SUMMARY
Our understanding of how stem cells are regulated to maintain appropriate tissue size and architecture is incomplete. We show that Yap is required for the actual maintenance of an adult mammalian stem cell. Without Yap, adult airway basal stem cells are lost through their unrestrained differentiation, resulting in the simplification of a pseudostratified epithelium into a columnar one. Conversely, Yap overexpression increases stem cell self-renewal and blocks terminal differentiation, resulting in epithelial hyperplasia and stratification. Yap overexpression in differentiated secretory cells causes them to partially reprogram and adopt a stem cell-like identity. In contrast, Yap knockdown prevents the dedifferentiation of secretory cells into stem cells. We then show that Yap functionally interacts with p63, the cardinal transcription factor associated with myriad epithelial basal stem cells. In aggregate, we show that Yap regulates all of the cardinal behaviors of airway epithelial stem cells and in so doing determines epithelial architecture.
INTRODUCTION
How adult tissues maintain their proper size and architecture is poorly understood. Here we explore how the regulation of adult stem cells is linked to epithelial architecture using the airway epithelium as a model system. Epithelial tissues are generally classified as simple, pseudostratified, or stratified. The murine tracheobronchial airway epithelium represents a model pseudostratified epithelium intermediate between that of a simple single-layered epithelium and a multi-layered stratified epithelium. Airway basal stem cells directly and broadly abut the basement membrane. In contrast, differentiated suprabasal secretory and ciliated cells have smaller zones of contact with the basement membrane and possess extensive luminal surfaces with their nuclei displaced towards the lumen. This arrangement of cells essentially creates a two-layered epithelium (Morrisey and Hogan, 2010; Rock et al., 2009). Theoretically, disturbances in the regulation of basal stem cells could, on the one hand, lead to a hypertrophic epithelium characterized by basal stem cell excess and stratified squamous metaplasia, as is frequently observed in conditions like chronic obstructive pulmonary disease. Conversely, decreased stem cell numbers would be predicted to result in epithelial hypoplasia, which is thought to play a role in conditions like bronchiolitis obliterans and airway fibrosis (O'Koren et al., 2013; Rock et al., 2010). Thus, tightly controlled mechanisms to regulate basal stem cell maintenance, proliferation and differentiation must exist in order to properly police epithelial size and architecture.
Yap (Yes associated protein 1) is a transcriptional coactivator in the conserved Hippo kinase cascade that has been shown to be involved in growth control as well as the regulation of stem and progenitors cells (Barry and Camargo, 2013; Halder and Johnson, 2011; Pan, 2007, 2010; Ramos and Camargo, 2012; Zhao et al., 2011). In epithelia, Yap modulation has diverse consequences on stem and progenitor cell behaviors (Ramos and Camargo, 2012; Zhao et al., 2011). In the embryonic neuroepithelium, Yap loss leads to decreased progenitor cell survival (Cao et al., 2008), whereas in the embryonic epidermis, Yap loss leads to decreased progenitor cell proliferation (Schlegelmilch et al., 2011). In contrast, Yap activation leads to the same phenotype in both of these tissues, namely increased progenitor and stem cell replication (Cao et al., 2008; Schlegelmilch et al., 2011; Zhang et al., 2011). Unexpectedly, Yap loss throughout the intestinal epithelium results in no obvious phenotype but causes hyperplasia and increased stem cell replication after injury (Barry et al., 2013). Surprisingly, Yap overexpression leads to a loss rather than a gain of intestinal stem cells (Barry et al., 2013). Thus, Yap acts in a tissue, cell, and context dependent manner, even within epithelia.
Here we use the airway epithelia to reveal that Yap, in concert with the cardinal basal stem cell transcription factor p63, participates in the maintenance of an adult stem cell and the regulation of stem cell identity itself. Furthermore, we demonstrate that stem cell behaviors including self-renewal and differentiation can be modulated by Yap to result in predictable alterations in epithelial architecture and size. These findings suggest that alterations in Yap activity may be involved in those diseases of the airways associated with alterations in epithelial architecture, such as pre-malignant squamous metaplasia.
RESULTS
Yap Is Required for the Maintenance of Adult Airway Basal Stem Cells and Yap Loss Results in the Simplification of a Pseudostratified Epithelium into a Columnar Epithelium
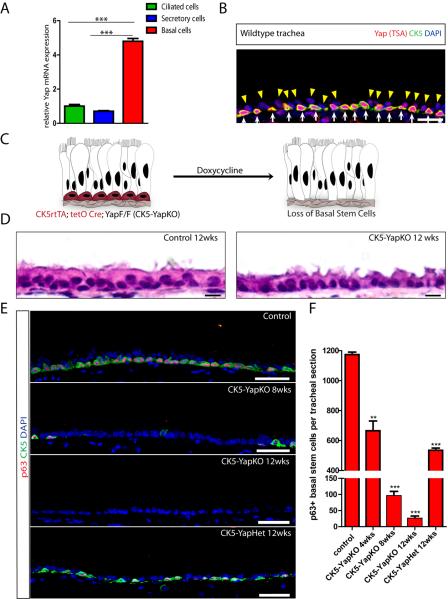
We defined the expression pattern of Yap in the three different cell types of the adult airway epithelium. Basal, secretory, and ciliated cells were sorted based upon GSIβ4, SSEA1 and CD24 surface expression respectively (Figure S1A). We verified the cell type-specific exclusive expression of mRNAs in each sorted cell population (Figure S1B). Yap mRNA was expressed at higher levels in basal stem cells than in secretory and ciliated cells (Figure 1A). We used three different Yap antibodies to establish cell type-specific Yap protein expression patterns using Tyramide Signal Amplification (TSA). Staining demonstrated that Yap protein is expressed most highly in basal stem cells (Figure 1B). The nuclear localization of Yap in basal stem cells (n=2893) suggests that Yap is actively performing its function as a transcriptional co-activator in these stem cells (Halder and Johnson, 2011; Pan, 2010; Zhao et al., 2011).
Figure 1.
Yap Is Required for the Maintenance of Adult Airway Basal Stem Cells and Yap Loss Results in the Simplification of a Pseudostratified Epithelium into a Columnar Epithelium
(A) Expression of Yap mRNA in basal and secretory cells relative to that in ciliated cells. (B) Immunostaining for Yap (red) and the basal stem cell marker Cytokeratin 5 (CK5, green). Yap protein is highly enriched in the nuclei of basal stem cells (white arrows) as compared to differentiated cells (yellow arrowheads). (C) A schematic of the strategy and phenotypic outcome of stem cell-specific Yap deletion. (D) Hematoxylin and eosin (H&E) staining of tracheal sections from control (left panel) and CK5-YapKO (right panel) animals. Control animals bear only the CK5 rtTA and tetO Cre alleles. (E) Immunofluorescence analysis of basal stem cell markers p63 (red) and CK5 (green) in control, CK5-YapKO and CK5-YapHet tracheal sections after doxycycline treatment. (F) Quantification of basal stem cells marked by p63 in control, CK5-YapKO and CK5-YapHet tracheal sections. Data are represented as mean +/− SEM. n=3 for each genotype per each time point. DAPI is in blue. Scale bar represents 10 μm in B and E, and 5 μm in D. See also Figure S1.
