Abstract
The white spotting locus (S) in dogs is colocalized with the MITF (microphtalmia-associated transcription factor) gene. The phenotypic effects of the four S alleles range from solid colour (S) to extreme white spotting (sw). We have investigated four candidate mutations associated with the sw allele, a SINE insertion, a SNP at a conserved site and a simple repeat polymorphism all associated with the MITF-M promoter as well as a 12 base pair deletion in exon 1B. The variants associated with white spotting at all four loci were also found among wolves and we conclude that none of these could be a sole causal mutation, at least not for extreme white spotting. We propose that the three canine white spotting alleles are not caused by three independent mutations but represent haplotype effects due to different combinations of causal polymorphisms. The simple repeat polymorphism showed extensive diversity both in dogs and wolves, and allele-sharing was common between wolves and white spotted dogs but was non-existent between solid and spotted dogs as well as between wolves and solid dogs. This finding was unexpected as Solid is assumed to be the wild-type allele. The data indicate that the simple repeat polymorphism has been a target for selection during dog domestication and breed formation. We also evaluated the significance of the three MITF-M associated polymorphisms with a Luciferase assay, and found conclusive evidence that the simple repeat polymorphism affects promoter activity. Three alleles associated with white spotting gave consistently lower promoter activity compared with the allele associated with solid colour. We propose that the simple repeat polymorphism affects cooperativity between transcription factors binding on either flanking sides of the repeat. Thus, both genetic and functional evidence show that the simple repeat polymorphism is a key regulator of white spotting in dogs.
Introduction
Coat colour variation has fascinated humans for centuries and consequently it has become one of the most extensively studied traits, mainly in mice [1] but also in domestic animals [2], [3]. There is an extensive diversity of white spotting patterns within and between dog breeds caused by genetic factors and stochastic effects during melanocyte development. White spotting indicates an absence of melanocytes in the hair follicles and/or skin due to a failure of melanoblast migration, proliferation or survival during development. In dogs, there is one major white spotting (S) locus that was defined by C. Little during the 1950s [4]. He described four different alleles at this locus with phenotypic effects ranging from solid (S, Figure 1A), to a completely white coat, caused by homozygosity for the Extreme white allele (sw, Figure 1B). The two intermediate phenotypes were named Irish spotting (si, Figure 1D) and piebald (sp, Figure 1E). Irish spotting is characterized by modest white spotting, often present as a white collar and a white belly, as demonstrated by breeds such as the Bernese Mountain dog and Basenji. Piebald-coloured dogs display limited to extensive white spotting and the phenotype is observed in several breeds, including the Beagle and Fox Terrier. Two S alleles are segregating in Boxers: Solid (S) and Extreme white (sw). These give rise to three different phenotypes: solid (S/S), flash (S/sw, Figure 1C) and extreme white (sw/sw). The flash (S/sw) phenotype is similar to Irish spotting (si/s i) and is therefore often called pseudo-Irish.
Figure 1. Overview of the different phenotypes included in the study.
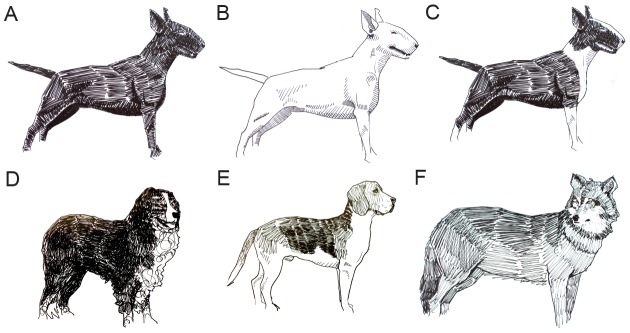
(A) Solid (S/S) Bull terrier, (B) white Bull terrier (sw/sw), (C) flash (S/sw) Bull terrier, (D) Irish spotting (si/si) in a Bernese Mountain Dog, (E) piebald (sp/sp) Beagle, (F) wolf. Drawings: Anders Sundström.
In 2006 and 2007, three groups independently mapped some or all of the white spotting phenotypes to the MITF (microphtalmia-associated transcription factor) locus [5]–[7]. The highest resolution was obtained by Karlsson et al. [6] who mapped the Spotting locus to a 1 Mb region on chromosome 20 based on a genome-wide association study comparing 10 homozygous white (sw/sw) and 10 homozygous solid (S/S) Boxers. Fine-mapping to a 100 kb region which included MITF was achieved by including a second breed, bull terrier, segregating for the same two alleles [6].
