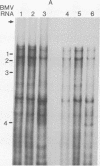
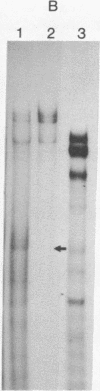
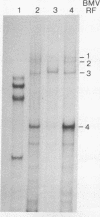
Abstract
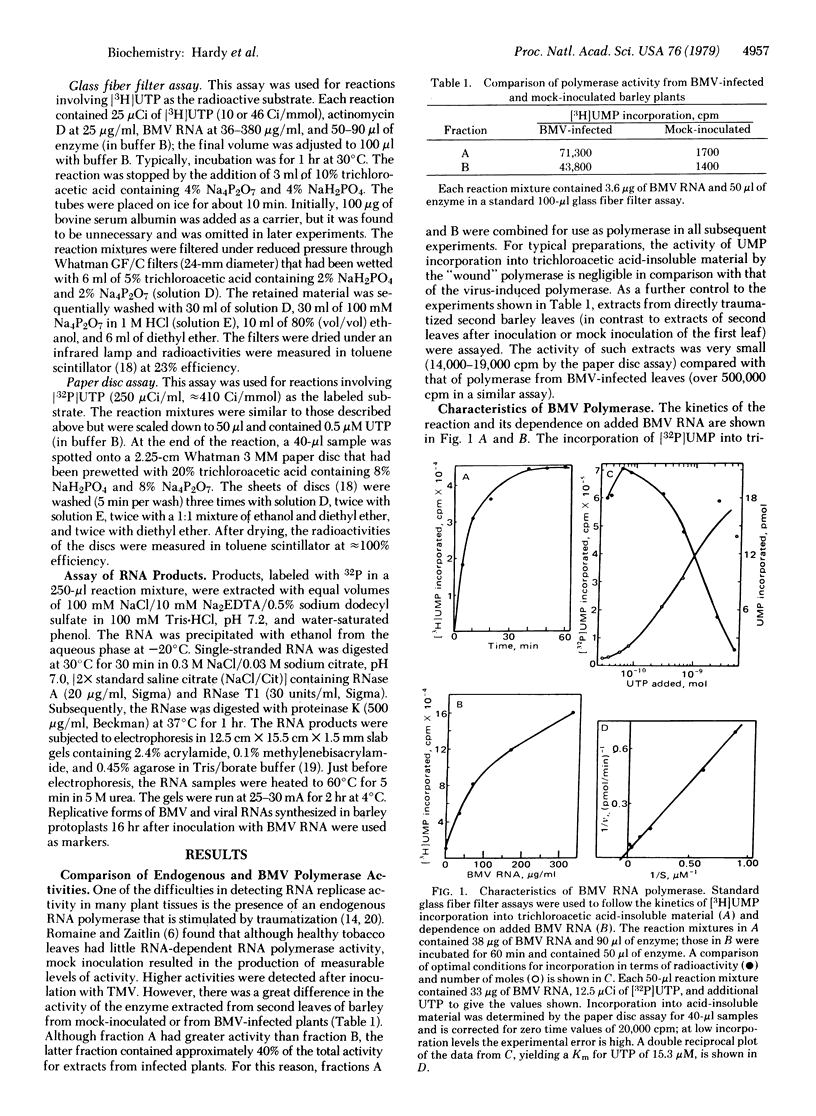
The extraction of a template-dependent and template-specific RNA-dependent RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) from a eukaryotic source is described. The enzyme, extracted from barley leaves infected with brome mosaic virus (BMV), is capable of incorporating high levels of radioactivity into trichloroacetic acid-insoluble products. The purification procedure included solubilization with nonionic detergent and precipitation with polyethylene glycol. The enzyme was more than 50 times more active than was a comparable preparation from mock-inoculated leaves and was stimulated more than 15-fold by the addition of BMV RNA to the reaction. Other viral RNA templates were less than 25% as efficient as was BMV RNA in stimulating UMP incorporation; poly(A), tRNA, and mRNA gave little stimulation and rRNA was inactive. Autoradiographic analysis after electrophoretic separation of the radioactive products from reaction mixtures containing BMV RNA template revealed prominent bands that coelectrophoresed with replicative forms of BMV RNAs. When BMV RNA template was enriched in RNA3 or RNA4, larger proportions of the products were replicative forms of RNA3 or RNA4, respectively.
