Abstract
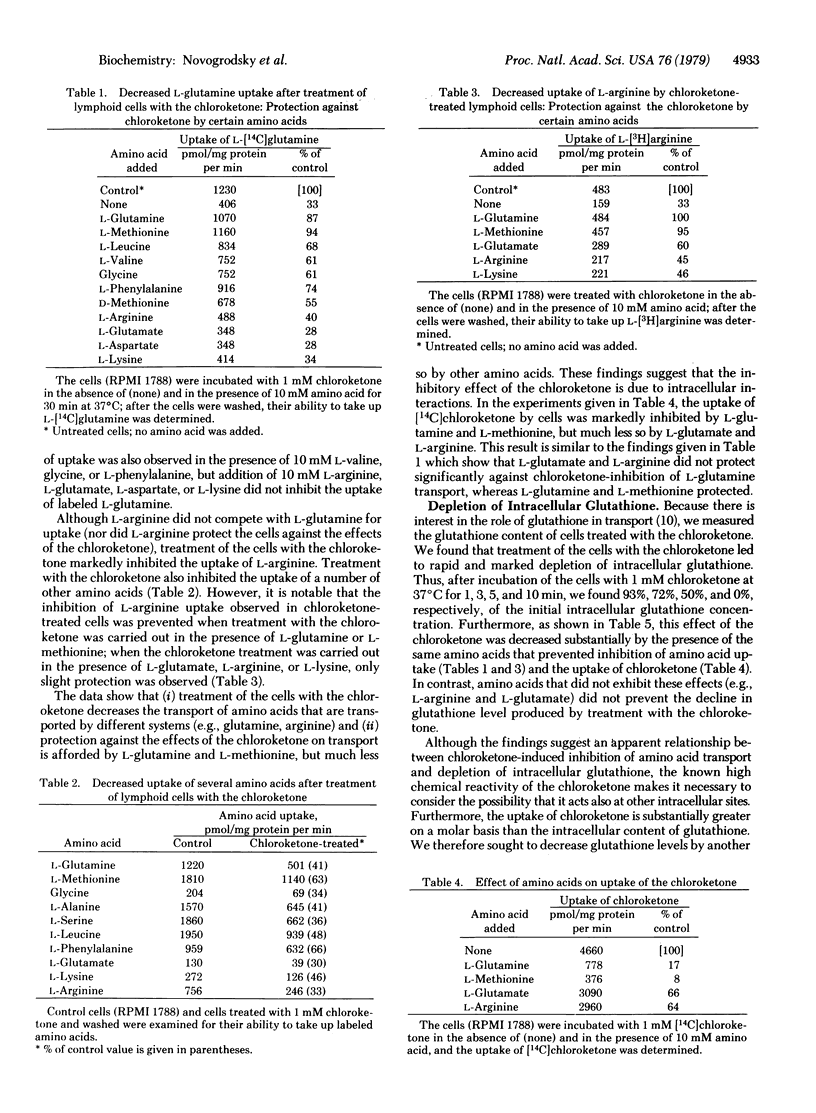
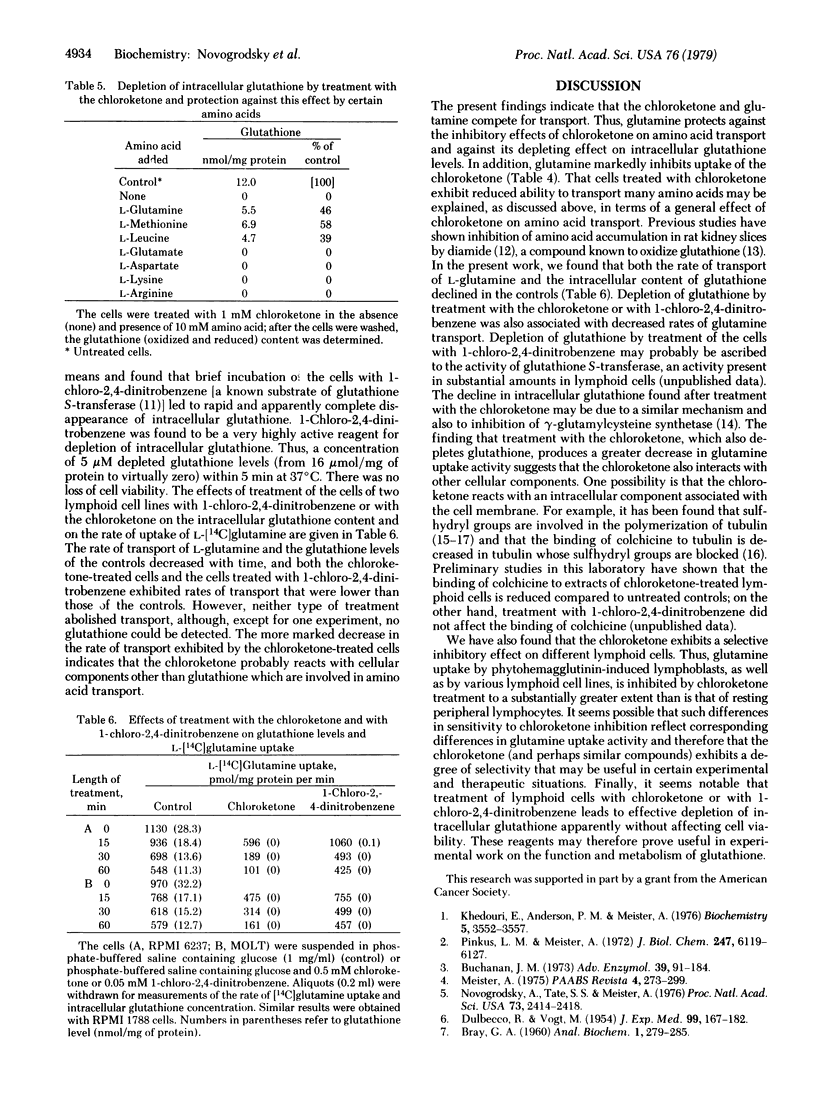
Transport of L-glutamine and of the chloroketone glutamine analog L-2-amino-4-oxo-5-chloropentanoate into lymphoid cells is mediated by the same system. Arginine and a number of other amino acids (e.g., glutamate, aspartate, and lysine) are transported to a much lesser extent by this system. However, after uptake of the chloroketone into the cells, the transport of glutamine, arginine, and other amino acids is markedly inhibited, due evidently to reaction of the chloroketone with intracellular components that are involved in amino acid transport. The chloroketone acts more effectively on growing than on resting cells. Treatment of lymphoid cells with the chloroketone or with L-chloro-2,4-dinitrobenzene leads to rapid and complete depletion of intracellular glutathione without affecting cell viability. These reagents appear to be useful experimental tools for studies of glutathione function and metabolism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan J. M. The amidotransferases. Adv Enzymol Relat Areas Mol Biol. 1973;39:91–183. doi: 10.1002/9780470122846.ch2. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J., Pillion D., Leibach F. H. Inhibition of amino acid accumulation in slices of rat kidney cortex by diamide. Biochim Biophys Acta. 1974 Sep 6;363(2):267–276. doi: 10.1016/0005-2736(74)90066-2. [DOI] [PubMed] [Google Scholar]
- Khedouri E., Anderson P. M., Meister A. Selective inactivation of the glutamine binding site of Escherichia coli carbamyl phosphate synthetase by 2-amino-4-oxo-5-chloropentanoic acid. Biochemistry. 1966 Nov;5(11):3552–3557. doi: 10.1021/bi00875a024. [DOI] [PubMed] [Google Scholar]