MITF was an obvious candidate gene because it encodes a transcription factor controlling neural crest-derived melanocyte development and migration [8], [9]. MITF has nine alternative promoters that produce multiple isoforms expressed in different tissues. All isoforms share the sequence encoded by exons 2–9, but have unique amino acids in their N-termini, determined by distinct first exons. There is clear conservation across mammals at this gene, as several of the MITF isoforms expressed in human and mouse have also been identified in dog [10], including the melanocyte-specific MITF-M isoform.
To date, 29 mutations in murine Mitf have been identified which affect the development and function of melanocytes in the skin, eye and inner ear [1]. Some variants affect vision by reducing eye size (microphtalmia) or cause early onset hearing disorders [11]. In humans, deleterious MITF mutations cause disorders of vision and hearing, including the Waardenburg and Tietz syndromes [9], [12], [13]. Deafness has also been recorded in white dogs, where approximately 2% of white dogs (sw/sw) present with bilateral deafness and 18% are unilaterally deaf [14]. The majority of mutations reported in mice and humans that cause severe pleiotropic effects are generally loss-of-function mutations affecting the coding regions [15]. This is not the case with dog MITF alleles. A comparison of S and sw haplotypes, using BACs from an S/sw heterozygote, across the 100 kb canine white spotting candidate region revealed 124 sequence polymorphisms, all of which were located in non-coding regions [6]. This demonstrated that the extreme white coat colour phenotype is controlled by one or several regulatory mutations. This hypothesis is strongly supported by the fact that coloured patches on white spotted dogs display normal pigmentation. Thus, this suggests that the canine MITF variants primarily affects migration and survival of melanocytes during development, but have no or only minor effects in mature melanocytes in the hair follicle; pigmentation of the hair requires MITF protein expression [16].
Further analysis of these 124 polymorphisms resulted in a short list of three candidate mutations within or in the vicinity of the MITF-M promoter, and one in the MITF-1B exon located upstream of MITF-M. The first of these is a canine-specific short interspersed nucleotide element (SINEC-Cf element), located about 3 kb upstream of the MITF-M transcription start site (TSS). The SINE insertion was only found in dogs presenting the extreme white (sw/sw) or piebald (sp/sp) phenotypes, and was absent in Irish-spotted (si/si) and solid (S/S) dogs [6]. This very strong association between the SINE insertion and the extreme white and piebald phenotypes was confirmed in a subsequent study based on 324 dogs from 45 breeds although a few exceptions from this rule were noted [17]. The second candidate (SNP#21), a SNP located approximately 1.2 kb upstream of MITF-M TSS, occurs in a highly conserved region and the A allele at this locus is associated with white spotting alleles [6]. The third polymorphism is a variable length polymorphism (Lp) approximately 100 bp upstream of the MITF-M TSS. Long variants of the Lp (LpWhite) are associated with all three white-spotting alleles (sw, sp and si), whereas all solid dogs examined carried short Lp variants [6], from here on named LpSolid. The fourth candidate mutation, a 12 bp deletion in exon 1B (Exon1B_del) also showed a very strong association with white spotting. It was found on all Extreme white and Piebald chromosomes tested, but it was also found in the heterozygous state in 4 out of 76 solid dogs. This previous work showed that the Extreme white and Piebald alleles share the same sequence variants for the SINE, SNP#21, Lp and Exon1B_del polymorphisms, implying that other sequence variants must explain the phenotypic differences between the two alleles.
The aims of the current study were two-fold: (i) to screen for the presence of the candidate mutations in wolves that are expected to have the S wild-type genotype and (ii) to evaluate the functional significance of the candidate mutations associated with the MITF-M promoter, alone and in combination. Given the high level of conservation across mammals at this region, any mechanistic insights gained in the canine model could also be used to further understand the role of the master melanocyte regulator, MITF.
Results
MITF haplotype diversity in wolves
A causal mutation with a major effect on white spotting is expected to be rare or absent in wolves. Wolves show the wild-type coat colour that ranges from dark grey to almost white in arctic populations, but without obvious patches of no pigmentation as is characteristic for dogs carrying the Extreme white or Piebald alleles. We therefore examined the frequency of the four candidate mutations in a set of 60 wolves with global distribution (Table S1). All tested individuals had a low copy number (CN) status at the AMY2B locus (Table S1), known to distinguish wolves (CN = 2) from the majority of domestic dogs (CN = 3–30) [18].