Full text
PDF
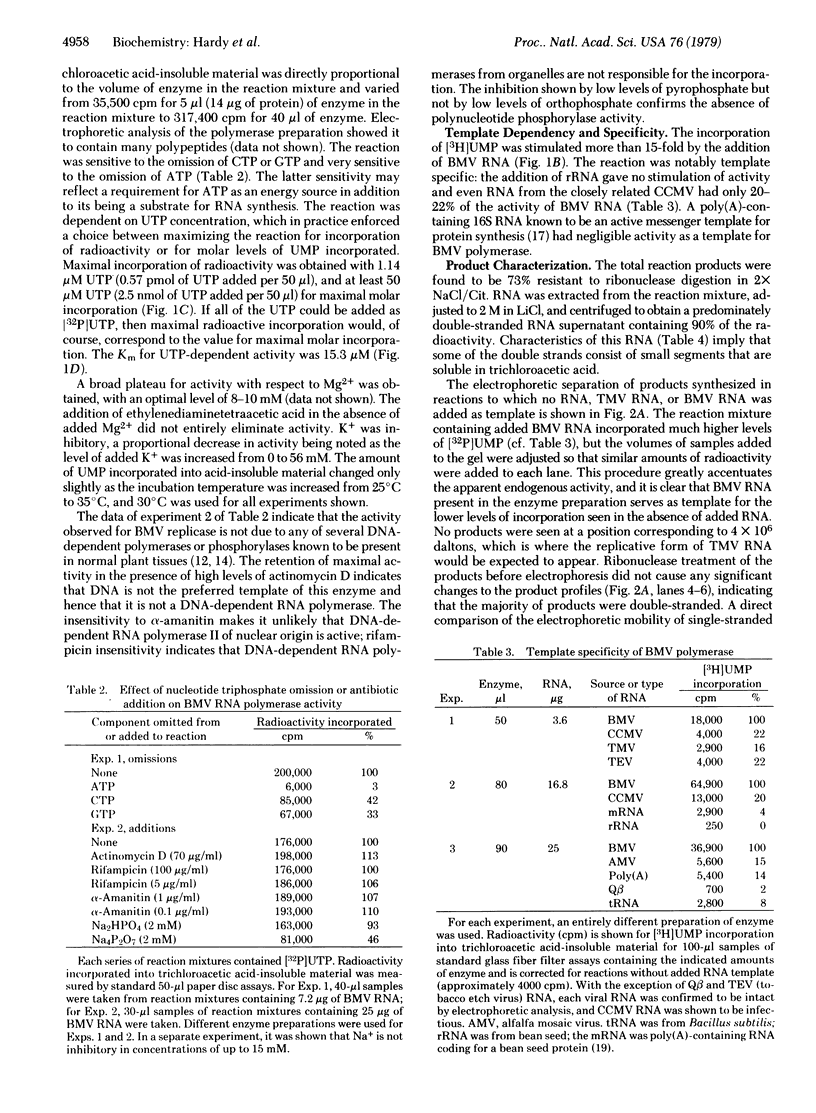
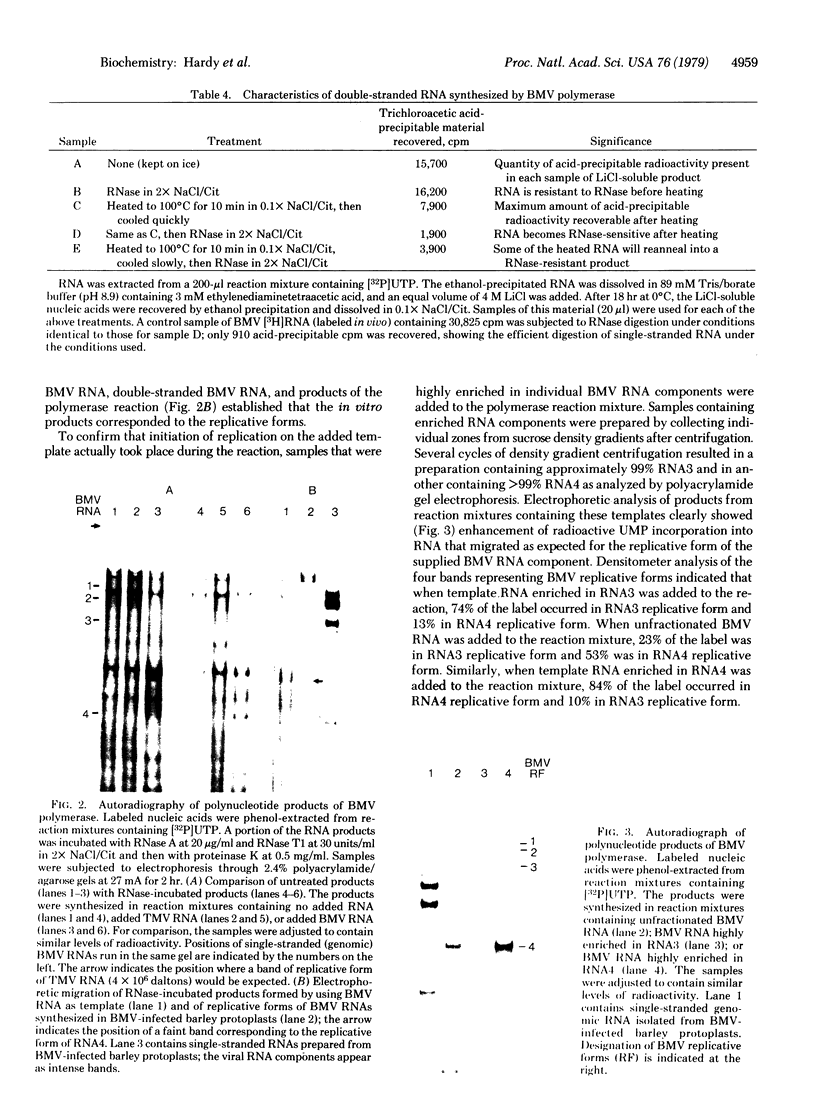

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastin M., Kaesberg P. A possible replicative form of brome mosaic virus RNA 4. Virology. 1976 Jul 15;72(2):536–539. doi: 10.1016/0042-6822(76)90185-9. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Kaesberg P. Isolation and properties of RNA from bromegrass mosaic virus. J Mol Biol. 1965 Aug;13(1):127–137. doi: 10.1016/s0022-2836(65)80084-5. [DOI] [PubMed] [Google Scholar]
- Clark G. L., Peden K. W., Symons R. H. Cucumber mosaic virus-induced RNA polymerase: partial purification and properties of the template-free enzyme. Virology. 1974 Dec;62(2):434–443. doi: 10.1016/0042-6822(74)90405-x. [DOI] [PubMed] [Google Scholar]
- Duda C. T., Zaitlin M., Siegel A. In vitro synthesis of double-stranded RNA by an enzyme system isolated from tobacco leaves. Biochim Biophys Acta. 1973 Aug 10;319(1):62–71. doi: 10.1016/0005-2787(73)90041-5. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus polyuridylic acid polymerase and RNA replicase have the same viral polypeptide. J Virol. 1979 Jan;29(1):352–360. doi: 10.1128/jvi.29.1.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel-Conrat H. RNA polymerase from tobacco necrosis virus infected and uninfected tobacco. Purification of the membrane-associated enzyme. Virology. 1976 Jul 1;72(1):23–32. doi: 10.1016/0042-6822(76)90308-1. [DOI] [PubMed] [Google Scholar]
- Hadidi A., Fraenkel-Conrat H. Characterization and specificity of soluble RNA polymerase of brome mosaic virus. Virology. 1973 Apr;52(2):363–372. doi: 10.1016/0042-6822(73)90331-0. [DOI] [PubMed] [Google Scholar]