To determine the effect of Yap removal specifically from CK5 positive (+) airway basal stem cells, we generated triple transgenic mice carrying the Cytokeratin5 (CK5) rtTA (Diamond et al., 2000), tetO Cre and floxed Yap (Schlegelmilch et al., 2011) alleles, referred to as CK5-YapKO throughout the text (Figure 1C). We first verified that Cre recombinase was expressed exclusively in basal stem cells using a YFP reporter (Figure S1C). Then, adult CK5-YapKO animals (age 8–12 weeks) were treated with both inhaled doxycycline (Tata et al., 2013b) and doxycycline in their drinking water to induce efficient Yap deletion. Yap deletion was verified by quantitative RT-PCR analysis performed on sorted basal stem cells one month after doxycycline administration (Figure S1D). Histology of tracheal sections revealed that the normally pseudostratified airway epithelium was simplified into a columnar epithelium three months after doxycycline treatment (Figure 1D). Following Yap loss, we found a significant decrease in the total number of airway basal stem cells as demonstrated by a loss of cells that express CK5 and p63 (Figure 1E). In control animals, 1174±16 basal stem cells marked by p63 were counted in standardized tracheal sections covering 12 cartilaginous rings. Following doxycycline treatment in the CK5-YapKO animals, the number of p63+ basal stem cells per section dropped to 666±65 in one month (p=0.0017), to 96±14 (p<0.0001) after two months, and to 25±8 (p<0.0001) after three months (Figure 1E and 1F). These results were further confirmed using the basal stem cell-specific markers NGFR and T1α (Figure S1E). Additionally, there was a significant decrease in the total number of basal stem cells in transgenic animals in which only a single copy of Yap was removed (referred to as CK5-YapHet) (Figure 1E). As compared to the controls above, we found only 535±15 basal stem cells marked by p63 per section (p<0.0001) in the trachea of CK5-YapHet animals three months after doxycycline treatment (Figure 1F). In summary, we found that basal stem cell-specific deletion of Yap led to a dose-dependent loss of stem cells, and resulted in a corresponding simplification of airway epithelial architecture.
Basal Stem Cells Undergo Unrestrained Differentiation Following Yap Loss
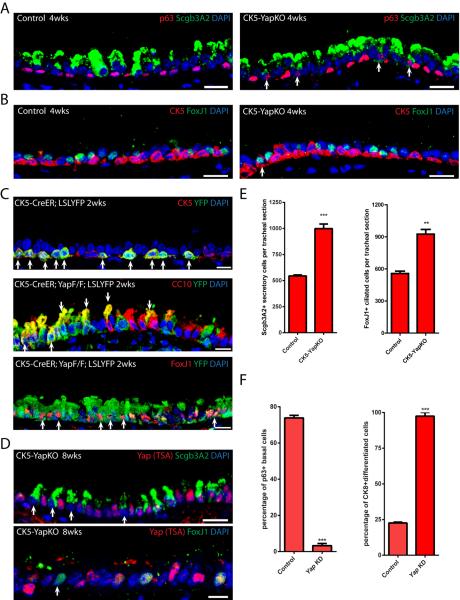
We hypothesized that the loss of basal stem cells following Yap inactivation could have occurred as a result of their death, decreased replication, or differentiation. We found no detectable changes in apoptosis as assessed by activated Caspase 3 and TUNEL staining at one, two and four weeks after doxycycline administration (Figure S2A and data not shown). With regard to replication, at homeostasis, cycling basal cells (i.e. positive for both Ki-67 and p63) accounts for 2.52±0.97% of total basal stem cells. The percentage of p63+Ki-67+ cells in CK5-YapKO trachea was unchanged after Yap deletion (2.10±0.81%, 2.94±0.60%, 2.88±1.05% at one, two and three months after Yap deletion, respectively). However, when we assessed markers of differentiation, we noted the appearance of presumptively differentiating stem cells positive for both p63 (basal) and Scgb3A2 (secretory) (Figure 2A and S2B) and cells positive for both CK5 (basal) and FoxJ1 (ciliated) (Figure 2B and S2B) in CK5-YapKO animals. In controls, these markers were mutually exclusive (Figure 2A and 2B). We further demonstrated that such transitional double-positive cells are also present during the course of normal airway epithelial regeneration following sulfur dioxide-induced injury, when stem cells begin to differentiate into suprabasal cells (Figure S2C).
Figure 2.
Basal Stem Cells Undergo Differentiation Following Yap Loss
(A) Expression of p63 (red) and secretory cell marker Scgb3A2 (green) are mutually exclusive in control epithelium. In CK5-YapKO trachea double-positive cells (arrows) are observed four weeks after continuous doxycycline administration. (B) Expression of CK5 (red) and ciliated cell marker FoxJ1 (green) are mutually exclusive in control epithelium. Double-positive cells (arrow) are detected in CK5-YapKO trachea. (C) Lineage tracing of basal stem cells following Yap loss. The YFP reporter (green) is restricted to CK5-expressing (red, top panel) basal stem cells two weeks after tamoxifen induction in control animals carrying the CK5-CreER and LSL-YFP alleles. In the experimental animals carrying the CK5-CreER, YapF/F and LSL-YFP alleles, YFP+ (green) and CC10-expressing (red, middle panel) secretory cells and the YFP+ (green) and FoxJ1-expressing (red, bottom panel) ciliated cells are frequently detected. White arrows point to double-positive cells. (D) Scgb3A2 (green, upper panel) and FoxJ1 (green, lower panel) are expressed in cells in which Yap (red) is absent, demonstrating that basal stem cells that have lost Yap (arrows) go on to terminally differentiate. (E) Quantification of Scgb3A2+ secretory cells and FoxJ1+ ciliated cells per tracheal section reveals a significant increase in differentiated epithelial cell numbers in CK5-YapKO animals. (F) Quantification of the percentage of p63+ cells as a proportion of virally infected GFP+ cells reveals that basal stem cells are lost following Yap knockdown (left panel). The large majority of these GFP+ infected cells differentiate into CK8+ cells (right panel). Data are represented as mean +/− SEM. Scale bar represents 15 μm in Figure A, B and D (top panel), and 10 μm in Figure C and D (bottom panel). See also Figure S2.
To definitively confirm that stem cells were being lost through differentiation following Yap loss, we specifically lineage traced the basal stem cells that had lost Yap. Triple transgenic mice bearing CK5-CreER (Van Keymeulen et al., 2011), floxed Yap and Rosa26-LSL-YFP alleles were generated so that a YFP reporter could be used to label and trace basal stem cells in which Yap had been deleted. Two weeks after tamoxifen induction, the majority of YFP labeled basal stem cells remained CK5+ basal stem cells in control mice (98.75%, n=240 YFP cells counted). However, YFP+ cells that expressed the secretory cell marker CC10 and the ciliated cell marker FoxJ1 were frequently detected in the experimental animals following Yap deletion (Figure 2C). Thus, following Yap loss, basal stem cells underwent unrestrained differentiation. As a further confirmation of these results, we also traced the lineage of basal stem cells in CK5-YapKO mice by identifying cells that completely lacked Yap staining after TSA amplification. When we used an increased antibody concentration for Yap staining, every cell normally shows a strong staining for Yap in control trachea (Figure S2D). Using this highly sensitive immunohistochemical assay for Yap protein expression, we found Yap-deleted cells that expressed secretory (Scgb3A2) and ciliated cell (FoxJ1) markers in CK5-YapKO trachea (Figure 2D). Such cells were never found in control airways. The complete lack of Yap staining in these differentiated cells indicated that they originated from basal stem cells in which Yap had been deleted. These results further confirm that basal stem cells that lose Yap subsequently differentiate. Consistent with this notion, we found that there was a significant increase in secretory and ciliated cells in CK5-YapKO animals (Figure 2E). In controls, we counted 544±10 Scgb3A2-expressing secretory cells per standard tracheal section. This number increased significantly to 998±44 (p<0.001) in CK5-YapKO tracheal sections two months after doxycycline treatment (Figure 2E). Similarly, control tracheal sections contained 557±22 FoxJ1+ ciliated cells per tracheal section, whereas there was a significant increase of this number to 925±44 (p=0.0017) in CK5-YapKO animals two months after doxycycline treatment (Figure 2E). Of note, at three months following doxycycline treatment, there was a significant increase in the proportion of ciliated to secretory cells (data not shown). In sum, since we found no significant changes in basal stem cell proliferation or cell death following Yap deletion, our results demonstrate that basal stem cell loss occurs predominantly through their differentiation.