We first screened the wolf samples for the SINE insertion and found that this polymorphism is widespread among both European and North-American wolf populations (Table 1). Thus, we can exclude the possibility that this is a derived mutation in domestic dogs with a major effect on the white spotting phenotype.
Table 1. Allele frequencies of three candidate MITF polymorphisms in wolves from different geographic regions.
| Geographic region | n | SINE1 | SNP#21*A 2 | Exon1B_Del |
| Scandinavia | 34 | 0.32 | 0 | 0.18 |
| Belarus | 4 | 0.50 | 0 | 0 |
| Russia | 5 | 0.20 | 0 | 0 |
| Bulgaria | 2 | 0.50 | 0 | 0 |
| Spain | 6 | 0.08 | 0.08 | 0 |
| Italy | 1 | 1.00 | 0 | 0 |
| Canada | 7 | 0.21 | 0.14 | 0 |
The frequency of the allele with the SINE insertion is presented.
The A and G alleles at SNP#21 are associated with white spotting and solid colour, respectively.
The SNP#21*A allele associated with white spotting in dogs was only found in one Spanish and two Canadian wolves from the 60 wolves tested (Table 1). This suggested that SNP#21 could be a derived mutation in dogs and therefore we screened a larger set of dogs (206 dogs from 17 breeds) with different coat colour phenotypes to assess the strength of association to white spotting (Table S2). This analysis confirmed the association between SNP#21*A and white spotting, but the association was far from complete, since the allele occurred at a high frequency in Irish Wolfhound that has solid colour and it occurred at a low frequency in some breeds showing white spotting (Bearded Collie and Border Collie) (Table S2). We conclude that the allele on its own cannot have a strong effect on white spotting.
The composition (CXAYG2AZ) of the length polymorphism (Lp) in the MITF-M promoter makes it very challenging to genotype, as the three mononucleotide repeats show extensive polymorphism as well as length instability during PCR amplification. In a previous study, the composition was determined in dogs using direct sequencing after PCR amplification [6]. In the present study, the repeat and unique flanking sequence was amplified, cloned and the most likely genotype inferred through the sequencing of many clones per individual. In this analysis, we used a conservative approach and so did not designate new alleles unless supported by multiple clones from the same individual. To confirm the utility of this approach we first confirmed the composition of some alleles previously determined by direct sequencing, followed by a screen of 17 wolves (Table 2). The screen revealed an extensive diversity in wolves since as many as 15 alleles were found.
Table 2. SINEC-Cf and length polymorphism (Lp) alleles in the MITF-M promoter among dogs and wolves.
| Lp base composition | |||||||||
| Colour | SINE | C | A | C | A | G | A | Alleles (bp)3 | Population |
| Dogs | Dog breed | ||||||||
| Solid (S) | − | 9 | - | - | 7 | 2 | 11 | 29A | Yorkshire Terrier1 |
| 10 | - | - | 8 | 2 | 11 | 31A | Various solid dogs1 , 2 | ||
| 10 | - | - | 9 | 2 | 11 | 32A | Golden Retriever, Keeshond1 | ||
| Irish (si) | − | 13 | - | - | 8 | 2 | 12 | 35C | Basenji1 |
| 14 | - | - | 8 | 2 | 12 | 36B | Basenji1 | ||
| 14 | - | - | 8 | 2 | 11 | 35D | Bernese Mountain Dog1 , 2 | ||
| Piebald (sp) | + | 11 | - | - | 10 | 2 | 12 | 35B | English Springer Spaniel1 |
| 12 | - | - | 10 | 2 | 12 | 36A | English Springer Spaniel, Fox Terrier1 | ||
| White (sw) | + | 11 | - | - | 7 | 2 | 12 | 32B | Dalmatian1 , 2 |
| 12 | - | - | 9 | 2 | 12 | 35A | Boxer1 , 2 | ||
| 11 | - | - | 10 | 2 | 12 | 35B | Bull Terrier1 | ||
| Wolves | Wolf origin | ||||||||
| Wild-type | + | 10 | - | - | 7 | 2 | 11 | 30A | Scandinavia2 |
| + | 11 | - | - | 7 | 2 | 12 | 32B | Belarus, Scandinavia2 | |
| − | 7 | 1 | 1 | 9 | 2 | 13 | 33A | Scandinavia2 | |
| − | 7 | 1 | 1 | 10 | 2 | 13 | 34A | Scandinavia, USA2 | |
| + | 11 | - | - | 10 | 2 | 12 | 35B | Scandinavia2 | |
| − | 7 | 1 | 1 | 10 | 2 | 14 | 35E | Scandinavia2 | |
| − | 7 | 1 | 1 | 9 | 2 | 11 | 31B | Belarus2 | |
| − | 10 | - | - | 8 | 2 | 12 | 32C | Belarus2 | |
| − | 7 | 1 | 1 | 9 | 2 | 12 | 32D | Spain, Belarus2 | |
| − | 11 | - | - | 8 | 2 | 11 | 32E | Spain2 | |
| − | 12 | - | - | 8 | 2 | 12 | 34B | Spain2 | |
| − | 14 | - | - | 8 | 2 | 11 | 35D | Belarus2 | |
| − | 6 | 1 | 3 | 8 | 2 | 12 | 32F | Canada2 | |
| + | 12 | - | - | 9 | 2 | 12 | 35A | Canada2 | |
| + | 12 | - | - | 7 | 2 | 12 | 33B | USA2 | |
Determined by direct sequencing in Karlsson et al. [6].