- Hall T. C., Ma Y., Buchbinder B. U., Pyne J. W., Sun S. M., Bliss F. A. Messenger RNA for G1 protein of French bean seeds: Cell-free translation and product characterization. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3196–3200. doi: 10.1073/pnas.75.7.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami M., Fraenkel-Conrat H. Characterization of the RNA-dependent RNA polymerase of tobacco leaves. J Biol Chem. 1979 Jan 10;254(1):149–154. [PubMed] [Google Scholar]
- Ikegami M., Fraenkel-Conrat H. RNA-dependent RNA polymerase of tobacco plants. Proc Natl Acad Sci U S A. 1978 May;75(5):2122–2124. doi: 10.1073/pnas.75.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M., Gallerani R., Weissmann C. Subunit structure of Q-beta replicase. Nature. 1970 Nov 7;228(5271):525–527. doi: 10.1038/228525a0. [DOI] [PubMed] [Google Scholar]
- Kummert J., Semal J. Properties of the products synthesized by a detergent-treated RNA polymerase preparation from barley leaves infected with bromegrass mosaic virus. Virology. 1977 Mar;77(1):212–220. doi: 10.1016/0042-6822(77)90419-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Roy C., Stussi-Garaud C., Hirth L. RNA-dependent RNA polymerases in uninfected and in alfalfa mosaic virus-infected tobacco plants. Virology. 1977 Oct 1;82(1):48–62. doi: 10.1016/0042-6822(77)90031-9. [DOI] [PubMed] [Google Scholar]
- McLeester R. C., Hall T. C. Simplification of amino acid incorporation and other assays using filter paper techniques. Anal Biochem. 1977 May 1;79(1-2):627–630. doi: 10.1016/0003-2697(77)90447-x. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Romaine C. P., Zaitlin M. RNA-dependent RNA polymerases in uninfected and tobacco mosaic virus-infected tabacco leaves: viral induced stimulation of a host polymerase activity. Virology. 