Finally, we performed ex vivo Yap knockdown experiments using a culture system that supports basal stem cell expansion and differentiation. Sorted basal stem cells in culture were infected with lentiviruses that expressed GFP and short hairpin RNAs targeting Yap. Co-staining for GFP and Yap demonstrated that infected cells lost Yap protein expression, whereas control cells showed robust Yap staining that was detectable even without amplification in culture (Figure S2E). We first examined the effect of Yap knockdown on cell death and cell proliferation. As we found in vivo, there was no discernible difference in apoptosis (as marked by the percentage of activated Caspase 3+ cells within the virally infected and therefore GFP+ cell population) following Yap knockdown (Figure S2F). However, knockdown of Yap did decrease cell proliferation as marked by Ki-67 in GFP+ transfected cells (Figure S2G), although a significant fraction of the infected cells were still proliferating (13.81±1.68%, Figure S2G). Presumably we detected this effect on basal cell replication in vitro and not following Yap loss in vivo since our culture conditions mimic injury and are associated with dramatically increased basal cell replication when compared to the low degree of basal cell replication during homeostasis in vivo. We next sought to determine the effect of Yap knockdown on differentiation. When scrambled virus was used, 73.85±1.43% of infected GFP+ cells continued to express p63, but this percentage dramatically decreased to 3.26±0.70% following Yap knockdown (p<0.0001, Figure 2F and S2H). Although our in vitro platform does not support the terminal differentiation of secretory or ciliated cells, infected GFP+ cells went on to express the early differentiation marker Cytokeratin 8 (CK8) (Rock et al., 2011) (Figure S2H). In the scrambled control, only 22.62±0.64% of the cells differentiated and expressed CK8 at day 9 of culture, whereas this fraction increased to 97.41±2.59% following Yap knockdown (p<0.0001, Figure 2F and S2H). This result further confirms that Yap loss promotes stem cell differentiation.
Yap Overexpression Promotes Stem Cell Proliferation and Inhibits Terminal Differentiation Resulting in Epithelial Hyperplasia and Stratification
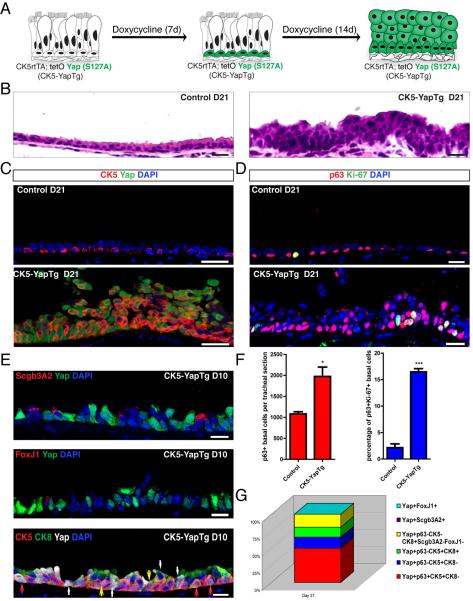
To assess the effect of Yap overexpression on basal stem cells, we generated double transgenic animals carrying both the CK5 rtTA and tetO Yap (S127A) (Camargo et al., 2007) alleles (referred to as CK5-YapTg). In these animals, the expression of constitutively active Yap (S127A) is induced specifically in CK5+ cells (Figure 3A and S3). Siblings carrying the CK5rtTA driver alone were used as controls. It is important to note that although TSA amplification is required to detect endogenous Yap, unamplified Yap staining unambiguously identifies only Yap-overexpressing cells in CK5-YapTg transgenic mice. Using unamplified Yap staining, ectopic Yap expression was detected only in CK5+ basal stem cells 7 days after doxycycline administration (Figure S3). Twenty one days after doxycycline initiation, a thickened and highly stratified airway epithelium was evident (Figure 3B).
Figure 3.
Yap Overexpression Promotes Stem Cell Proliferation and Inhibits Terminal Differentiation Resulting in Epithelial Hyperplasia and Stratification
(A) Schematic representation of the experimental design and phenotypic outcome of Yap overexpression in CK5+ basal stem cells. (B) H & E staining of tracheal sections from CK5-YapTg animals demonstrates epithelial stratification following 21 days of Yap overexpression. (C) CK5 (red) and Yap (green) co-staining demonstrates an increase in CK5+ cells in CK5-YapTg tracheal epithelium 21 days after doxycycline induction. (D) Immunostaining for p63 (red) and Ki-67 (green) reveals an increase in basal stem cells and their proliferation in CK5-YapTg tracheal epithelium 21 days after doxycycline induction. (E) Expression of Scgb3A2 (red, top panel) and FoxJ1 (red, middle panel) is not detected in Yap-overexpressing cells (green) 10 days after doxycycline treatment. Some cells overexpressing Yap (white in lower panel) also co-express CK8 (green), indicating that a preliminary differentiation program has occurred but that terminal differentiation has been blocked. Red arrows point to Yap+CK5+CK8− cells. Yellow arrows point to Yap+CK5+CK8+ cells. White arrows point to Yap+CK5−CK8+ cells. (F) Graphs showing the total number of p63+ cells (left) and the percentage of proliferating basal stem cells (p63+ Ki-67+, right) demonstrate that basal stem cell numbers and their proliferation rates increase after Yap overexpression. (G) A column chart showing the relative abundance of p63+CK5+CK8− basal stem cells (red), p63−CK5+CK8− progenitor cells (blue), p63−CK5+CK8+ progenitor cells (green), p63−CK5−CK8+Scgb3A2−FoxJ1− progenitor cells (yellow), Scgb3A2+ secretory cells (purple) and FoxJ1+ ciliated cells (cyan) in Yap-overexpressing epithelial cells 21 days after doxycycline treatment. Data are represented as mean +/− SEM. Scale bar represents10 μm in B, D and E, and 20 μm in C. See also Figure S3.
We then assessed the effect of Yap overexpression specifically on basal stem cells. Immunohistochemical characterization of CK5 and p63 in the stratified epithelium at day 21 revealed that there was a significant increase in the total number of both CK5+ and p63+ cells (Figure 3C and 3D). As compared to the controls, which contained 1082±53 p63+ basal stem cells per tracheal section, this number increased to 1975±223 (p=0.0178) in the CK5-YapTg mice 21 days after continuous doxycycline administration (Figure 3D and 3F, left graph). We also examined the effect of Yap overexpression on basal stem cell proliferation in CK5-YapTg animals. Following Yap overexpression, the percentage of p63+ basal stem cells that were positive for Ki-67 increased from 1.23±0.40% in the control to 16.47±0.60% (p=0.0001) at day 21 following doxycycline initiation (Figure 3D and 3F, right graph). Thus, Yap overexpression promotes stem cell self-renewal.
We then assessed the effect of Yap overexpression on differentiation. Only 0.26% (n=2667) of Yap-overexpressing cells were positive for the secretory cell marker Scgb3A2 at day 10 after doxycycline administration (Figure 3E, upper panel). At day 21, only a single Scgb3A2+ cell was found out of 1299 Yap-overexpressing cells. With respect to ciliated cell differentiation, only 0.22% (n=1349) of Yap-overexpressing cells were co-labeled with FoxJ1 at day 10 (Figure 3E, middle panel). Not even a single FoxJ1+Yap+ cell was detected at day 21 (n=2097). Therefore, Yap overexpression in CK5-YapTg trachea blocks terminal differentiation.
