Abstract
Background
Sirolimus is used in patients with renal insufficiency following liver transplantation (LT), especially in those with calcineurin inhibitor (CNI) associated nephrotoxicity.
Aims
We conducted a systematic review of all randomized controlled trials (RCTs) and observational studies to test the hypothesis that use of sirolimus is associated with an improvement in renal function at 1 year in LT recipients with renal insufficiency (GFR<60ml/min or creatinine≥1.5mg/dL).
Methods
We performed a search of all major databases, conference proceedings and relevant journals through December 2009 and contacted content experts, corresponding authors, and the pharmaceutical manufacturer. A random effects model was utilized to determine the pooled estimate of the change in renal function and pooled risk estimates of adverse events that may be associated with sirolimus based therapy at 1 year.
Results
Eleven studies (3 RCTs and 8 observational) met final inclusion criteria. A non significant improvement of 3.38 ml/min (95% CI -2.93 to 9.69) was observed among methodologically sound observational studies and controlled trials reporting the primary outcome. Among controlled trials, sirolimus use was associated with a 10.35 ml/min (95% CI 3.98 - 16.77) improvement in GFR or creatinine clearance. Sirolimus was not significantly associated with death, RR=1.12 (95% CI 0.66-1.88) or graft failure, RR=0.80 (95% CI 0.45-1.41), though reporting was incomplete. It was associated with a statistically significant risk of infection (RR=2.47, 95% CI 1.14-5.36), rash (RR=7.57, 95% CI 1.75-32.70), ulcers (RR=7.44, 95% CI 2.03-27.28) and discontinuation of therapy (RR=3.61, 95% CI 1.32-9.89).
Conclusion
Conversion to sirolimus from CNI is associated with a non significant improvement in renal function in LT recipients with renal insufficiency, though results are limited by heterogeneity, risk of bias, and lack of standardized reporting.
Keywords: side effects, rapamycin, mTOR Inhibitor, calcineurin inhibitors
BACKGROUND
Renal insufficiency in liver transplant (LT) recipients is associated with progression to end stage renal disease and a decrease in patient and graft survival.(1-4) After LT, calcineurin inhibitors (CNI) contribute further to the development of chronic renal failure.(5-8) Minimizing the nephrotoxicity of immunosuppressive regimens may help decrease the number of patients developing chronic renal failure and its associated morbidity and mortality following LT.
Sirolimus is used as an alternative to CNI based immunosuppression, especially given its non-nephrotoxic side effect profile. However, outcomes with sirolimus based conversion in LT recipients have not been systematically studied. Part of the hesitation may be due to the adverse events associated with sirolimus. In 2002, the Food and Drug Administration (FDA) issued a black box warning regarding the risk of hepatic artery thrombosis with de novo sirolimus based primary immunosuppression. In June 2009, the FDA notified healthcare professionals of preliminary data suggesting increased mortality in stable LT patients after conversion from a CNI-based immunosuppression regimen to sirolimus.(9) Regardless, sirolimus is actively used in many liver transplant centers for patients with renal insufficiency based on data from small single center studies, often with contrasting results.
We hypothesized that the use of sirolimus in LT recipients with renal insufficiency will be associated with improvement in renal function at 1 year as compared to CNI based therapy or other standard immunosuppression. Additionally, we review reported side effects and adverse events associated with sirolimus.
METHODS
We utilized the recommendations of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) as well as the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group for reporting of the study.(10, 11)
Inclusion and exclusion criteria
In primary adult LT recipients, we examined the use of sirolimus either as primary immunosuppression in patients with renal insufficiency or conversion from a reference immunosuppression regimen due to nephrotoxicity. We defined renal insufficiency as either a serum creatinine measurement ≥ 1.5mg/dL or estimated glomerular filtration rate (GFR) < 60ml/min/1.73 m2 based on National Kidney Foundation K/DOQI criteria.(12, 13) The comparison or reference group could receive CNI (tacrolimus or cyclosporine at low dose or standard dose), mycophenolate mofetil, or interleukin 2 inhibitors (daclizumab or basiliximab), separately or in combination without sirolimus. All doses of steroid or other secondary immunosuppression (e.g. azathioprine) were eligible.
We included all randomized controlled trials and observational studies with a comparison group. Review articles, editorials and letters that did not report original data were excluded.
Search Strategy and Selection
We assembled a team with content (RHW, WRK) and methodological expertise (CPW, MHM). A medical librarian with expertise in conducting systematic reviews (PJE) was consulted to devise a sensitive search strategy. We searched PUBMED, EMBASE, Scopus, Web of Science, Cochrane central register of controlled trials, and the Hepatobiliary Group in the Cochrane library through December 25, 2009 without restriction on publication status or language of publication. We combined database specific search terms for sirolimus (sirolimus or rapamycin or mTOR inhibitors or rapamune) and liver transplantation (liver or liver transplantation or hepatic transplantation or hepatic graft or LT or olt). A hand search of relevant journals and annual meetings was also conducted. Further details regarding search strategy are provided in appendix 1 (Supplementary Information). Authors of relevant abstracts were contacted to reveal any unpublished data if available. All reference sections of eligible studies and pertinent reviews were hand reviewed for potential studies. We also requested information on potentially eligible studies from content experts and contacted the manufacturer of sirolimus for potential trials. We included relevant studies reported in foreign languages for translation into English (JT). Finally, authors of included studies were contacted for data clarifications and input as to the accuracy of our extraction.
Two reviewers (SKA and MDL) independently considered the eligibility of potential abstracts and titles. Inter-rater reliability was calibrated and retrieval strategies were refined by utilizing a smaller set of reports. All studies with disagreement, or lack of information to accurately assess eligibility, were carried to the full text stage for evaluation.
