Abstract
Background
α-Klotho (αKl) regulates mineral metabolism such as calcium ion (Ca2+) and inorganic phosphate (Pi) in circulation. Defects in mice result in clinical features resembling disorders found in human aging. Although the importance of transmembrane-type αKl has been demonstrated, less is known regarding the physiological importance of soluble-type αKl (sαKl) in circulation.
Objectives
The aims of this study were: 1) to establish a sandwich ELISA system enabling detection of circulating serum sαKl, and 2) to determine reference values for sαKl serum levels and relationship to indices of renal function, mineral metabolism, age and sex in healthy subjects.
Results
We successively developed an ELISA to measure serum sαKl in healthy volunteers (n=142, males 66) of ages (61.1 ± 18.5 yr). The levels (mean ± SD) in these healthy control adults were as follows: total calcium (Ca; 9.46 ± 0.41 mg/dL), Pi (3.63 ± 0.51 mg/dL), Blood urea nitrogen (BUN; 15.7 ± 4.3 mg/dL), creatinine (Cre; 0.69 ± 0.14 mg/dL), 1,25 dihydroxyvitamin D (1,25(OH)2D; 54.8 ± 17.7 pg/mL), intact parathyroid hormone (iPTH; 49.2 ± 20.6 pg/mL), calcitonin (26.0 ± 12.3 pg/mL) and intact Fibroblast growth factor (FGF23; 43.8 ± 17.6 pg/mL).
Serum levels of sαKl ranged from 239 to 1266 pg/mL (mean ± SD; 562 ± 146 pg/mL) in normal adults. Although sαKl levels were not modified by gender or indices of mineral metabolism, sαKl levels were inversely related to Cre and age. However, sαKl levels in normal children (n=39, males 23, mean ± SD; 7.1 ± 4.8 years) were significantly higher (mean ± SD; 952 ± 282 pg/mL) than those in adults (mean ± SD; 562 ± 146, P<0.001). A multivariate linear regression analysis including children and adults in this study demonstrated that sαKl correlated negatively with age and Ca, and positively with Pi. Finally, we measured a serum sαKl from a patient with severe tumoral calcinosis derived from a homozygous missense mutation of α-klotho gene. In this patient, sαKl level was notably lower than those of age matched controls.
Conclusion
We established a detection system to measure human serum sαKl for the first time. Age, Ca and Pi seem to influence serum sαKl levels in a normal population. This detection system should be an excellent tool for investigating sαKl functions in mineral metabolism.
Keywords: alpha-Klotho, ELISA, calcium, phosphate, tumoral calcinosis
Introduction
α-kl gene encodes a type I membrane protein with expression restricted to parathyroid glands, the choroid plexus and the kidney [1-4]. αKl binds to Na+,K+-ATPase to regulate PTH secretion and is involved in transepitherial calcium concentration. In response to altered extra-cellular calcium concentrations, αKl is rapidly translocated from endosomal organella to the plasma membrane together with Na+,K+-ATPase and simultaneously the extracellular domain of αKl is cleaved and secreted into the blood circulation and cerebrospinal fluid (CSF) [5,6]. The increased Na+ gradient produced by elevated Na+,K+-ATPase activity drives PTH secretion in parathyroid glands and transepithelial transport of calcium in the kidney and choroid plexus [5]. Accordingly, it is assumed that αKl levels in the serum and CSF mirror the molecular actions of the cellular form of αKl in these tissues.
αKl also binds to fibroblast growth factor 23 (FGF23), which was discovered in studies of autosomal dominant hypophosphatemic rickets (ADHR) [7] and later tumor-induced osteomalacia (TIO) [8, 9]. FGF23 (i) is produced and secreted from bone in response to serum levels of phosphorus and 1,25(OH)2D [10-12], (ii) binds to FGF receptor1 (FGFR1), both suppressing 1α-hydroxylase (CYP27B1) expression and stimulating 24-hydroxylase (CYP24A1) expression in kidney [13,14], and (iii) downregulates protein amounts of Na+ dependent-phosphate transporter (NaPi) IIa/c to the brush border membrane of proximal tubules thus decreasing phosphate reabsorption [11].