We also note that Yap overexpression results in an abundance of CK5+p63−(negative) cells not normally present in the homeostatic airway epithelium (Figure 3C and 3D). This suggests the presence of a population of progenitor cells that are not basal stem cells since they are p63−, but which are blocked from executing a terminal differentiation program. Quantification revealed that 81.09% (n=3229) of Yap overexpressing cells were CK5+, but only 50.13% were double-positive for p63 and CK5 and thus bona fide basal stem cells. We then assessed whether the remaining 30.96% of p63−CK5+ Yap-overexpressing cells expressed the luminal progenitor cell marker CK8. Of the total 30.96% p63−CK5+ progenitor cells, 16.30% were p63−CK5+CK8− and 14.66% were p63−CK5+CK8+ (Figure 3G). The remaining CK5− Yap overexpressing cells were exclusively CK8+ luminal progenitor cells (Figure 3E, bottom panel and Figure 3G) that did not initiate a terminal differentiation program. These results, in aggregate, suggest the presence of a group of stem cell-derived progenitor cells in varying states of differentiation, which were prevented from completing terminal differentiation (Figure 3G). Of note, these progenitors (p63−CK5+CK8−, p63−CK5+CK8+, p63−CK5−CK8+ cells), are exceedingly rare in the homeostatic tracheal epithelium, but they do occur as orderly transient intermediates in regenerating airway epithelium (Rock et al., 2011).
Normalizing Yap Expression Restores Pseudostratified Epithelial Architecture and a Normally Sized Basal Stem Cell Pool
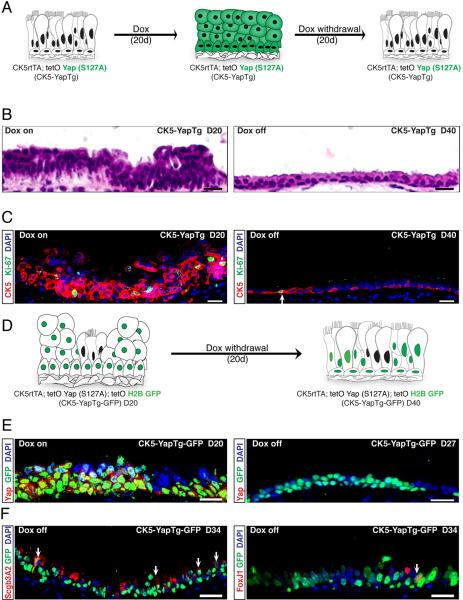
To determine whether the maintenance of Yap-induced stem cell proliferation and epithelial stratification requires persistent Yap overexpression, we first administered doxycycline to experimental CK5-YapTg animals for 20 days and then withdrew doxycycline for 20 days. At the end of the chase period, we observed that the airway epithelium was now morphologically normal (Figure 4A and 4B). There was no significant difference between control and CK5−YapTg animals in the total number of CK5+ basal stem cells per section (Figure 4C and S4A). Additionally, the proportion of CK5+ cells per total cells present in the epithelium and the percentages of proliferating basal cells marked by both CK5 and Ki-67 were not statistically different from those in control animals (Figure 4C, S4B and S4C). Thus, persistent Yap overexpression is required to maintain excess stem cell proliferation and epithelial stratification.
Figure 4.
Normalizing Yap Expression Restores Pseudostratified Epithelial Architecture and a Normally Sized Basal Stem Cell Pool
(A) A schematic of the experimental strategy and phenotypic result of an experiment designed to test whether normalizing Yap signaling in a stratified epithelium restores normal basal stem cell numbers and pseudostratified epithelial architecture. (B) H&E staining reveals the restoration of a normal pseudostratified epithelium after the discontinuation of doxycycline-induced Yap overexpression. (C) Immunostaining for CK5+ basal stem cells (red) and Ki-67 (green) reveals a normalization of stem cell numbers and proliferation rates following doxycycline withdrawal. The arrow points to a basal stem cell that is double-positive for CK5 and Ki-67. (D) Experimental strategy for using long-lived H2BGFP expression as a lineage tracing tool for tracing the behavior of stem and progenitor cells that have previously overexpressed Yap. (E) Expression of GFP (green) is detected in the majority of the cells that overexpress Yap (red, left panel) following doxycycline treatment. Ectopic Yap expression is no longer detected following one week of doxycycline withdrawal whereas strong GFP lineage label perdures (green, right panel). (F) Scgb3A2 (red, left panel) and FoxJ1 (red, right panel) occur in H2BGFP+ (green) cells two weeks after doxycycline removal (arrows point to double-positive cells). Scale bar = 20 μm in B, C, E and F. See also Figure S4.
We next examined the effect of Yap normalization on terminal differentiation using lineage analysis. Triple transgenic mice possessing CK5 rtTA, tetO Yap (S127A), and tetO histone 2B GFP (H2BGFP) alleles (referred to as CK5-YapTg-GFP) were generated. In these animals, the expression of the active Yap allele and the H2BGFP reporter is induced in concert, under the control of the CK5rtTA driver. Since the H2BGFP protein has a very long half-life, GFP labeling persists in the descendants of basal stem cells. This perdurance of the GFP label allows the use of GFP expression as a lineage-tracing tool to identify cells that had once overexpressed Yap (Figure 4D). Following 20 days of doxycycline administration, 98.56% (n=761) of GFP+ cells overexpressed Yap protein (Figure 4E, left panel). Although the GFP lineage label persisted two weeks after doxycycline withdrawal, ectopic Yap expression was lost within a week (Figure 4E, right panel), confirming that persistent H2BGFP can be used as an effective lineage tracing tool for marking cells that previously overexpressed Yap. Two weeks after doxycycline withdrawal, GFP+ lineage-traced stem cell progeny included cells positive for mature secretory (Scgb3A2) and ciliated (FoxJ1) cell markers (Figure 4F). Thus, stem and progenitor cells that previously expressed ectopic Yap underwent terminal differentiation after Yap withdrawal. We further confirmed this result with stains for CC10 (secretory cells) and acetylated tubulin (A-tub, ciliated cells) to ensure that differentiated cells expressed a full panel of markers associated with terminal differentiation (Figure S4D). Additionally, immunostaining revealed normally positioned and shaped CK5+ basal stem cells, CC10+ secretory cells and A-tub+ ciliated cells in an architecturally normal pseudostratified epithelium 20 days after doxycycline withdrawal (Figure S4E). Therefore, when Yap expression was normalized, a normally sized epithelium was restored with a normally sized basal stem cell pool and the epithelium was characterized by a proper pseudostratified architecture.
The Overexpression of Yap in Secretory Cells Causes Them to Adopt a Basal-like Program
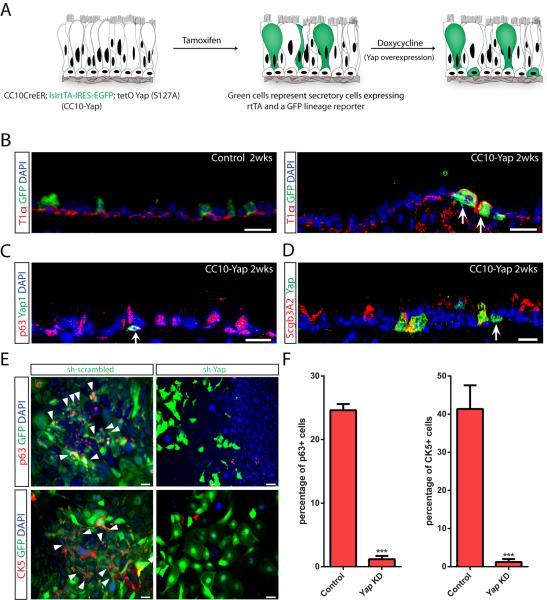
Thus far, we have demonstrated that Yap is required for the maintenance of adult basal stem cells and that Yap overexpression promotes stem cell proliferation at the expense of terminal differentiation. These findings suggest the possibility that Yap overexpression in a differentiated cell could promote a stem cell-like program. To test this hypothesis, we overexpressed Yap in secretory cells. We generated triple transgenic mice carrying the CC10-CreER (Rawlins et al., 2009), Rosa26-LSL-rtTA-IRES-GFP, and tetO Yap (S127A) alleles (referred to as CC10-Yap mice). In these mice, CreER protein is expressed exclusively in secretory cells. Upon tamoxifen administration, a multi-cistronic transcript containing rtTA and GFP is permanently induced in these cells and doxycycline administration then induces Yap overexpression specifically in these GFP lineage-tagged secretory cells (Figure 5A).