Both reviewers worked independently and considered the full text reports for eligibility. The kappa statistic (measure of agreement) was 0.83 with 94.4% observed agreement. Disagreements were harmonized by consensus and if not possible, by arbitration (WRK). The excluded studies and the reasons for exclusion are included in appendix 2 (Supplementary Information).
Data Extraction
All data extraction was done in duplicate by both reviewers independently. We extracted inclusion and exclusion criteria, participants enrolled (patient flow, age, sex, reason for transplantation, previous CNI dose, length of follow up), sirolimus associated characteristics (timing of intervention, bolus and maintenance dose, taper vs. abrupt cessation of previous therapy), and reference group immunosuppression regimens. Next, we assessed the risk of bias. For all trials, we assessed loss to follow up and how missing data was handled. For randomized controlled trials, we applied the Cochrane Collaboration risk assessment tool.(14) For assessing the quality of observational studies, we modified criteria suggested by the Newcastle–Ottawa quality assessment tool for observational studies.(15) The primary outcome was change in renal function from 1 year as compared to baseline (creatinine or glomerular filtration rate). The secondary outcomes were survival, graft failure, episodes of acute rejection, and requirement of renal replacement therapy at 1 year. We also examined the following adverse events: dyslipidemia requiring statin use, infection, edema, rash, oromucosal ulcers, proteinuria, poor wound healing, pneumonitis and hepatic artery thrombosis.
Statistical Analysis
The primary analysis determined the mean change in renal function from baseline to 1 year in the sirolimus group as compared to the reference group. We generated relative risk estimates for the secondary dichotomous outcomes (e.g. acute rejection episodes). A random effects model for pooled estimates and associated confidence intervals was utilized. Heterogeneity was explored by the I2 test.(16) We calculated separate pooled estimates for randomized controlled trials.
We also calculated a standardized mean difference (SMD) to obtain a pooled estimate for all controlled trials and observational studies. The SMD is used as a summary statistic in meta-analysis when studies measure the same outcome (i.e. change in renal function) but measure it in a variety of ways (e.g. glomerular filtration rate, creatinine or creatinine clearance). The SMD expresses the size of the intervention effect relative to the variability or the standard deviation observed in that study.(14) Given that serum creatinine and eGFR have an inverse relationship, the mean values in studies reporting serum creatinine were multiplied by -1 to obtain the SMD. After calculation of the SMD, it was back transformed and re-expressed in familiar scales (estimated GFR) by multiplying the SMD by the baseline standard deviation of a representative observational study.(17)
To explore causes of inconsistency and subgroup-treatment interactions, a priori subgroup analyses were performed based on trial design (randomized trials vs. observational studies), interventions (taper vs. abrupt cessation of CNI, bolus vs. no sirolimus bolus, timing of intervention, CNI vs. non CNI), and patient characteristics (>50% male). A sensitivity analysis was performed to examine whether the results changed when borderline eligible articles were excluded. We used RevMan 5 to conduct the analyses.
Missing information
We encountered two main types of missing information, regarding either precision of the estimates of the change in renal function (no standard deviation provided) or incomplete description of all adverse events of interest. To reconcile this, we provided all authors with a data extraction sheet and requested entry of missing items. If information was still lacking, standard deviations were imputed (appendix 3, supplementary information). We also included information from trials reporting no events in either arm over the follow up period. Though inclusion of these zero events trials may drive the results towards the null, they can increase precision of the estimate and allows for the inclusion and evaluation of the totality of the evidence.(18) To ensure that our imputations did not change our conclusion, we conducted sensitivity analyses varying the assumptions utilized in the imputations.
RESULTS
Ascertainment of studies
Figure 1 summarizes the retrieval process utilized for the systematic review. Of the total 16 eligible studies, 2 reports were excluded as they characterized the same study(19, 20), 1 report was excluded given that it was a non-English study only indexed at the National Library of Medicine(21) and 1 study was excluded as Wyeth pharmaceutical was unable to share unpublished data.(22) One study was available only in abstract form and no further data regarding renal function was available despite author contact.(23) The sponsor of the study, Genentech, was unable to share unpublished data.
Figure 1. Flow of included studies.
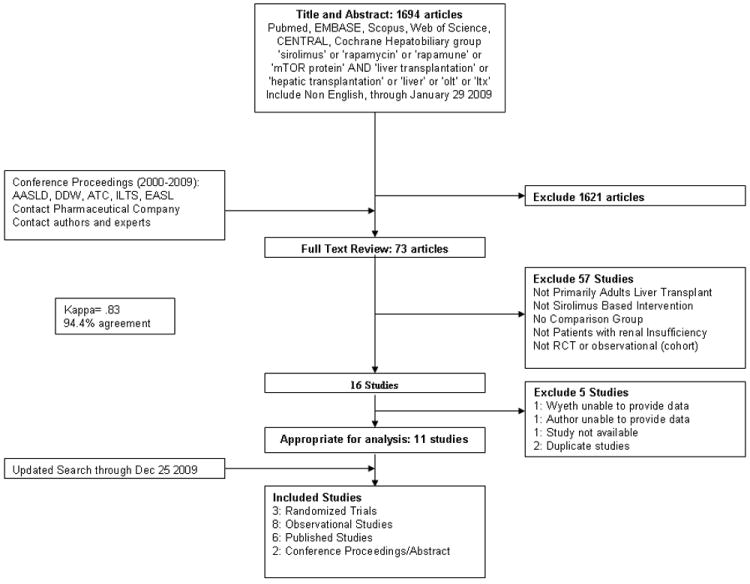
In total, 11 studies (3 randomized controlled trials (24-26) and 8 observational studies (17, 27-33)) were included for the systematic review and meta-analysis. Within these 11 studies, authors provided recently accepted unpublished manuscripts for 2 studies(24, 29), and only abstract data was available for 2 studies(30, 31). Authors of 7/11 studies provided further information.