Determined as the most prevalent clone after cloning PCR products in the present study; only one dog per breed and coat colour type was sequenced.
Allele designations are based on the length of the repeat. Alleles with same length but different repeat compositions are distinguished by capital letters. Alleles present in both wolves and dogs are marked in bold.
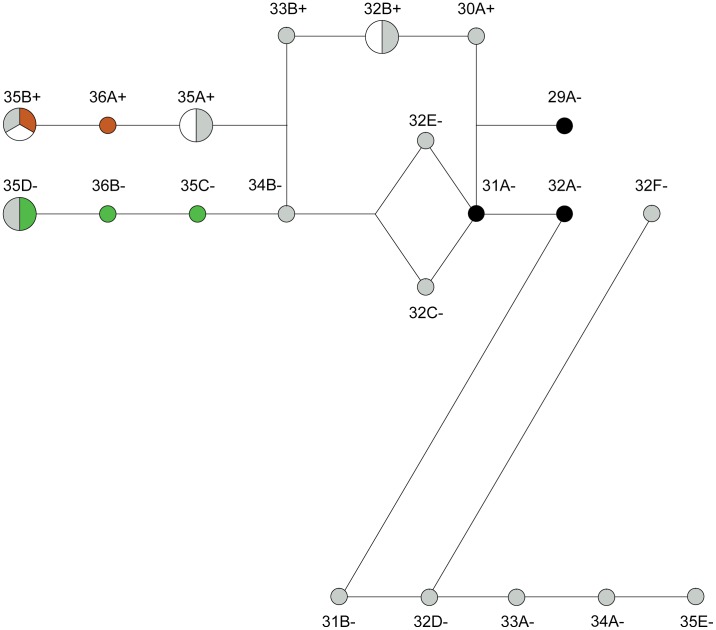
We generated weighted conservative median joining network in order to visualize the relationships between the 21 haplotypes generated from the combined SINE and Lp genotypes of dogs and wolves (Figure 2, Table 2). Haplotypes shared between wolves and dogs (i.e. 35B+, 35A+, 35D− and 32B+) are drawn proportionately larger than those that are private (e.g. wolf, 34B−; dog, 35C−). The disrupted CX mononucleotide repeat (C7AC or C6AC3) observed in 50% of wolf Lp alleles is responsible for the long branch, which separates these haplotypes from the torso. This altered motif is yet to be identified in dog (Table 2). Even with this compositional change, the total repeat length was found to be similar between wolves (30–35 bp) and dogs (29–36 bp).
Figure 2. Median joining network for haplotypes generated from the combined SINE and Lp alleles.
The designation of Lp alleles is defined in Table 2. + and − after the allele designations indicates the presence/absence of the SINE insertion. Species membership in the network is designated with increasing node size (private<shared). Branch lengths are proportional to the number of mutational steps between the nodes. Colour reflects the white spotting phenotype of the individual sampled (Wolf = grey; for dog: extreme white = white; piebald = brown; Irish = green; solid = black).
The network was additionally coloured to reflect the white spotting phenotype of the individual sampled. Whilst only six haplotypes contained the SINE element, these included all haplotypes associated with the piebald and extreme white phenotypes. Three of these (32B+, 35A+ and 35B+) were also observed in wolf. Strikingly, a fourth haplotype (35D−) found in wolves was associated with Irish white spotting, in contrast none of the haplotypes found in solid dogs were shared with wolves (Figure 2, Table 2). This result shows that Lp alleles cannot on their own be causal for the extreme white and piebald phenotypes, since we found several wolves sharing the Lp alleles associated with these phenotypes.