Figure 5.
The Overexpression of Yap in Secretory Cells Causes Them to Adopt a Basal-like Program
(A) Schematic of the strategy to inducibly overexpress Yap in GFP lineage tagged secretory cells. Following Yap induction, some GFP tagged secretory cells adopt a basal stem cell-like identity. (B) Co-staining of the secretory cell lineage tag GFP with the basal stem cell marker T1α (red) shows that GFP is associated with normal columnar cells and not basal stem cells in control animals (left panel). In experimental animals, GFP+ T1α+ basal-like cells derived from Yap-overexpressing secretory cells are observed (right panel, arrows). (C) A pyramidal basal-like cell expressing p63 (red) and ectopic Yap (green) reveals that the stem-like cell was derived from a Yap-overexpressing secretory cell (arrow). (D) Expression of Scgb3A2 (red) is lost in pyramidal Yap-overexpressing (green) secretory-derived cells (arrow). (E) Yap knockdown using GFP-expressing lentivirus prevents the dedifferentiation of secretory cells into basal stem cell as demonstrated by the absence of p63 (red, top panels) and CK5 (red, bottom panels) staining in GFP+ infected cells as opposed to controls. Arrowheads point to GFP+ cells infected with control viruses that do dedifferentiate into p63+ and CK5+ basal stem cells. (F) Quantification of p63+ and CK5+ basal stem cells as a fraction of all GFP+ infected cells in scrambled control and Yap knockdown secretory cell cultures reveals that inhibition of Yap prevents secretory cell dedifferentiation into stem cells. Data are represented as mean +/− SEM. Scale bar represents 20 μm in B, C, D and 100 μm in E. See also Figure S5.
Tamoxifen was administered to adult CC10-Yap transgenic mice (8–10 week old) and then followed by doxycycline induction. FACS analysis demonstrated that GFP+ cells accounted for 3.94% of the total epithelial cells (Figure S5A). In control animals possessing the CC10-CreER and Rosa26-LSL-rtTA-IRES-GFP alleles, GFP expression was appropriately restricted to columnar secretory cells (Figure 5B, left panel) and entirely absent in basal stem cells. In experimental animals, some GFP+ cells exhibited the characteristic pyramidal morphology of basal stem cells and expressed the basal stem cell-specific marker T1α (Figure 5B, right panel). These pyramidal cells established a broad base in direct contact with the basement membrane and stained for ectopic Yap, as assessed by Yap staining without amplification (Figure S5B). Furthermore, the pyramidal GFP+ Yap-overexpressing cells also expressed the basal stem cell-specific marker p63 (Figure 5C). Of all GFP+ cells, we note that only 79.30% continued to express Scgb3A2 (Figure 5D) and 8.06% became p63+ basal-like cells (n=589). Thus, in addition to adopting a basal-like program, secretory cells that had once overexpressed Yap also suppressed elements of their secretory cell program.
To test whether the secretory cell-derived partially reprogrammed basal-like cells persisted following the normalization of Yap expression, we administered doxycycline to CC10-Yap mice for two weeks and then withdrew doxycycline for three weeks. After this period, there were no GFP+ basal-like cells (n=561). Thus, persistent overexpression of Yap was required to maintain the partially reprogrammed basal-like state that is transiently induced by Yap overexpression in secretory cells.
The Inhibition of Yap Blocks the Dedifferentiation of Secretory Cells into Basal Stem Cells
We recently demonstrated that secretory cells cultured ex vivo dedifferentiate into p63+ CK5+ basal stem cells (Tata et al., 2013a). To further examine the role of Yap in regulating stem cell identity, we performed Yap lentiviral knockdown experiments in secretory cell cultures to assess whether Yap loss alters the propensity of a secretory cell to dedifferentiate. In contrast to controls, secretory cells infected with a Yap knockdown lentivirus failed to dedifferentiate into stem cells and did not activate p63 expression 9 days after plating (Figure 5E and 5F). Among the GFP+ cells infected with scrambled virus, p63+ basal stem cells were easily detected and accounted for 24.61±0.96% of all GFP+ cells. Among the GFP+ cells infected with Yap virus, p63+ cells decreased to only 1.18±0.51% of the total population (p<0.0001, Figure 5F). We confirmed the finding with another basal stem cell marker CK5 (Figure 5E and 5F). In cells infected with the scrambled control virus, 41.33±6.2% of GFP-expressing cells were CK5+ 9 days after culture. This number dramatically decreased to 1.23±0.79% (p<0.0001) when Yap was knocked down (Figure 5E and 5F). These results suggest that Yap is required for the dedifferentiation of secretory cells and their adoption of a stem cell program.
Yap and p63 Interact in Basal Stem Cells and Regulate Common Target Genes
Since the transcription factor p63 is the most well established regulator of basal stem cells (Koster et al., 2004; Romano et al., 2012; Su et al., 2013), we sought to determine whether there was a direct mechanistic interaction between p63 and Yap. Although p63 has previously been shown to interact with Yap in cell lines (Chatterjee et al., 2010; Strano et al., 2001; Yuan et al., 2010), the functional relevance of such an interaction has not been established in vivo.
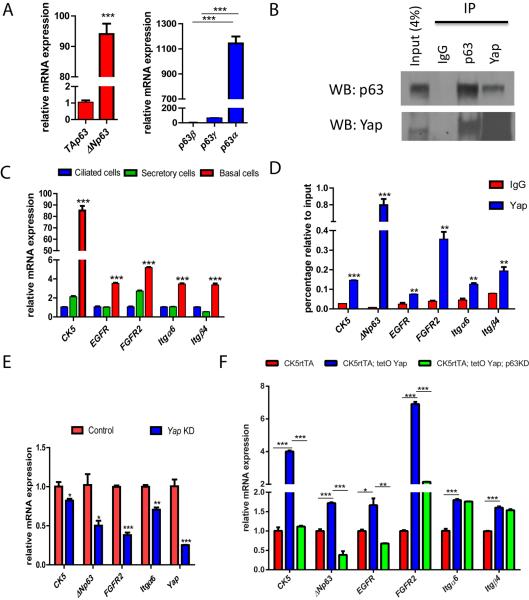
p63 has two isoforms, TA and delta N (ΔN), each of which has three splice variants: α, β, and γ (Yang et al., 1998). Quantitative PCR revealed that TAp63 splice variants were expressed at very low levels in basal stem cells, and that ΔNp63α was by far the most abundant transcript in basal cells in vivo and in cultured basal cells (Figure 6A and S6A). Staining with two ΔNp63-specific antibodies showed that the expression of ΔNp63 was exclusively restricted to the basal stem cell population (Figure S6B). We then verified the previously reported interaction of Yap and ΔNp63α in HEK cells using Myc-tagged Yap and Flag-tagged ΔNp63α (Figure S6C). To extend these finding to our model system, we performed reciprocal immunoprecipitations using primary human basal stem cells and found that Yap and p63 physically interact with one another specifically in airway epithelial basal stem cells (Figure 6B). Of note, the size of the precipitated p63 in the Yap pulldown demonstrates that the Yap partner is ΔNp63.
Figure 6.
Yap and p63 Interact in Basal Stem Cells and Regulate Common Target Genes
(A) Quantitative-PCR analysis demonstrates that ΔNp63α is the major p63 transcript expressed in sorted basal stem cells. (B) Immunoprecipitation demonstrates that Yap interacts with p63 in human basal stem cells. (C) Quantitative-PCR analysis of p63 target genes demonstrates that these targets are more enriched in basal stem cells. (D) Chromatin immunoprecipitation demonstrates that Yap binds to the promoters of p63 target genes. (E) Decrease in mRNA expression of p63 target genes following Yap knockdown. (F) Gene expression analysis shows an increase in mRNA expression of p63 target genes following Yap overexpression, but this effect is abolished when p63 is knocked down. Data are represented as mean +/− SEM. See also Figure S6.