Study Characteristics
Table 1 describes the characteristics of the included studies; further details regarding patient selection, comparison group and outcomes are provided in appendix 4 (Supplementary Information). Most of the studies were single center and the total number of patients per study ranged from 16 to 148. A total of 86 patients were included in the 3 controlled trials. A majority of patients were male and transplanted for either alcoholic liver disease or hepatitis C.
Table 1.
Characteristics of included studies
| Study and Country | Sirolimus N | Reference N | Age, years Mean (SD) | Female % | ALD/HCV % | Baseline Renal Function, Mean (SD): Sirolimus | Baseline Renal Function, Mean (SD): Reference | Measurement of Renal Function |
|---|---|---|---|---|---|---|---|---|
| CONTROLLED TRIALS | ||||||||
| Watson 20071 UK | 13 | 17 | 59 (54-66)2 | 41 | 30/0 | 50.0 (9.3) | 47.2 (13.4) | GFR: 6 point EDTA clearance |
| Shenoy 2007 USA | 20 | 20 | 59 (7) | 52 | 20/13 | 64(18) | 60(12) | 24 hour CrCl |
| Eisenberger 2009 Switzerland | 8 | 8 | 56 (9) | 25 | 313/314 | 55.4 (24) | 66.9 (17) | CrCl: Cockcroft–Gault formula |
| OBSERVATIONAL STUDIES | ||||||||
| Montalbano 2004 USA | 12 | 8 | 53 | n/a | 55/10 | 43.6 (N/A) | 55 (N/A) | CrCl |
| Kneipess 20055 Austria | 22 | 36 | 54 (26-70)2 | 24 | 31/29 | 1.7 (N/A) | 1.4 (N/A) | CrCl |
| Smallwood 20056 USA | 27 | 34 | 58 (10) | n/a | n/a | 1.97 (1.26) | 2.58 (0.78) | Serum Creatinine |
| Zaghla 2006 USA | 28 | 101 | 54 (10) | 35 | 9/40 | 2.3 (1.5) | 1.3 (0.82) | Serum Creatinine |
| Dubay 2008 Canada | 57 | 57 | 52 (21-70)2 | 45 | 9/33 | 37.0(12.1) | 37.3(10.5) | CrCl: Cockcroft–Gault formula |
| Thompson 20087 USA | 42 | 58 | n/a | n/a | 20/31 | 40.3 (N/A) | 33.3 (N/A) | eGFR: MDRD |
| Zhang 2008 China | 20 | 19 | 50 (3) | 28 | 5/2.5 | Early: 35.33 (5.93) | Early: 37.2 (9.37) | CrCl |
| Late: 49.53 (12.69) | Late: 52.4 (11.73) | |||||||
| Rogers 2009 USA | 728 | 658 | 52 (10) | n/a | n/a | Early: 40.7 (24.7) | 73.4 (34.24) | eGFr: MDRD |
| Late: 30.6 (12.6) | ||||||||
Abbreviations: N/A=not available; SD=Standard Deviation; ALD: Alcoholic Liver Disease; HCV: Hepatitis C virus infection; GFR: glomerular filtration rate, ml/min; CrCl: Creatinine Clearance; MDRD: Modification of Diet in Renal Disease
Willcocks et al. 2007-duplicate study (20)
Median age and range
Includes cryoptogenic cirrhosis
Includes hepatitis B cirrhosis
Kneipess et al. 2004-duplicate study (19)
Abstract data only
Abstract data only
smaller number of participants evaluated for renal function: 43 in sirolimus arm and 40 in the reference arm.
Table 2 shows details regarding the immunosuppression regimen. Two studies examined de novo sirolimus use at time of transplantation (32, 34) and 9 studies examined patients converted to sirolimus from other immunosuppression regimens. Most studies restricted their analysis to late initiation of sirolimus (> 6 months after transplantation). Two studies had separate subgroups of patients that underwent early initiation (< 6 months).(29, 33) The comparison group either received only CNI (17, 24-26, 28, 29), CNI and Mycophenolate Mofetil (MMF) (27, 33) or only MMF.(30, 31) Zaghla et al. 2006 had two sirolimus arms: sirolimus alone +/- MMF and sirolimus with a CNI +/- MMF; information from the former sirolimus group was compared to the reference arm.(32) The studies differed in method of switching from CNI to sirolimus (abrupt versus taper) and administration of sirolimus (bolus vs. no bolus, target sirolimus level and dose).
Table 2.