αKl contributes to integrate mineral homeostasis. Consequently, disturbances of αKl expression impair mineral metabolism via multiple mechanisms involving FGF23 signaling [13, 14], PTH secretion and transepithelial calcium transport. Recently, a patient with autosomal recessive hyperphosphatemic tumoral calcinosis shed new light on the impact of αKl [15]. Mutation analysis revealed a missense mutation in αKl, and in vitro studies indicated that αKl translocation to the plasma membrane was impaired [16]. Therefore, analysis of serum αKl levels may lead to greater understanding of disorders of mineral homeostasis.
In the present study, we developed an ELISA system to measure circulating sαKl concentrations in serum from human subjects for the first time. We further analyzed and compared sαKl levels of both healthy volunteers and a case with the α-klotho gene mutation [16]. Finally, we discuss the potential utility in measuring serum αKl in clinical disorders.
Materials and methods
Plasmid construction
Human full length α-Klotho (fl-αKl; 1012 amino acids(a.a), RefSeq ID: NP_004786)-cDNA and cDNA encoding extracellular domain of α-Klotho (sαKl; 1-979a.a.) were amplified from total human kidney cDNAs by PCR and consequently cloned into pLP-CMVneo and pLP-IRESneo, respectively, by In fusion PCR kit (Clonetech).
Cell culture
pLP-CMVneo-fl-αKl was transfected into HEK293 cells by the calcium-phosphate method. pLP-IRESneo-sαKl was transfected into CHO cells, by the Lipofectamine method (Invitrogen). Then, cells stably expressing either fl-αKl or sαKl were selected by G418 and cloned by limiting dilution. HEK293 cells expressing fl-αKl were grown in DMEM supplemented with 10% Fetal Calf Serum (FCS) and 1mg/mL of G418. CHO cells expressing sαKl were grown in MEMα supplemented with 10% FCS and 2mg /mL of G418.
sαKl protein purification
Recombinant sαKl protein was purified from serum-free conditioned media (SFMII, Gibco) of CHO cells stably expressing sαKl by a series of chromatographic steps employing Q Sepharose XL, Butyl Sepharose FF, Heparin Sepharose HP, Q Sepharose HP, SP Sepharose HP, DEAE Sepharose FF, Phenyl Sepharose HP, Q Sepharose HP, Lentil Lectin sepharose 4B, and DEAE Sepharose FF (All sepharoses were purchased from GE healthcare). The concentration of purified sαKl protein was determined by absorbance at 280 nm.
Animals
All mice were purchased from Charlesriver Inc. Japan and were maintained in SPF conditions according to the institutional guidelines of Kyowa Hakko Kirin Co., Ltd.
Antibodies
Monoclonal anti-human α-Klotho antibodies were generated by immunizing B6D2F1 mice once a week for 5 weeks with pLP-CMVneo-fl-αKl vector by TransIT in vivo gene delivery system (Takara Bio). Hybridomas were obtained by fusing spleen cells and Sp2/o mouse myeloma cells (ATCC ID CRL1581) with polyethylene glycol. Then, hybridomas producing anti-α-Klotho antibody were selected by cell ELISA and flow cytometry analysis by using HEK293 cells stably expressing fl-αKl. Finally, nine monoclonal antibodies were purified with Protein G Sepharose 4FF (GE healthcare) and we next tested all pairs of antibodies with high affinity against recombinant sαKL for sandwich ELISA by using conjugated antibodies with biotin as detection antibodies. A pair of antibodies that have the highest reactivity to sαKl protein were named 67G3 (IgG1, kappa) as a capture antibody and 91F1 (IgG1, kappa) as a detection antibody, respectively. Rabbit anti-human α-Klotho polyclonal antibodies (N116 and C939) were generated by immunization of two different human α-Klotho partial polypeptides from N-terminal region (PLQPATGDVASDSYNNVFRDT, 116-136a.a.) and C-terminal region (ASMKHYRKIIDSNGFPGPETLERFC, 939-963a.a.), respectively.