We then examined whether Yap modulation had an effect on the transcription of p63 target genes in mouse basal stem cells. In other contexts, p63 has been shown to regulate the transcription of CK5, EGFR, FGFR2, Integrin alpha 6 (Igtα6), Integrin β4 (Igtβ4) and p63 itself (Carroll et al., 2006; McDade et al., 2012; Romano et al., 2009). All of these p63 target genes were more enriched in basal stem cells relative to secretory and ciliated cells (Figure 6C). We verified that p63 binds upstream of the transcriptional start sites of these genes by ChIP-PCR (Figure S6D). Additionally, ΔNp63 knockdown in mouse basal stem cell cultures led to a decrease in the expression of these target genes after 6 days (Figure S6E, S6F, S6G and S6H). Using ChIP-PCR analysis, we found that Yap also binds upstream of the transcriptional start sites of the aforementioned p63 target genes (Figure 6D). Furthermore, Yap knockdown resulted in a decrease in the expression of p63 target genes (Figure 6E). Conversely, the transcription of all p63 target genes was significantly increased 12 hours after doxycycline was administered to induce the expression of Yap in sorted basal stem cells from CK5-YapTg trachea, as compared to those from CK5rtTA controls (Figure 6F). To directly test whether the transcriptional effect caused by Yap overexpression was mediated by p63, we knocked down p63 while overexpressing Yap. We harvested and analyzed these cells after 6 days of culture, since at this time point the majority of infected cells remained p63+CK5+ basal stem cells, although p63 expression was decreased given the knockdown (Figure S6E). We found that increases in the transcription of p63 target genes in Yap-overexpressing basal cells were significantly suppressed when p63 was inhibited (Figure 6F). In aggregate, these data demonstrate that Yap and p63 interact in basal stem cells to regulate common target genes.
p63 and Yap Genetically Interact
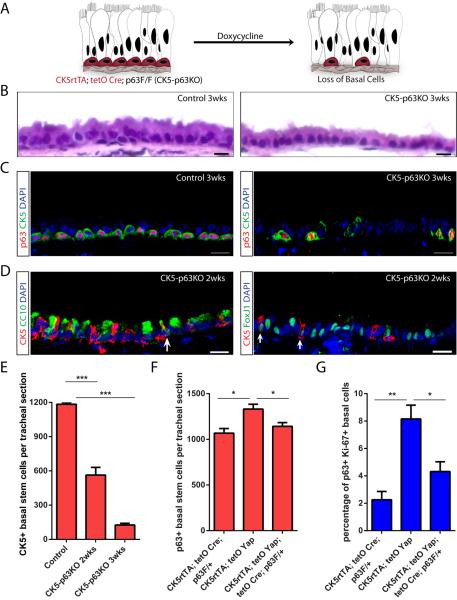
Finally, we sought to establish whether genetic and phenotypic data could confirm an in vivo relevance for our biochemical data demonstrating an interaction of Yap and p63. Specifically, we asked whether p63 loss would phenocopy Yap loss. Transgenic animals carrying the CK5 rtTA, tetO Cre and floxed p63 alleles (Mills et al., 2002) (referred to as CK5-p63KO) were generated, and we hypothesized that stem cell loss would occur following p63 deletion in analogy to the phenotype associated with Yap deletion (Figure 7A). Using animals bearing only the CK5 rtTA and tetO Cre alleles as controls, our histological analysis revealed that p63 deletion resulted in a simplification of the normal pseudostratified epithelium into a simple columnar epithelium three weeks after doxycycline treatment (Figure 7B). This was accompanied by a significant decrease in the total number of p63+ cells (Figure 7C), demonstrating efficient deletion of p63. A loss of CK5+ and T1α+ cells (Figure 7C and S7A) confirmed the loss of basal stem cells in CK5-p63KO animals. Controls had 1184±15 CK5 expressing cells per standard tracheal section. After doxycycline treatment, this number decreased to 563±67 (p<0.001) in two weeks and to 126±15 (p<0.0001) in three weeks (Figure 7E). Although we functionally demonstrated that ΔNp63 was the predominant isoform of p63 in basal stem cells, to further verify this point, we analyzed the effect of TAp63 loss on the tracheal epithelium of TAp63 null animals (Su et al., 2009). No changes in the histology of the tracheal epithelium and the expression of basal stem cell markers including ΔNp63 and CK5 were found in the mutant TAp63 trachea (Figure S7B and S7C). Furthermore, quantification of CK5+ and ΔNp63+ cells in the mutant and control trachea revealed no significant differences in the number of basal stem cells per standard section (Figure S7D), indicating that TAp63 is dispensable for the maintenance of basal stem cells in the trachea during homeostasis. These data together demonstrate that p63, specifically ΔNp63, is required for the maintenance of an adult airway basal stem cell.
Figure 7.
p63 and Yap Genetically Interact
(A) A schematic of the strategy for basal stem cell-specific p63 deletion. It is hypothesized that p63 deletion results in a loss of basal stem cells (red) mirroring Yap deletion. (B) H&E staining of tracheal sections from control (left panel) and experimental CK5-p63KO (right panel) animals reveals a simplification of a normally pseudostratified epithelium into a columnar epithelium following p63 deletion. Controls animals bear only the CK5 rtTA and tetO Cre alleles. (C) Immunostaining for p63 (red) and CK5 (green) reveals a significant decrease in total basal stem cell numbers in CK5-p63KO tracheal sections as compared to that in control mice possessing only the CK5 rtTA and tetO Cre alleles. (D) Detection of transitional cells double-positive for CK5 (red) and CC10 (green) (left panel) as well as CK5 (red) and FoxJ1 (green) (right panel) in CK5-p63KO trachea two weeks after doxycycline administration. Arrows point to the double-positive cells. (E) Quantification of CK5+ basal stem cells per standard tracheal section following p63 loss. (F) Quantification of p63+ cell numbers reveals that a loss of one copy of p63 significantly suppresses the increase in basal stem cells numbers caused by Yap overexpression. (G) Quantification of p63+ Ki-67+ cells reveals that the loss of one copy of p63 significantly suppresses the increased basal stem cell proliferation caused by Yap overexpression. Data are represented as mean +/− SEM. Scale bar represents 5 μm in B, and10 μm in C and D. See also Figure S7.
We next sought to determine whether basal stem cells underwent differentiation following p63 loss by assessing whether transitional cells expressing both basal and differentiated cell markers were induced. Indeed, a significant increase in cells double-positive for CK5 and CC10 or CK5 and FoxJ1 occurred (Figure 7D), suggesting that basal stem cells were differentiating into both secretory and ciliated cells in the absence of p63. Similarly, p63 knockdown in basal stem cell cultures at day 10 decreased the number of cells that were positive for the basal stem cell marker CK5 (Figure S7E, upper panel) and increased the number of cells expressing the early differentiation marker CK8 (Figure S7E, bottom panel). Taken together, these results demonstrate that p63, like Yap, is required for the maintenance of adult basal stem cells.
Finally, we sought to determine whether Yap and p63 genetically interact. To do this, we examined whether the removal of one copy of p63 could suppress the phenotype generated by Yap overexpression. Transgenic animals bearing CK5rtTA, tetO Yap (S127A), tetO Cre and a single floxed p63 allele were generated. Siblings carrying CK5rtTA and tetO Yap (S127A) as well as animals with CK5rtTA, tetO Cre and floxed p63 were used as controls. All animals were given doxycycline continuously for 10 days. There was no obvious effect on basal stem cell maintenance or airway epithelial architecture when one copy of p63 was deleted at homeostasis (Figure S7F, upper panel). As we observed previously, epithelial stratification was induced when Yap was activated in CK5-YapTg animals (Figure S7F, middle panel). Epithelial stratification was suppressed when one copy of p63 was removed (Figure S7F, bottom panel). Similarly, the increases in basal stem cell numbers and their proliferation rates following Yap activation were significantly lessened when one copy of p63 was removed (Figure 7F and 7G). Ten days after doxycycline treatment, 1331±54 basal stem cells marked by p63 per tracheal section were detected following Yap overexpression. This number significantly decreased to 1143±41 (p=0.049) when one copy of p63 was removed (Figure 7F). With regard to basal stem cell proliferation, 8.16±1.00% of p63+ cells were also Ki-67+ when Yap was overexpressed. This number significantly decreased to 4.32±0.71% (p=0.036) when one copy of p63 was removed (Figure 7G). These data suggest that the effects of Yap overexpression are mediated by p63 and that the two factors genetically interact in the airway epithelium.