Immunosuppression in included studies
| Study and Country | Immunosuppression in Sirolimus Arm prior to initiation or Conversion (N) | Method of switch from CNI in Sirolimus Arm | Time to Sirolimus Initiation1 | Sirolimus Bolus/dose/trough | CNI in Sirolimus Arm after initiation/conversion | Immunosuppression in reference group initiation/conversion (N) |
|---|---|---|---|---|---|---|
| CONTROLLED TRIALS | ||||||
| Watson 2007 UK | CYC - 3 | Abrupt | Med 3.1 yrs (IQR 0.88-7.4) | none/2mg/5-15 ng/ml | None | CYC - 2 |
| TAC - 10 | TAC - 12 | |||||
| Shenoy 2007 USA | CYC - 15 | Overlap (1-2wks) | 4.4 yrs | 5mg/3mg 6-10ng/ml | None | CYC - 16 |
| TAC - 5 | TAC - 4 | |||||
| Eisenberger 2009 Switzerland | CYC - 6 | Abrupt | Med 50 mo (range 6-123) | 10-15mg/3-5mg 6-16ng/ml | None | CYC - 5 |
| TAC - 2 | TAC - 3 | |||||
| OBSERVATIONAL STUDIES | ||||||
| Montalbano 2004 USA | Not applicable | Not applicable | At transplant | mean dose 8.7ng/ml | TAC - 12 | TAC in all? |
| Kneipess 2005 Austria | CYC - 1 | n/a | 59 mo (range 1–126) | n/a/n/a 4-10ng/ml | CYC - 1 | CYC - 31 |
| TAC - 14 | TAC - 14 | TAC - 5 | ||||
| MMF - 20 | MMF - 20 | MMF - 29 | ||||
| Smallwood 20052 USA | n/a | n/a | 4.5 yrs (SD 3) | 4mg?/ 9.1 (4.3) n/a | None | MMF – 34 |
| Zaghla 2006 USA | Not applicable | Not applicable | At transplant | none/5-7mg n/a | None | n/a |
| Dubay 2008 Canada | CYC - 38 | overlap and abrupt | med 45 mo (range 3-204) | none/1-2mg 5-15μg/d | Low dose CNI - 5 | CYC - 36 |
| TAC - 19 | TAC - 21 | |||||
| Thompson 20082 USA | n/a | n/a | 47.8 mo | n/a | None | MMF - 58 |
| Zhang 2008 China | CYC/TAC -20 | Taper (3 days) | 0-9 months after LT | n/a/2mg n/a | None | CYC/TAC-193 |
| MMF-all | MMF-all | |||||
| Rogers 2009 USA | n/a | Taper | Early: 50 (20) d | n/a/n/a 3-8ng/dL | None | CYC – n/a |
| Late: 309 (SD 292) d | TAC – n/a | |||||
Abbreviations: N/A=not available; SD=Standard Deviation; CNI=calcineurin inhibitors; CYC=cyclosporine; TAC=tacrolimus; MMF: mycophenolate mofetil; LTx=liver transplantation.
Mean time stated unless otherwise specified
Reference group received only mycophenolate mofetil and did not receive calcineurin inhibitors
target was lower limit of range for tacrolimus (10-12ng/ml) or CYC (250-300ng/ml)
Risk of Bias
We evaluated the risk of bias in the 3 published randomized controlled trials (Table 3). Allocation sequence generation was either described or confirmed by author contact for all studies. Allocation concealment was clearly described only by Watson et al. 2007. None of the randomized controlled trials were blinded to observer or patient. Neither Eisenberger et al. 2009 nor Shenoy et al. 2007 were intent to treat analyses. Though Watson et al. 2007 was meant to be intent to treat analysis, randomization was not preserved as three patients who were randomized to CNI were not analyzed. Also, the trial was stopped at the interim analysis and not powered for the primary outcome.
Table 3.
Risk of Bias in published controlled trials
| Study | Allocation Sequence Described |
Allocation Concealment |
Blinding Patient |
Blinding Personnel |
Blinding Assessor |
Selective Outcome Reporting1 |
Other Potential Bias |
Handling of Missing Data |
|---|---|---|---|---|---|---|---|---|
| Watson 2007 | Yes | Yes | No | No | Yes | No | Not Intention to Treat2 | Unclear |
| Not powered, stopped at interim analysis | ||||||||
| Eisenberger 2009 | Yes3 | No | No | No | No | No | Not Intention to Treat | Unclear |
| Shenoy 2007 | Yes | No | No | No | No | No | Last value forward |
Selective outcome reporting: discrepancy in findings reported in the final manuscript versus prior conference proceedings
Three patients who were randomized to CNI were not analyzed
Author provided information
Risk of bias was evaluated by modification of the Newcastle-Ottawa scale (Table 4) for the 6 observational studies. Only abstract data was available for the other 2 studies.(30, 31) The comparison group in all observational studies was obtained from LT recipients that did not receive sirolimus. Comparability of sirolimus and comparison group was obtained by restriction in three of the six studies.(17, 28, 32) No other method to ensure comparability (adjusting for confounders, stratification, or multivariable analysis) was described for the other studies. Selection bias may have influenced the outcome in all trials as reasons for allocation to either sirolimus or reference group may have varied. Allocation may have been at physician’s discretion in most studies though Rogers et al. 2009 reported that a protocol was in place for sirolimus initiation. Not all patients had data available for the primary outcome at 1 year. Methods for handling missing data were not adequately described in any study. Patient flow was adequately described in one study.(17)
Table 4.
Risk of Bias in observational studies using Ottawa-Newcastle rules and other criteria
| Study | Representative Cohort/ Reference |
Exposure Ascertainment |
Comparabili ty of Sir group and reference |
Outcome Assessment |
Sufficient Duration? |
Follow Up | Selection Bias1 |
Missing Data and Other |
|---|---|---|---|---|---|---|---|---|
| Montalbano 2004 | Yes | Medical Records | Restriction to early initiation | Record Linkage | Yes | Details Provided | Allocation to sirolimus group may be biased | Unclear if only positive reports presented |
| Same patient base | Match = ambiguous | |||||||
| Kneipess 2005 | Yes | Unclear | Unclear | Unclear | Yes | Unclear | Allocation to sirolimus group may be biased | Unclear |
| Same patient base | ||||||||
| Zaghla 2006 | Yes | Medical Records | Restriction to early initiation, exclude patients not on Sirolimus plus CNI | Record Linkage | Yes | Unclear-Large Dropout | Allocation to sirolimus group may be biased | Unclear |
| Same patient base | Unsure how RRT handled | |||||||
| Dubay 2008 | Yes | Medical Records | Restriction to late initiation | Record Linkage | Yes | Unclear | Allocation to sirolimus group may be biased | Unclear if data available for all pts at 12 months |
| Same patient base | Match on Gender, Year of Transplant Creatinine | |||||||
| Zhang 2008 | Unclear | Unclear | No restriction or matching | Unclear | No | No 12 month data available | Allocation to sirolimus group may be biased | Unclear |
| Same patient base | Controls reported but matching not described | |||||||
| Rogers 2009 | Yes | Medical Records | No restriction or matching | Record Linkage | Yes | Only 42/72 have >3 months follow up for renal | Same patient base | Unclear |
| Same patient base |
Abbreviations: RRT: renal replacement therapy
Selection bias: physician deciding allocation to arm, no protocol present
Change in Renal Function over 1 year
Given the variability in reporting of the outcome (4 studies reported change in creatinine, 3 studies reported either change in creatinine clearance or glomerular filtration rate and 4 studies reported both), we calculated the SMD. Figure 2 shows that when all controlled trials and observational studies with complete data are considered, sirolimus use was associated with a non significant improvement in renal function at 1 year, SMD 0.30 [95% CI -0.26 to 0.86]. As an illustration, after transformation into the eGFR scale, this implies that sirolimus was associated with an improvement of 3.38 ml/min (95% CI -2.93 to 9.69).