Subjects
Healthy Asian volunteers (n=181, 89 males) between 0.1 and 88 years of age participated in our study. Subjects had no known medical conditions. A patient with a missense mutation of the α-klotho gene was described in a previous paper [16]. The study protocol was approved by the ethics committee on human research at Kyoto University Graduate School of Medicine, Tokyo Metropolitan Children's Medical Center, Osaka University Hospital, Nara Medical University Hospital and Indiana University. Written informed consent was obtained from subjects prior to enrollment. All subjects had normal serum Ca, Pi, 1,25(OH)2D, iPTH, calcitonin, FGF23 levels and normal renal function as assessed by BUN and serum Cre levels.
Measurement of serum parameters
Blood samples were drawn from a forearm vein in the morning after overnight fasting. Sera were obtained by centrifugation and immediately stored at −30°C. Serum FGF23 concentrations were measured by a FGF23 kit (Kainos) which can only measure the intact FGF23 [9]. Ca, Pi, BUN, Cre and Alb were measured at SRL Inc. Japan using standard clinical methods. Serum levels of iPTH, calcitonin and 1,25(OH)2D were measured only in adults (>20 years) at SRL Inc. because of limited serum quantity in children. The mean values of age and serum biochemical tests from each group are shown in Table 1.
Table 1.
Yamazaki Y et al
| number: | total (male/female) | all 181 (89/92) | children (<17yrs) 39 (23/16) | adults (>20yrs) 142 (66/76) | P children vs adults |
|---|---|---|---|---|---|
| Age | year | 49.4 ± 27.7 | 7.1 ± 4.8 | 61.1 ± 18.5 | <0.001 |
| Ca | mg/dL | 9.50 ± 0.44 | 9.65 ± 0.52 | 9.46 ± 0.41 | 0.018 |
| Pi | mg/dL | 3.90 ± 0.75 | 4.88 ± 0.68 | 3.63 ± 0.51 | <0.001 |
| Ca x Pi | (mg/dL)2 | 37.1 ± 8.0 | 47.2 ± 7.9 | 34.4 ± 5.3 | <0.001 |
| BUN | mg/dL | 15.2 ± 4.3 | 13.3 ± 3.6 | 15.7 ± 4.3 | 0.002 |
| Cre | mg/dL | 0.62 ± 0.19 | 0.36 ± 0.12 | 0.69 ± 0.14 | <0.001 |
| Alb | mg/dL | 4315 ± 384 | 4190 ± 474 | 4349 ± 349 | 0.021 |
| 1,25(OH)2D | pg/mL | ND | ND | 54.8 ± 17.7 | ND |
| iPTH | pg/mL | ND | ND | 49.2 ± 20.6 | ND |
| calcitonin | pg/mL | ND | ND | 26.0 ± 12.3 | ND |
| FGF23 | pg/mL | 39.6 ± 18.4 | 24.2 ± 12.0 | 43.8 ± 17.6 | <0.001 |
ND: not determined
Immunoprecipitation and Western blot
500μL of sera from two healthy subjects and purified sαKl protein were incubated with resin (NHS-activated Sepharose 4 FF; GE healthcare) conjugated with 67G3, 91F1 or isotype-matched control antibody (mouse IgG1) at 4°C for 2h. The specifically retained proteins on the resin were solubilized by boiling in SDS-PAGE sample buffer with the reducing agent and separated by SDS-PAGE. Following electrophoresis, proteins were transferred to PVDF membrane and bound with the biotin labeled N116 or C939 antibodies followed by HRP-conjugated streptavidin (DAKO). Protein bands were visualized by the ECL system (GE healthcare)
ELISA procedure
Wells of microtiter plates were coated with 1 μg/mL of 67G3 monoclonal antibody in 100 μL of carbonate buffer (pH9.5) for 18h at 4 °C and then blocked with 1% BSA in PBS for 1h at 37°C. Samples (50μL) were premixed with dilution buffer (50μL; PBS, pH7.4, containing 0.05% Tween20, 1% BSA, and 50μg /mL purified normal mouse IgG). The mixtures were loaded and incubated for 1h at room temperature (RT), followed by the addition of 100μL 91F1-Fab’ conjugated with HRP by using N-(6-Maleimidocaproyloxy)succinimide (EMCS, Dojindo) in PBS for 30 min at RT, and the reaction was visualized by the addition of 100μL of chromogenic substrate (TMB+, Dako) for 30 min at RT. The reaction was stopped with 100μL of 1N H2SO4 and absorbance at 450nm was measured with a reduction at 570 nm using ELISA plate reader. Plates were washed 6 times with washing buffer (PBS, pH7.4, containing 0.1% (v/v) Tween 20) after each step. A standard curve was established by a serial dilution of recombinant sαKl.