1978 May 1;86(1):241–253. doi: 10.1016/0042-6822(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Ríman J., Beaudreau G. S. Viral DNA-dependent DNA polymerase and the properties of thymidine labelled material in virions of an oncogenic RNA virus. Nature. 1970 Oct 31;228(5270):427–430. doi: 10.1038/228427a0. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Lane L. C., Kaesberg P. Origin of the small component of brome mosaic virus RNA. J Mol Biol. 1972 Mar 14;64(2):353–362. doi: 10.1016/0022-2836(72)90503-7. [DOI] [PubMed] [Google Scholar]
- Traub A., Duskin B., Rosenberg H., Kalmar E. Isolation and properties of the replicase of encephalomyocarditis virus. J Virol. 1976 May;18(2):375–382. doi: 10.1128/jvi.18.2.375-382.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. L., Dawson W. O. Characterization of RNA-dependent RNA polymerases in uninfected and cowpea chlorotic mottle virus-infected cowpea leaves: selective removal of host RNA polymerase from membranes containing CCMV RNA replicase. Virology. 1978 Jul 1;88(1):33–43. doi: 10.1016/0042-6822(78)90107-1. [DOI] [PubMed] [Google Scholar]
- White J. L., Murakishi H. H. In vitro replication of tobacco mosaic virus RNA in tobacco callus cultures: solubilization of membrane-bound replicase and partial purification. J Virol. 1977 Feb;21(2):484–492. doi: 10.1128/jvi.21.2.484-492.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., van Kammen A. In Vitro Replication of Cowpea Mosaic Virus RNA III. Template Recognition by Cowpea Mosaic Virus RNA Replicase. J Virol. 1979 Jan;29(1):21–33. doi: 10.1128/jvi.29.1.21-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]