Figure 2. Standardized Mean Difference of change in renal function from initiation of sirolimus to 1 year.
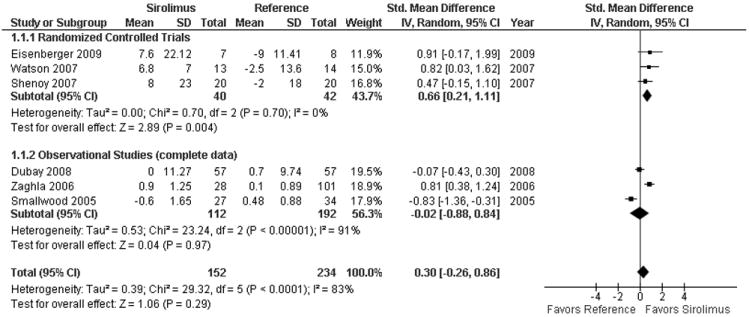
Test for subgroup interaction was non significant (p=0.17). After back transformation into the eGFR scale, sirolimus was associated with an improvement of 3.38 ml/min (95% CI -2.93 to 9.69). Borderline eligible observational studies are not included (see appendix 6). Smallwood 2005 and Zaghla 2006 only reported creatinine. The mean values in studies reporting serum creatinine were multiplied by -1 to make the scale uniform.
All controlled trials had late initiation of sirolimus. In all three trials, the mean eGFR at baseline was >50 ml/min (table 1). When limited to the 3 controlled trials (figure 3), sirolimus use was associated with a 10.38 ml/min (95% CI 3.98-16.77, I2=0%) improvement in renal function.(24-26)
Figure 3. Pooled estimate of change in glomerular filtration rate or creatinine clearance from initiation of sirolimus to 1 year in controlled trials (ml/min).
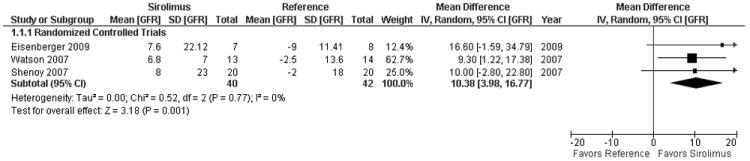
Imputation for mean change and standard deviation of the change was required in most studies. Additionally, it is unclear whether patients that progressed to renal replacement therapy (and therefore deterioration in renal function) were included in the change in renal function from baseline to 1 year. Data could not be included from Thompson et al. 2008 (endpoint not well specified) or Montalbano et al. 2004 and Kniepeiss et al. 2005 (no estimate of variability for baseline or 1 year).(28, 31) Further only 6 month data was available for Zhang et al. 2008 (33) and the GFR in the reference group for Rogers et al. 2009 was only available prior to transplantation (rather than prior to sirolimus conversion) and end of follow up (mean 616 days) rather than 1 year after sirolimus. However, inclusion of these borderline eligible observational studies yielded a more favorable result, SMD 0.89 [95% CI 0.23 - 1.55] or an improvement of 10.03 ml/min (95% CI 3.27 to 17.47) (Appendix 5, Supplementary information).
Secondary Outcomes
Reporting on secondary outcomes was incomplete across all studies. We assessed the relative risk of adverse outcomes if described at 1 year after initiation of sirolimus (Figure 4). Specific details regarding the studies included for each outcome are provided in appendix 6 (Supplementary Information). At 1 year, the relative risk (RR) of patient death (RR=1.12, 95% CI 0.66-1.88), graft failure (RR=0.80, 95% CI 0.45-1.41) and rejection (RR=0.88, 95% CI 0.44-1.76) were not significantly increased in the sirolimus arm as compared to the reference arm. However, sirolimus use was associated with a non significant relative risk of renal replacement therapy (RR=1.71, 95% CI 0.83 - 3.51) and the need for statin therapy (RR=2.93, 95% CI 0.52-16.55). It was associated with a statistically significant risk of infection (RR=2.47, 95% CI 1.14-5.36), rash (RR=7.57, 95% CI 1.75-32.70), edema (RR=2.49, 95% CI 1.09-5.66) and oromucosal ulcers (RR=7.44, 95% CI 2.03-27.28). Incidence of proteinuria and poor wound healing were similar for the sirolimus and reference groups. Insufficient details were provided to obtain the relative risk of either pneumonitis or hepatic artery thrombosis.
Figure 4. Pooled relative risk estimates (95% confidence interval) of side effects and adverse events from initiation of sirolimus to 1 year (mg/dL).