Results
Establishment of anti-αKl mouse monoclonal antibodies for sandwich ELISA
To establish mouse monoclonal antibodies with strong affinity for human αKl protein, we immunized mice with a human full-length αKl expressing vector and screened the resulting hybridomas by measuring the binding affinities to human αKl expressing cells. Nine antibodies which showed high and specific affinities to an extracellular domain of human αKl protein were tested for compatibility as a capture antibody or detection antibody in sandwich ELISA to detect sαKl protein. We finally selected a pair of antibodies (67G3 for a capture antibody and 91F1 for a detection antibody). While these antibodies are not useful for Western blot assays, both antibodies have a high affinity in immunoprecipitation to sαKl protein as well as in Cell ELISA and Flow cytometry to a transmembrane type of αKl (data not shown), indicating that both antibodies specifically recognize a tertiary protein structure of an extracellular domain of αKl.
Sandwich ELISA for the detection of sαKl in human sera
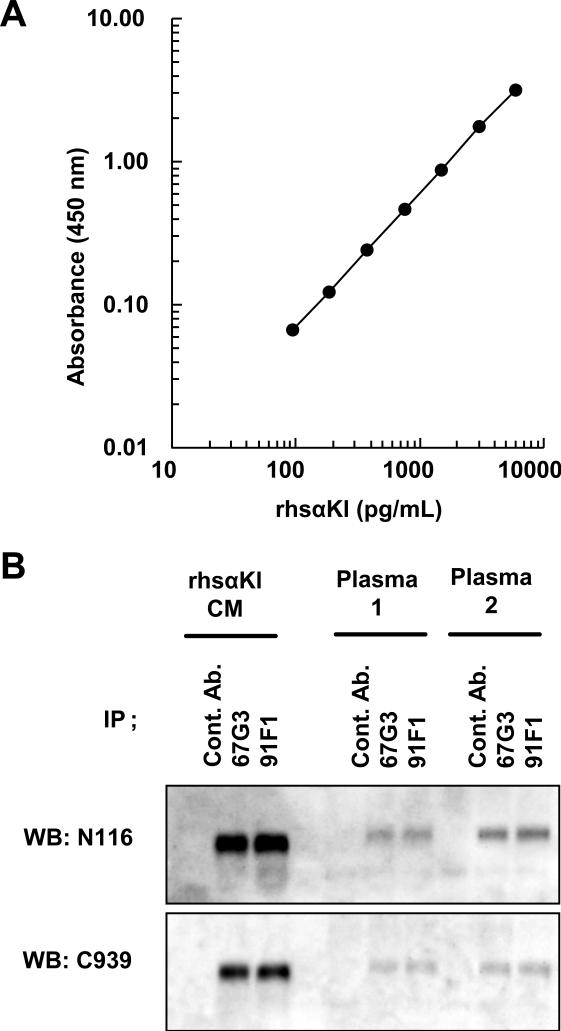
A typical standard curve is shown in Fig.1A of the purified recombinant human sαKl protein concentration between 94 pg/mL and 6000 pg/mL. The intra- and inter-assay coefficients of variation (CV) were calculated by measuring three different doses with purified human sαKl protein. As shown in Table S1A and S1B, both CVs ranged from 2.7 to 9.8%. The accuracy of this assay in the presence of human serum and plasma was determined by serially diluting recombinant human sαKl in 50% human serum and plasma. The measured sαKl levels were then compared with theoretical sαKl levels contributed by the addition of recombinant sαKl and the endogenous sαKl in serum and plasma. As shown in Table S1C, measured sαKl levels were 92.6% (in serum) and 94.1% (in plasma) of expected values. Furthermore, the serum dose-response curves were parallel to the purified recombinant sαKl dose-response curves (data not shown). Moreover, addition of recombinant human purified FGF23 (1μg/mL) did not affect a measurement (data not shown). Serum samples subjected to quick freezing at −80°C and thawing at RT did not show a significant decrease during three cycles (data not shown). These results indicate that sαKl can be measured with acceptable accuracy in the presence of 50% human serum or plasma.