DISCUSSION
We demonstrate that the regulation of basal stem cell behavior is intimately associated with epithelial size and architecture in the adult airway epithelium. On the one hand, basal stem cell loss results in the simplification of a pseudostratified airway epithelium into a columnar one. Conversely, Yap overexpression increases stem cell proliferation, resulting in epithelial hyperplasia and stratification. Although one might assume that epithelial size and stratification are largely governed by the increased replicative activity of a stem cell, we note that suprabasal progenitor cells (not normally present in the homeostatic airway epithelium) also divide, just as they do in embryonic epidermis (Lechler and Fuchs, 2005). Indeed, after airway injury, stratification is similarly accompanied by replication in both a stem cell and a newly revealed progenitor cell compartment. The nature of the cell divisions (i.e. symmetric versus asymmetric) occurring in these two compartments are not understood and are possibly distinct. Interestingly, in other instances of epithelial stratification such as in the human larynx or esophagus, parabasal cells are the dominant replicating cell population, and the putative basally located stem cells are more quiescent (data not shown). Thus, stem and progenitor cell behaviors may be differentially regulated to produce alternative paths to epithelial stratification. Of note, pre-malignant airway squamous metaplasia is also associated with both stem and progenitor cell replication and excess (Rock et al., 2010) and our Yap overexpression system may serve to model some aspects of this pathology.
The size of a steady state stem cell pool is dictated by balanced rates of stem cell self-renewal, stem cell survival, and stem cell differentiation. Previous reports have largely focused on the role of Yap in promoting stem cell and progenitor cell replication and survival (Barry et al., 2013; Cai et al., 2010; Cao et al., 2008; Schlegelmilch et al., 2011). In contrast, our loss-of-function study points to a requirement for Yap in restraining stem cell differentiation as a mechanism to ensure the maintenance of stem cells during normal epithelial homeostasis. Indeed, we also demonstrated a dose-dependent effect of Yap on the maintenance of basal stem cells, suggesting that the tight control of Yap levels is critical to maintain a normally sized basal stem cell pool. The notion of a set point for the airway stem cell pool contingent on particular levels of Yap expression is further evidenced by the return of a normal stem cell pool size when Yap expression is normalized after its overexpression.
Given that Yap overexpression promotes basal stem cell proliferation, it will be of interest to determine whether Yap levels are transiently increased following airway epithelial injury repair since the early stratification that accompanies regeneration is also associated with an increased rate of basal cell proliferation. This notion would be consistent with our finding that Yap knockdown inhibits stem cell proliferation in vitro, as dissociation and culture of basal cells induces a proliferative response similar to that encountered after airway injury. Analogously, the differentiation associated with late epithelial repair might be associated with a tuning down of Yap levels.
Furthermore, we revealed a role for Yap in regulating stem cell identity per se. Previously, we demonstrated that secretory cells can stably dedifferentiate into stem cells (Tata et al., 2013a). We now show that Yap loss prevents this dedifferentiation. Conversely, Yap overexpression in secretory cells promotes a stem cell-like identity and concomitantly suppresses the secretory cell program. Interestingly, persistent Yap overexpression is required to maintain the stem cell-like identity despite the induction of the basal stem cell transcription factor p63. Thus, the induction of p63 in secretory cells is insufficient to stably reprogram differentiated cells into stem cells within the period of time we assessed. We note that following Yap overexpression in secretory cells, there are more cells that suppress their secretory cell identity than cells that adopt a basal cell-like program, implying the existence of transitional progenitor cells. Similarly, Yap overexpression from basal stem cells produces more basal cells, but Yap overexpression is also associated with an excess of intermediate progenitor cells, again suggesting that Yap alone cannot enforce a basal stem cell identity in which differentiation is fully blocked. It will be of considerable interest to determine why Yap overexpression partially reprograms secretory cells, while stem cell ablation induces a permanent conversion to a stem cell state. Although in aggregate very high levels of Yap expression seem to drive cells towards a stem cell-like identity, the low levels of Yap detected in secretory cells, as well as ciliated cells, of the homeostatic epithelium may be of some functional relevance that we have not yet assessed.
The interaction of the Yap transcriptional coactivator with TEAD partners has been well established to have an in vivo physiologic relevance (Yu and Guan, 2013). Other partner transcription factors have been shown to interact with Yap in cultured cells, including Smad 1 (Alarcon et al., 2009), Smad 7 (Ferrigno et al., 2002), RUNX 1/2 (Yagi et al., 1999), ErbB4 (Komuro et al., 2003), p73, and p63 (Strano et al., 2001). In this study, we have now demonstrated that a WW domain (Yap)-PPxY (p63) interaction is physiologically relevant in an in vivo setting (Chen and Sudol, 1995; Sudol and Harvey, 2010). Previously, it had been shown that p63 null mutants die at birth without embryonic basal stem cell progenitors in their airway epithelium (Daniely et al., 2004), either because basal stem cells were never specified or because they could not be maintained after specification. We now show that p63, like Yap, is required for the actual maintenance of adult airway basal stem cells. In addition, both Yap loss and p63 loss are ultimately associated with an excess of ciliated cell numbers relative to secretory cell numbers. Furthermore, p63 overexpression had been shown to promote epithelial stratification in the murine lung (Koster et al., 2004; Romano et al., 2009). In aggregate, this suggests a functionally relevant role for an interaction of Yap with a new transcription factor partner. This interaction may be relevant in the myriad epithelia that contain p63+ basal cells. Indeed, prior work in the epidermis reveals a role for both p63 and Yap in epidermal basal stem cell proliferation and epithelial stratification (Koster et al., 2004; Romano et al., 2012; Schlegelmilch et al., 2011; Zhang et al., 2011). Finally, since Yap and p63 both have roles as tumor suppressors and oncogenes (Overholtzer et al., 2006; Pan, 2010; Su et al., 2013), our results have potential implications for understanding tumorigenesis.
EXPERIMENTAL PROCEDURES
Detailed protocols are described in Extended Experimental Procedures in the Supplemental Information.
Animal Models
The CK5 rtTA (Diamond et al., 2000), CK5-CreER (Van Keymeulen et al., 2011), CC10-CreER (Rawlins et al., 2009), tetO Yap (S127A) (Camargo et al., 2007), floxed Yap (Schlegelmilch et al., 2011), floxed p63 (Mills et al., 2002) and TAp63 knockout (Su et al., 2009) mice have been previously described. Transgenic mice bearing tetO Cre (006234), tetO H2BGFP (005104), Rosa26-LSL-YFP (006148) and Rosa26-LSL-rtTA-IRES-GFP (005670) were purchased from Jackson laboratory (Bar Harbor, ME). Doxycycline was administered by drinking water (1 g/L) and inhalation (Tata et al., 2013b). Tamoxifen (2mg/20g body weight) or corn oil was injected intraperitoneally for three consecutive days in transgenic animals carrying CK5-CreER, floxed Yap and Rosa-LSL-YFP and their respective control animals, and for five consecutive days in CC10-Yap animals and their respective controls. Mice were euthanized using CO2. All animal work was approved by the committee on Research Animal Care of the Massachusetts General Hospital according to federal and institutional policies and regulations.
