, Nandakumar KS, Holmdahl R, Blomhoff R, Carlsen H. In vivo imaging of reactive oxygen and nitrogen species in inflammation using the luminescent probe L-012. Free Radic Biol Med. 2009;47(6):760–6. doi: 10.1016/j.freeradbiomed.2009.06.013. [DOI] [PubMed] [Google Scholar]
- [31].Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- [32].Nahrendorf M, Sosnovik DE, Waterman P, Swirski FK, Pande AN, Aikawa E, et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ Res. 2007;100(8):1218–25. doi: 10.1161/01.RES.0000265064.46075.31. [DOI] [PubMed] [Google Scholar]
- [33].Li Z, Wang F, Roy S, Sen CK, Guan J. Injectable, highly flexible, and thermosensitive hydrogels capable of delivering superoxide dismutase. Biomacromolecules. 2009;10(12):3306–16. doi: 10.1021/bm900900e. [DOI] [PubMed] [Google Scholar]
- [34].Daiber A, August M, Baldus S, Wendt M, Oelze M, Sydow K, et al. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic Biol Med. 2004;36(1):101–11. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- [35].Thurber GM, Figueiredo JL, Weissleder R. Multicolor fluorescent intravital live microscopy (FILM) for surgical tumor resection in a mouse xenograft model. PLoS One. 2009;4(11):e8053. doi: 10.1371/journal.pone.0008053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Three-dimensional rendering of luminol bioluminescence from polystyrene bead implantation. (a) Image of whole mouse with sagittal (blue), coronal (red) and transverse (green) planes indicated. (b) Cross-sectional views of the coronal (i), sagittal (ii) and transverse (iii) planes. Polystyrene beads were implanted in the region where all three planes intersect.