Fig.1.
Enzyme-linked immunosorbent assay (ELISA) and immunoprecipitation analysis by two useful anti-sαKl monoclonal antibodies. (A) Typical dose-response curve of recombinant human sαKl (rhsαKl). Sandwich ELISA for sαKl was performed by 67G3 as a capture antibody and 91F1 as a detection antibody. The results are expressed as the mean ± standard deviation (SD) of triplicate measurements. (B) Immunoprecipitation of plasma sαKl by 67G3 and 91F1 antibodies. Conditioned medium from CHO cells stably expressing FGF23 (rhsαKl) and plasma from healthy volunteers (plasma 1, plasma 2) were immunoprecipitated anti-Klotho antibodies (67G3 and 91F1) and control mouse IgG1 (cont. Ab.), and analyzed by Western blot with anti-human Klotho rabbit polyclonal antibodies, N116 and C939.
Immunoprecipitation of plasma sαKl protein from healthy subjects
To address whether both antibodies bind to the endogenous sαKl in human plasma as well as the recombinant extracellular (soluble) αKl, immunoprecipitation was performed. Both 67G3 and 91F1 antibodies immunoprecipitated sαKl from plasma from healthy adult subjects (Fig.1B) as well as the recombinant human sαKl (rhsαKl). Furthermore, the densities of the immunopositive bands for precipitated sαKl (about 130kDa) precisely corresponded to the concentrations of sαKl represented by ELISA (rhsαKl: 5000 pg/mL, plasma 1: 575 pg/mL and plasma 2: 1272 pg/mL). These data suggest that both capture and detection antibodies can bind to endogenous sαKl in blood, indicating that our sandwich ELISA system can specifically detect and accurately measure the circulating sαKl.
Circulating sαKl concentrations in healthy adults
In order to determine the reference range of serum sαKl and to address the correlation of several mineral parameters with sαKl in healthy adults, we next analyzed serum levels of sαKl and indices of mineral metabolism in 142 healthy adults (age 20 years old or over) (Table 1). The serum sαKl concentrations of healthy adults ranged from 239 to 1266 pg/mL (mean ± SD; 562 ± 146 pg/mL). There was no apparent correlation of sαKl levels with gender, iPTH, 1,25(OH)2D, calcitonin, Ca, Pi, BUN, Cre or FGF23 by simple regression analyses. However, sαKl levels were slightly, but significantly correlated with age (r=-0.199, P=0.017) and Cre (r=-0.183, P=0.030) by simple regression analyses.