Cell Dissociation and Sorting of Different Fractions of Epithelial Cells
Mouse tracheas were dissected, minced manually, and then incubated in Papain dissociation solution. Cells were sorted using a FACS Aria flow cytometer (BD, San Jose, CA) or a Vantage Cell Sorter (BD, San Jose, CA). Epithelial cells were gated as EpCAM+ cells. Basal stem cells were sorted as EpCAM+ GSIβ4+ cells (confirmed by staining for p63 after cytospin). Secretory cells were sorted as EpCAM+ SSEA1+ cells (verified by staining with CC10 after cytospin). Ciliated cells were sorted as EpCAM+ CD24+ cells (verification with both Foxj1 and A-tub staining after cytospin).
Yap TSA Amplification
Immunohistochemical staining for Yap protein was performed using a TSA Biotin Tyramide Amplification system (SAT700001EA, Perkin Elmer). The following Yap antibodies were used: mouse anti-Yap (101199, Santa Cruz); mouse anti-Yap (H00010413-M01, Abnova), and rabbit anti-Yap (kindly provided by Joseph Avruch). When used at a 1:500 dilution, immunohistochemical staining in the case of each antibody revealed a strong nuclear signal in basal stem cells as compared to weaker staining in differentiated cells. In order to confirm that cells were Yap-negative following Yap deletion, we deployed antibody staining at a 1:100 dilution so that we could detect even very low levels of Yap protein expression.
Cell Counting
Cells were manually counted based upon immunofluorescent staining for markers of specific cell types in at least three different tracheas with 3–5 representative regions per tracheal section. For the quantification of total basal stem cells, p63+ cells were counted along the full length of the tracheal epithelium extending from cartilage ring 1 to 12 in 3 to 5 tracheas with 2–3 6-μm thick sections counted per trachea.
Co-Immunoprecipitation and Chromatin Immunoprecipitation
Detailed protocols for Co-Immunoprecipitation and Chromatin immunoprecipitation is provided in Extended Experimental Procedures.
Statistical Analysis
Data was analyzed and compared between groups using two tailed, unpaired Student's t-tests (Prism, Graphpad). p-value of less than 0.05 was considered statistically significant and is presented as * p<0.05, **p<0.01, or ***p<0.001.
Supplementary Material
HIGHLIGHTS.
Yap is required for the maintenance of airway basal stem cell identity
Yap overexpression in basal stem cells promotes airway epithelial stratification
Yap overexpression causes differentiated cells to assume a stem cell-like identity
Yap interacts with p63 in basal stem cells and p63 loss phenocopies Yap loss
ACKNOWLEDGMENTS
We thank Adam Glick and Alea Mills for providing the CK5rtTA and p63 floxed mice, Elsa Flores for the TAp63 knockout mice, Brigid Hogan for the CK5-CreER and CC10-CreER mice, Barry Stripp for providing the goat anti-CC10 antibody, Shioko Kimura for the rabbit Scgb3A2 antibody, Satrajit Sinha for the rabbit ΔNp63 antibody and Joseph Avruch for the rabbit Yap antibody. We would like to acknowledge Xu Wu and Jenna Galloway for their comments on the manuscript. We thank Nicole Forster, Karin Schlegelmilch, Laura Prickett-Rice, Kat Folz-Donahue and David Dombkowski for technical assistances. J. R. is a New York Stem Cell Foundation-Robertson Investigator. This study was supported by an NIH-NHLBI Early Career Research New Faculty (P30) award (5P30HL101287-02), a grant from NHLBI Progenitor Cell Biology Consortium (PCBC, 5U01HL099997-04) (to J.R.), an R01 (1R01HL116756-01a) from NIH/NHLBI (to J.R.), an NIDCR R01 DE015945 (to L.W.E. and S.V.S.), and a Harvard Stem Cell Institute (HSCI) Junior Investigator Grant (to J.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION Supplemental information includes Extended Experimental Procedures and seven figures.
REFERENCES
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139:757–769. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25:247–253. doi: 10.1016/j.ceb.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll DK, Carroll JS, Leong CO, Cheng F, Brown M, Mills AA, Brugge JS, Ellisen LW. p63 regulates an adhesion programme and cell survival in epithelial cells. Nat Cell Biol. 2006;8:551–561. doi: 10.1038/ncb1420. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Sen T, Chang X, Sidransky D. Yes-associated protein 1 regulates the stability of DeltaNp63alpha. Cell Cycle. 2010;9:162–167. doi: 10.4161/cc.9.1.10321. [DOI] [PubMed] [Google Scholar]
- Chen HI, Sudol M. The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A. 1995;92:7819–7823. doi: 10.1073/pnas.92.17.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniely Y, Liao G, Dixon D, Linnoila RI, Lori A, Randell SH, Oren M, Jetten AM. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- Diamond I, Owolabi T, Marco M, Lam C, Glick A. Conditional gene expression in the epidermis of transgenic mice using the tetracycline-regulated transactivators tTA and rTA linked to the keratin 5 promoter. J Invest Dermatol. 2000;115:788–794. doi: 10.1046/j.1523-1747.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- Ferrigno O, Lallemand F, Verrecchia F, L'Hoste S, Camonis J, Atfi A, Mauviel A. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879–4884. doi: 10.1038/sj.onc.1205623. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- Koster MI, Kim S, Mills AA, DeMayo FJ, Roop DR. p63 is the molecular switch for initiation of an epithelial stratification program. Genes Dev. 2004;18:126–131. doi: 10.1101/gad.1165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade SS, Henry AE, Pivato GP, Kozarewa I, Mitsopoulos C, Fenwick K, Assiotis I, Hakas J, Zvelebil M, Orr N, et al. Genome-wide analysis of p63 binding sites identifies AP-2 factors as co-regulators of epidermal differentiation. Nucleic Acids Res. 2012;40:7190–7206. doi: 10.1093/nar/gks389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Qi Y, Bradley A. Conditional inactivation of p63 by Cre-mediated excision. Genesis. 2002;32:138–141. doi: 10.1002/gene.10067. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Koren EG, Hogan BL, Gunn MD. Loss of basal cells precedes bronchiolitis obliterans-like pathological changes in a murine model of chlorine gas inhalation. Am J Respir Cell Mol Biol. 2013;49:788–797. doi: 10.1165/rcmb.2012-0369OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. Hippo signaling in organ size control. Genes Dev. 2007;21:886–897. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Camargo FD. The Hippo signaling pathway and stem cell biology. Trends Cell Biol. 2012;22:339–346. doi: 10.1016/j.tcb.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano RA, Ortt K, Birkaya B, Smalley K, Sinha S. An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate. PLoS One. 2009;4:e5623. doi: 10.1371/journal.pone.0005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, Sinha S. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J Biol Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Su X, Chakravarti D, Flores ER. p63 steps into the limelight: crucial roles in the suppression of tumorigenesis and metastasis. Nat Rev Cancer. 2013;13:136–143. doi: 10.1038/nrc3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Paris M, Gi YJ, Tsai KY, Cho MS, Lin YL, Biernaskie JA, Sinha S, Prives C, Pevny LH, et al. TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell. 2009;5:64–75. doi: 10.1016/j.stem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudol M, Harvey KF. Modularity in the Hippo signaling pathway. Trends Biochem Sci. 2010;35:627–633. doi: 10.1016/j.tibs.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, Vinarsky V, Cho JL, Breton S, Sahay A, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013a;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata PR, Pardo-Saganta A, Prabhu M, Vinarsky V, Law BM, Fontaine BA, Tager AM, Rajagopal J. Airway Specific Inducible Transgene Expression Using Aerosolized Doxycycline. Am J Respir Cell Mol Biol. 2013b doi: 10.1165/rcmb.2012-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- Yagi R, Chen LF, Shigesada K, Murakami Y, Ito Y. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–2562. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Luong P, Hudson C, Gudmundsdottir K, Basu S. c-Abl phosphorylation of DeltaNp63alpha is critical for cell viability. Cell Death Dis. 2010;1:e16. doi: 10.1038/cddis.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.