- Kosower E. M., Correa W., Kinon B. J., Kosower N. S. Glutathione. VII. Differentiation among substrates by the thiol-oxidizing agent, diamide. Biochim Biophys Acta. 1972 Mar 30;264(1):39–44. doi: 10.1016/0304-4165(72)90114-6. [DOI] [PubMed] [Google Scholar]
- Kuriyama R., Sakai H. Role of tubulin-SH groups in polymerization to microtubules. Functional-SH groups in tubulin for polymerization. J Biochem. 1974 Sep;76(3):651–654. doi: 10.1093/oxfordjournals.jbchem.a130609. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Meister A., Tate S. S. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Rebhun L. I. Sulfhydryls and the in vitro polymerization of tubulin. J Cell Biol. 1976 Jul;70(1):226–238. doi: 10.1083/jcb.70.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novogrodsky A., Tate S. S., Meister A. gamma-Glutamyl transpeptidase, a lymphoid cell-surface marker: relationship to blastogenesis, differentiation, and neoplasia. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2414–2418. doi: 10.1073/pnas.73.7.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus L. M., Meister A. Identification of a reactive cysteine residue at the glutamine binding site of carbamyl phosphate synthetase. J Biol Chem. 1972 Oct 10;247(19):6119–6127. [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Sherline P., Kipnis D. M. A sensitive method for measuring polymerized and depolymerized forms of tubulin in tissues. J Cell Biol. 1977 Aug;74(2):341–350. doi: 10.1083/jcb.74.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekura R., Meister A. Covalent interaction of L-2-amino-4-oxo-5-chloropentanoate at glutamate binding site of gamma-glutamylcysteine synthetase. J Biol Chem. 1977 Apr 25;252(8):2606–2610. [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]