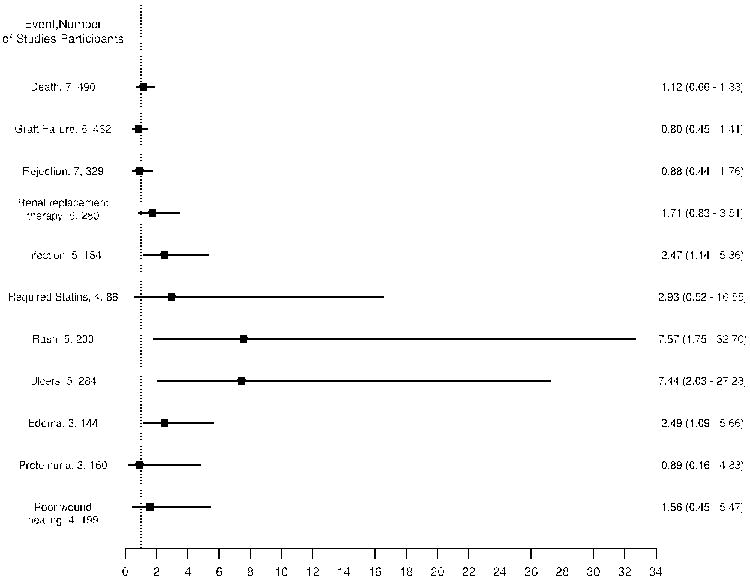
Only studies reporting 1 year data are presented.
Rates of discontinuation due to tolerability and potential side effects of therapy were significant in the sirolimus arm, RR=3.61, 95% CI 1.32-9.89. In addition, 3 studies reported discontinuation rates for the sirolimus arm but did not provide data regarding the reference arm. In these studies, discontinuation rates in the sirolimus arm ranged from 33-55%.(17,28-29)
Subgroup and sensitivity analyses
We conducted pre-specified subgroup and sensitivity analyses to explore heterogeneity based on study design, patient population, sirolimus administration and comparison group. Pooled estimates by trial design are provided in Figure 2.
In certain cases, subgroup analysis was not possible due to the limited number of trials or missing information. For ease of interpretation, estimates expressed as SMD were transformed into the eGFR scale. Subgroup analysis by early initiation was not possible due to incomplete information. All controlled trials had late initiation of sirolimus. Sirolimus was associated with a non significant change in GFR of 4.62 ml/min (95% CI -1.01 – 10.26) when limited to studies with late initiation of sirolimus as compared to a CNI only reference group.(17, 24-26) Sirolimus was associated with a non significant improvement in GFR of 1.35 ml/min (95 % CI -10.81 - 13.52) in studies where sirolimus bolus was used (24, 25, 30) and an improvement of 9.58 ml/min (95% CI 2.37 - 16.69) where CNI cessation was abrupt.(24, 26) Results were consistent across sensitivity analyses for imputation of standard deviations and studies with no events in either arm at the end of the follow up period (appendix 3, Supplementary Information).
DISCUSSION
Reducing the nephrotoxicity associated with current immunosuppressive regimens, especially in patients with underlying renal insufficiency is important in optimizing the long term outcome of liver transplant recipients. We conducted a systematic review and meta-analysis to assess the role of sirolimus in improving renal function after LT. Our study has several important findings: First, there is a paucity of well controlled trials and studies examining this issue. Current studies were heterogeneous, compared different interventions to sirolimus use and were susceptible to bias. Second, initiation of sirolimus is associated with a non significant improvement in renal function. Third, the risk of developing various infections, rash, oromucosal ulcerations and discontinuation of therapy were higher for patients treated with sirolimus. Finally, reporting of outcomes of renal function and adverse events is non-uniform and severely limits the conclusions.
A recent manufacturer sponsored randomized open label trial of conversion from CNI to sirolimus treatment versus continued CNI treatment was completed.(23) Patients with GFR >40ml/min were included. At the end of 12 months, baseline adjusted GFR was similar in patients receiving sirolimus (62ml/min) as those maintained on CNI (63ml/min). However details regarding the baseline GFR and percent change were not available to the authors despite contact with Wyeth pharmaceuticals. Whether these results will be published, remain to be seen. This trial formed the basis of a recent FDA sponsored alert regarding the elevated risk of death seen in the sirolimus arm. The number of deaths, though not statistically significant, was higher in the sirolimus arm (3.8% vs. 1.4%). Therefore, the use of sirolimus must be tempered with the risk of adverse events. In our meta-analysis, the risk of death (7 studies) or graft failure (6 studies) at 1 year was not significantly higher in the sirolimus arm. However, the inherent weaknesses of the individual studies as well as the lack of complete reporting of the outcomes may be responsible for the non significant effect seen in the meta-analysis.
Keeping the limitations of the individual studies and trials in mind, sirolimus use was associated with an improvement in renal function among the 3 controlled trials. When combined with methodologically sound observational studies, the improvement in renal function was no longer significant. All 3 trials examined late conversion (>6 months) from CNI based therapy to sirolimus. However, the mean GFR in the study was >50 ml/min and hence it is unclear whether the degree of improvement will be seen in patients with worse renal function. Furthermore, only 86 patients were analyzed in the 3 trials. In addition, the absence of differences in the open label trial sponsored by Wyeth as well as the unavailability of this data lends uncertainty to the true benefit of sirolimus in improving renal function. Whether the marginal improvement in renal function is sustained over the long term (beyond 1 year) is also unknown. This is especially important as the rates of discontinuation for sirolimus was high.