Circulating sαKl concentrations in healthy children
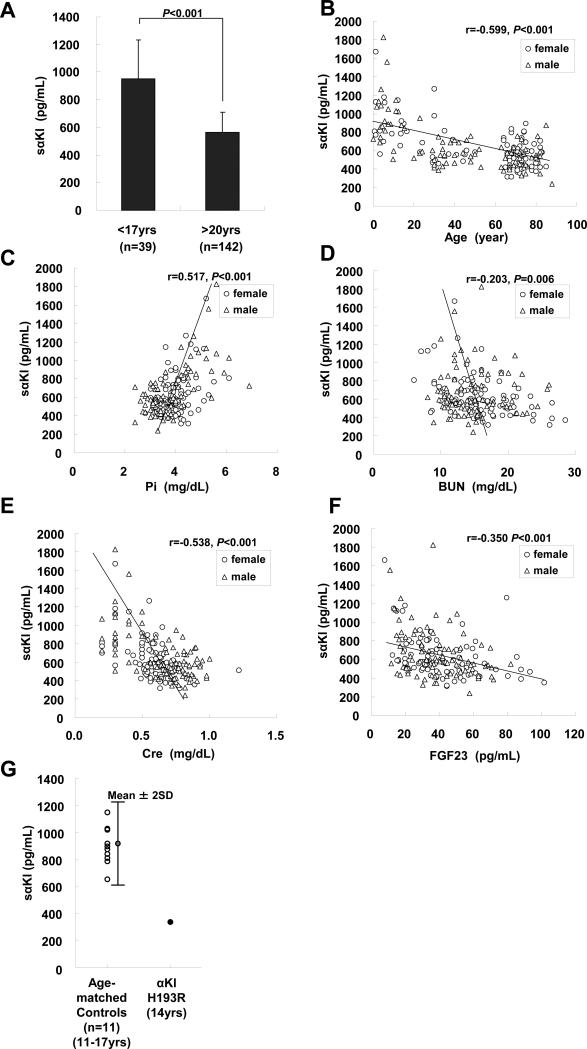
As mineral metabolism dramatically changes in childhood, we compared concentrations of sαKl between adults and children (<17 years) (Table 1). As expected, all factors measured in this study (Ca, Pi, Cre, BUN and FGF23) were significantly different between adults and children. The serum sαKl concentrations of normal children were significantly higher than those of adults (mean ± SD; 952 ± 282 pg/mL in children vs 562±146 pg/mL in adults, student's t-test, P<0.001, Fig.2A), indicating that serum sαKl correlated with age. The extent of correlation of sαKl with other serum factors examined by simple regression analysis was shown in Fig.2B-F. SαKl levels were found to correlate considerably with Pi (Fig.2C r=0.517, P<0.001), Cre (Fig.2E, r=−0.538, P<0.001) and FGF23 (Fig.2F r=−0.350, P<0.001) concentrations as well as age (Fig2B, r=−0.599, P<0.001). In addition, sαKl levels also slightly, but significantly, correlated with BUN (Fig.2D, r=−0.203, P=0.006) but sαKl levels did not significantly correlate with calcium.
Fig.2.
Serum sαKl levels (A) Comparison of serum sαKl levels between healthy adults and children. Serum sαKl levels of healthy adults (n=142, age: >20 years) and children (n=39, age: <17years) were measured. Results represent mean ± standard deviation (SD). Statistical analysis was performed by student's t-test. P values less than 0.05 were taken as statistically significant. Correlation of serum sαKl with (B) age (C) Pi (D) BUN (E) Cre and (F) FGF23 levels in simple regression analysis in healthy subjects. The open circles and triangles represent male and female subjects, respectively. Statistical analysis was performed by Pearson correlation. P values less than 0.05 were taken as statistically significant. (G) Comparison of serum sαKl levels between a patient with klotho missense mutation and age-matched healthy subjects. Serum sαKl levels from 11 healthy subjects (left, age: from 11.2 to 16.6 years) and a patient with klotho homozygous gene mutation (right, age: 14 years) were shown. An error bar along the values for healthy controls represents mean ± 2 fold of standard deviation (2SD) in order to indicate a reference range.
Next, factors independently associated with serum sαKl levels were examined using a multiple regression model. Serum sαKl exhibited significant and independent associations with age (P<0.001), Ca (P=0.004) and Pi (P=0.001), but no significant associations with BUN (P=0.411), Cre (P=0.051), or FGF23 (P=0.089) as shown in Table S2. Pi was positively correlated with sαKl. Interestingly, Ca and age were negatively correlated with sαKl in this model, while Ca was a not significant factor with sαKl by simple regression analysis.