Alternatively, there may be a renal threshold or a point of no return, beyond which sirolimus initiation may not provide benefit. Indeed, a higher percent progressed to requiring renal replacement therapy in patients assigned to sirolimus, though this was non significant. This was primarily seen in observational studies suggesting a selection bias. The increased rate of renal replacement therapy may imply that sirolimus was used in persons identified by their providers as having rapidly declining renal function requiring active changes to the immunosuppression regimen. Sirolimus may have been utilized too late in persons that may have derived a potential benefit from early initiation, when the renal function was preserved. Hence, progression to renal replacement therapy could not be prevented. Indeed, as reported by Dubay et al. 2008, patients exposed to CNI for more than 5 years or those with an initial creatinine clearance of less than 30 mL/minute who were converted to sirolimus did worse than control patients maintained on low-dose calcineurin inhibitors.(17) Among the three controlled trials (n=86), there was only 1 instance of RRT in the sirolimus arm.
Therefore, despite our rigorous methodology, the inherent limitations of the individual studies and trials preclude a stronger conclusion and further emphasize the need for large well-designed studies with longer follow up and well characterized end-points.
This study has several strengths. We conducted an extensive literature search and provided the most up to date information. Using two reviewers, we ensured inter-rater reliability and assessed inclusion of articles and extracted data independently. We aggressively contacted authors of included studies and content experts to accurately represent our findings. We improved inter-rater reliability by creating standard data extraction sheets and conducting pilot searches. We minimized publication bias by inclusion of non English studies, unpublished data and conference proceedings over a large time period. We applied known criteria to judge the quality of the trials and observational studies. We incorporated the MOOSE and PRISMA guidelines for the reporting of our systematic review. Finally, we contacted all authors of eligible trials. We assessed the appropriateness of our imputations in dealing with missing data. We were able to examine a multitude of adverse events.
However, our study may have limitations. Only 50% of the authors that were contacted provided information. Nonetheless, author contact is rarely done in meta-analyses and is a significant strength of our methodology. Imputations were required for many of the studies. However, we conducted sensitivity analysis to ensure that the imputations did not alter the results. We were unable to include two unpublished reports and their authors could not be reached. At least for one of them, the results would have enhanced our primary outcome. Finally, pooling may not have been appropriate in all cases given the heterogeneity. However, we felt that providing an estimate with a statement of the limitations of the primary studies would provide useful information to the reader. Given the small number of studies, we did not feel that a funnel plot would provide useful information. Through a rigorous and sensitive search strategy as outlined above any publication bias should have been averted. We were unable to conduct a subgroup analysis on patients with early initiation of sirolimus (less than 6 months) due to incomplete data. Whether a larger benefit would have been seen with earlier initiation remains to be seen. On the other hand, the incremental improvement in GFR may not be sustained in patients with worse renal function. However, stage specific information was not available in the trials.
In summary, the use of sirolimus was associated with a non significant improvement in GFR. However, sirolimus use was associated with development of oral ulcers, rash, various infections and discontinuation of therapy. A high-quality RCT limited to early initiation of sirolimus, standardized definition of renal insufficiency, and identification of degree of renal dysfunction beyond which sirolimus may not be beneficial is needed to better define its role in treatment of these patients.
Supplementary Material
Acknowledgments
Funding Source: This study was supported by a grant from the NIH (R01DK-34238, WRK) and a NIH digestive diseases training grant (T32 DK07198, SKA)
List of Abbreviations
- CNI
calcineurin inhibitors
- FDA
Food and Drug Administration
- GFR
glomerular filtration rate
- LT
liver transplant
- MMF
Mycophenolate Mofetil
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- PRISMA
Preferred Reporting Items for Systematic reviews and Meta-Analyses
- RCT
Randomized controlled trials
- RR
relative risk
- SMD
standardized mean difference
Footnotes
Financial Disclosure: None of the authors have conflicts of interest or any specific financial interests relevant to the subject of this manuscript.
Contributor Information
Sumeet K Asrani, Email: asrani.sumeet@mayo.edu.
Michael D Leise, Email: leise.michael@mayo.edu.
Colin P West, Email: west.colin@mayo.edu.
M. Hassan Murad, Email: murad.mohammad@mayo.edu.
Rachel A Pedersen, Email: pedersen.rachel@mayo.edu.
Patricia J Erwin, Email: erwin.patricia@mayo.edu.
Jianmin Tian, Email: tian.jianmin@mayo.edu.
Russell H. Wiesner, Email: rwiesner@mayo.edu.
References
- 1.Fisher NC, Nightingale PG, Gunson BK, Lipkin GW, Neuberger JM. Chronic renal failure following liver transplantation: a retrospective analysis. Transplantation. 1998;66:59–66. doi: 10.1097/00007890-199807150-00010. [DOI] [PubMed] [Google Scholar]
- 2.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AJ, Stegall MD, Rosen CB, Wiesner RH, Leung N, Kremers WK, Zein NN. Chronic renal dysfunction late after liver transplantation. Liver Transpl. 2002;8:916–921. doi: 10.1053/jlts.2002.35668. [DOI] [PubMed] [Google Scholar]
- 4.Gonwa TA, Mai ML, Melton LB, Hays SR, Goldstein RM, Levy MF, Klintmalm GB. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLT) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation. 2001;72:1934–1939. doi: 10.1097/00007890-200112270-00012. [DOI] [PubMed] [Google Scholar]
- 5.Encke J, Uhl W, Stremmel W, Sauer P. Immunosuppression and modulation in liver transplantation. Nephrol Dial Transplant. 2004;19(Suppl 4):iv22–25. doi: 10.1093/ndt/gfh1037. [DOI] [PubMed] [Google Scholar]
- 6.Eckardt KU. Renal failure in liver disease. Intensive Care Med. 1999;25:5–14. doi: 10.1007/s001340050780. [DOI] [PubMed] [Google Scholar]
- 7.Porayko MK, Textor SC, Krom RA, Hay JE, Gores GJ, Richards TM, Crotty PH, et al. Nephrotoxic effects of primary immunosuppression with FK-506 and cyclosporine regimens after liver transplantation. Mayo Clin Proc. 1994;69:105–111. doi: 10.1016/s0025-6196(12)61034-9. [DOI] [PubMed] [Google Scholar]
- 8.Varo E, Lopez A, Rivero C. Initial immunosuppression in liver transplant recipients with impaired renal function. Transplant Proc. 2005;37:3909–3912. doi: 10.1016/j.transproceed.2005.09.115. [DOI] [PubMed] [Google Scholar]
- 9.FDA. 2009 http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/ucm165015.htm.
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Stevens LA. Kidney function estimating equations: where do we stand? Curr Opin Nephrol Hypertens. 2006;15:276–284. doi: 10.1097/01.mnh.0000222695.84464.61. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]: The Cochrane Collaboration. 2008 Available from www.cochrane-handbook.org.
- 15.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2009 http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 16.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DuBay D, Smith RJ, Qiu KG, Levy GA, Lilly L, Therapondos G. Sirolimus in liver transplant recipients with renal dysfunction offers no advantage over low-dose calcineurin inhibitor regimens. Liver Transpl. 2008;14:651–659. doi: 10.1002/lt.21429. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich JO, Adhikari NK, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol. 2007;7:5. doi: 10.1186/1471-2288-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kniepeiss D, Iberer F, Schaffellner S, Jakoby E, Duller D, Tscheliessnigg K. Dyslipidemia during sirolimus therapy in patients after liver transplantation. Clin Transplant. 2004;18:642–646. doi: 10.1111/j.1399-0012.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- 20.Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, Watson CJ, Smith KG. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant. 2007;7:2006–2011. doi: 10.1111/j.1600-6143.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Wu YM, Xu ZC, Zhang B, Li ZC, Lu L, Zheng YS. Application of rapamycin in liver transplant patients with acute kidney malfunction. [Chinese] World Chinese Journal of Digestology. 2006;14:2974–2976. [Google Scholar]
- 22.A Randomized, Open-Label, Comparative Evaluation of Conversion From Calcineurin Inhibitor Treatment to Sirolimus Treatment Versus Continued Calcineurin Inhibitor Treatment in Liver Allograft Recipients Undergoing Maintenance Therapy. In: Clinicaltrials.gov identifier: NCT00086346 Wyeth Pharmaceuticals.
- 23.A Randomized, Open-Label Study of the Effect of a Long-Term Calcineurin Inhibitor-Free Maintenance Regimen With CellCept and Sirolimus on Preservation of Renal Function and Prevention of Acute Rejection in Recipients of an Orthotropic Liver Transplant. Clinicaltrials.gov identifier: NCT00118742 2009.
- 24.Eisenberger U, Sollinger D, Stickel F, Burckhardt B, Frey FJ. Relationship between renal resistance index and renal function in liver transplant recipients after cessation of calcineurin inhibitor. Clin Transplant. 2009;23:499–504. doi: 10.1111/j.1399-0012.2009.00986.x. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy S, Hardinger KL, Crippin J, Desai N, Korenblat K, Lisker-Melman M, Lowell JA, et al. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single-center trial. Transplantation. 2007;83:1389–1392. doi: 10.1097/01.tp.0000261630.63550.41. [DOI] [PubMed] [Google Scholar]
- 26.Watson CJE, Gimson AES, Alexander GJ, Allison MED, Gibbs P, Smith JC, Palmer CR, et al. A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transplantation. 2007;13:1694–1702. doi: 10.1002/lt.21314. [DOI] [PubMed] [Google Scholar]
- 27.Kniepeiss D, Iberer F, Schaffellner S, Jakoby E, Duller D, Tscheliessnigg KH. Nonnephrotoxic immunosuppression in patients after liver transplantation. Int Immunopharmacol. 2005;5:133–136. doi: 10.1016/j.intimp.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Montalbano M, Neff GW, Slapak-Green G, Berney T, Meyer D. Sirolimus therapy in orthotopic liver transplant (OLT) recipients with acute renal insufficiency. Dig Dis Sci. 2004;49:1986–1989. doi: 10.1007/s10620-004-9606-z. [DOI] [PubMed] [Google Scholar]
- 29.Rogers CC, Johnson SR, Mandelbrot DA, Pavlakis M, Horwedel T, Karp SJ, Egbuna O, et al. Timing of sirolimus conversion influences recovery of renal function in liver transplant recipients. Clin Transplant. 2009 doi: 10.1111/j.1399-0012.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- 30.Smallwood GA, Fasola C, S AC, Heffron TG. Is Sirolimus Nephrotoxic in Liver Transplant recipients? Am J Transplant. 2005;5:573. [Google Scholar]
- 31.Thompson JA, Radosevich DM, Humar A, Lake JR. Calcineurin Inhibitor (CNI) withdrawal in Adult Liver Transplant recipients: An Analysis of Long term Efficacy. Liver Transplantation. 2008;14:s107. [Google Scholar]
- 32.Zaghla H, Selby RR, Chan LS, Kahn JA, Donovan JA, Jabbour N, Genyk Y, et al. A comparison of sirolimus vs. calcineurin inhibitor-based immunosuppressive therapies in liver transplantation. Aliment Pharmacol Ther. 2006;23:513–520. doi: 10.1111/j.1365-2036.2006.02770.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XJ, Fu ZR, Wang ZX. Application of sirolimus in patients with renal injury after liver transplantation. [Chinese] Academic Journal of Second Military Medical University. 2008;29:461–462. [Google Scholar]
- 34.Montalbano M, Neff GW, Yamashiki N, Meyer D, Bettiol M, Slapak-Green G, Ruiz P, et al. A retrospective review of liver transplant patients treated with sirolimus from a single center: an analysis of sirolimus-related complications. Transplantation. 2004;78:264–268. doi: 10.1097/01.tp.0000128628.31556.b1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