Examination of sαKl levels in a patient with a αKl mutation
Ichikawa et al. have recently reported a case of hyperphosphatemic tumoral calcinosis with hypervitaminosis D due to missense mutations in human α-klotho gene (a single amino acid substitution from histidine193 to arginine). The authors concluded that, at least in part, disruption of the architecture of αKl protein results in a failure to translocate to the plasma membrane [16]. Consequently the subject's sαKl level was expected to be low. To test this prediction, we measured serum from the patient and found that the serum sαKl level was 337 pg/mL. Because the patient was 14 years old, we prepared serum from age-matched controls (n=11, mean ±SD: 13.5±1.7 years from 11.2 to 16.6 years old). As shown in Fig.2G, sαKl levels of age-matched controls ranged from 652 to 1146 pg/mL, and these values all fit within the mean value ± 2SD. As anticipated, the subject with α-Klotho mutation had low concentrations of sαKl compared with age-matched controls.
Discussion
We developed an ELISA to successfully measure sαKl in human subjects and demonstrated positive relationships with phosphorus, and negative relationships with age and Ca2+ in multiple linear regression analysis. Since low calcium stimuli induce αKl secretion [5], the negative relationship appears compatible. We have previously proposed a novel negative feedback system involving FGF23 and αKl for maintenance of normal phosphate and 1,25(OH)2D levels [16,13]. Based on the fact that FGF23 is a negative regulator of phosphate concentration (high FGF23 suppresses phosphate reabsorption in kidney), FGF23 correlates with phosphate in a positive manner (high phosphate induces high FGF23 level). Predictably, sαKl correlates negatively with FGF23, but positively with phosphate in simple regression analysis. We have proposed a mineral homeostatic system integrated by αKl, where Ca2+ and PTH levels are acutely regulated by Na+,K+-ATPase activity [5] and phosphate and 1,25(OH)2D levels are suppressed by FGF23 signals at late phase [8]. In this system, FGF23 signals lead to the suppression of α-klotho gene expression and the decreased synthesis of 1,25(OH)2D, an enhancer of α-klotho gene expression. A negative correlation between sαKl and FGF23 levels could reflect this putative feedback system.
FGF23 concentrations are maintained at a low or normal level regardless of high phosphate concentrations in children (Fig.S1). Since high phosphate has been reported to induce high FGF23, low or normal FGF23 concentrations in children seem to be paradoxical. Although the mechanism is unknown, this is postulated to be related to a higher need for adequate bone mineralization during childhood. In contrast, sαKl is maintained at a high concentration in children. Based on the preceding statistics that sαKl correlates positively with phosphate, the high value of sαKl is not inconsistent with a high phosphate level even at a young age. The balance of mineral metabolism in children is different from that in adults. It is very likely that a shift of metabolic equilibrium sustains higher phosphate concentration and optimizes Ca2+ reabsorption in tubules. Further studies are required to delineate the mechanism.
The manifestations seen in the patient with the α-klotho gene mutation resembled features observed in α-klotho ablated mice, although the patient's phenotype was milder than that observed in the mouse model. In an attempt to understand the manifestations seen in this patient, we verified sαKl levels using the ELISA system. Since the mutated protein impairs the intracellular trafficking ability in vitro, the sαKl level of the patient was expected to be low. Indeed, the sαKl value was more than 50% lower than the mean value of healthy age-matched subjects. Accordingly, the ELISA may be useful in studying disorders of mineral metabolism.
SαKl levels are higher in adolescence and lower in older adults and negatively correlate with Cre and BUN, which are increased in an age-dependent fashion even in healthy humans. Therefore, sαKl concentrations in serum are expected to be noted as a biomarker for kidney function. In the present study, we are not able to analyze the effect of renal function on sαKl and vice versa, since our subjects had normal renal function. Notably, in renal failure, α-klotho mRNA expression is decreased [18], though this may be due to death of the cells in the nephron expressing αKl. Further study is necessary to determine the utility of this ELISA in renal failure.
Kurosu et al reported that an increase of sαKl levels by genetic manipulation of full-length α-Klotho resulted in a prolonged life span in mice [19]. In that paper, since the levels of normal sαKl in mice were measured at >10ng/ml by radioimmunoassay [19], they were estimated to be approximately 20 fold greater than the average normal human sαKl concentration in this study. However, as previously reported, murine sαKl levels were calculated to be similar to that of humans by immunoprecipitation followed by western blot analysis [6]. These findings indicate that serum sαKl concentration should be carefully estimated to evaluate the roles of sαKl level in life span elongation. In addition, whereas two to three-fold elevations in sαKl level were noted in longevity-elongated mice [19], this magnitude would be within the divergence seen in healthy human subjects. Furthermore, this cross-sectioned study identified that younger subjects have higher sαKl, and that older subjects (who have lived longer) have lower sαKl. Therefore, careful prospective longitudinal studies would be needed to determine whether future longevity can be predictable from sαKl concentrations at one point in time.
Acknowledgements
This work was supported in part by the grants from Ministry of Education, Science and Culture in Japan, 21026017, 21390058 (to A.I.) and 17109004 (to Y.N.) and the Ministry of Health, Labour and Welfare of Japan KH20Q007a-1 (to K.O.). We thank K Sakuma, K Ono and N Yoshii for excellent technical support and Dr. T Sakai for helpful suggestion. We thank the coordinators of Fujiwara-kyo Study [20] from which almost healthy adults were recruited. Notably, subjects from Fujiwara-kyo Study had no history of cardiovascular diseases and diabetes mellitus and no other known medical conditions. Their blood pressures were within the normal range and their serum creatinine levels were less than 1.2mg/dL.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Kato Y, Arakawa E, Kinoshita S. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem. Biophys. Res. Commun. 2000;267:597–602. doi: 10.1006/bbrc.1999.2009. [DOI] [PubMed] [Google Scholar]
- 3.Takeshita K, Fujimori T, Kurotaki Y. Sinoatrial node dysfunction and early unexpected death of mice with a defect of klotho gene expression. Circulation. 2004;109:1776–1782. doi: 10.1161/01.CIR.0000124224.48962.32. [DOI] [PubMed] [Google Scholar]
- 4.Li SA, Watanabe M, Yamada H. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct. Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 5.Imura A, Tsuji Y, Murata M. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–1618. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 6.Imura A, Iwano A, Tohyama O. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 7.ADHR Consortium Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat. Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Mizutani S, Muto T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamazaki Y, Okazaki R, Shibata M. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J. Clin. Endocrinol. Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 10.Riminucci M, Collins MT, Fedarko NS. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J. Clin. Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada T, Hasegawa H, Yamazaki Y. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 12.Shimada T, Yamazaki Y, Takahashi M. Vitamin D receptor-independent FGF23 actions in regulating phosphate and vitamin D metabolism. Am. J. Physiol. Renal Physiol. 2005;289:F1088–1095. doi: 10.1152/ajprenal.00474.2004. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama K, Maeda R, Urakawa I. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl. Acad. Sci. U. S. A. 2010;107:1666–1671. doi: 10.1073/pnas.0913986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urakawa I, Yamazaki Y, Shimada T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 15.Chefetz I, Sprecher E. Familial tumoral calcinosis and the role of O-glycosylation in the maintenance of phosphate homeostasis. Biochim. Biophys. Acta. 2009;92:847–852. doi: 10.1016/j.bbadis.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa S, Imel EA, Kreiter ML. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J. Clin. Invest. 2007;117:2684–2691. doi: 10.1172/JCI31330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsujikawa H, Kurotaki Y, Fujimori T. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 18.Koh N, Fujimori T, Nishiguchi S. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 19.Kurosu H, Yamamoto M, Clark JD. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto N, Morikawa M, Okamoto K. Tooth loss is associated with mild memory impairment in the elderly. the Fujiwara-kyo study of successful aging’ Brain Research. 2010 doi: 10.1016/j.brainres.2010.06.054. in press. [DOI] [PubMed] [Google Scholar]