The Exon1B-Del polymorphism was screened in all wolf samples and the deletion was only found in wolves from Scandinavia and the allele frequency was estimated at 18% (Table 1). Therefore, this 12 bp deletion could be a derived mutation in dogs, since we cannot exclude the possibility that the presence of the deletion in Scandinavian wolves could be due to dog-wolf hybridization in the past. However, this result also indicates that it is very unlikely that the Exon1B_Del polymorphism is the sole causal mutation underlying extreme white or piebald white spotting, since such phenotypes are not observed in the Scandinavian wolf population.
Transcriptional activity is affected by the SINE insertion and in particular the length polymorphism (Lp)
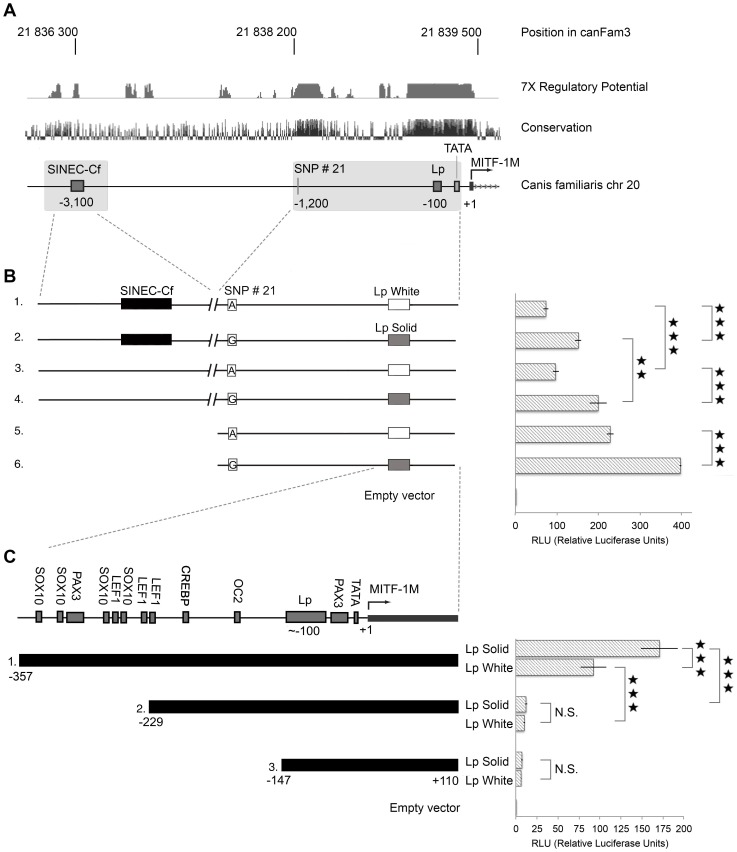
Luciferase reporter assays in human melanoma 624mel cells were performed to test the functional significance of the region containing the SINE insertion located about 3.1 kb upstream of the MITF-M TSS and the Lp located within the MITF-M promoter (Figure 3A). Six different reporter constructs were designed: (1) SINE+LpWhite (35A), corresponding to a sw allele, (2) SINE+LpSolid (31A), (3) no SINE+LpWhite (35A), (4) no SINE+LpSolid (31A), corresponding to a S allele, as well as two constructs without the SINE region (5) LpWhite or (6) LpSolid (Figure 3B).
Figure 3. Schematic overview of candidate causative mutations upstream of the canine MITF-M promoter and luciferase reporter activity.
(A) The SINEC-Cf, SNP#21 and Lp sequences included in constructs are indicated together with a comparative human-dog sequence alignment over the region. Tracks representing 7X regulatory potential and mammalian conservation for the corresponding region in humans are indicated. The broken vertical lines indicate the border between the promoter region and the upstream region combined in individual constructs. The region −1400 to −2800 bp upstream of the MITF-M promoter was not included in the construct. (B) Overview of the six luciferase reporter constructs used to assess the regulatory potential of different combinations of the SINE, SNP#21 and Lp variants and results of reporter assays. (C) Critical elements of the canine MITF-M promoter. Schematic overview of the canine MITF-M minimal promoter. Three different insert fragments (1, 2 and 3) and two variants of each fragment were designed corresponding to the S and sw haplotypes. The insert borders were defined based on the predicted transcription factor binding sites as indicated. Firefly luciferase reporter levels in B and C are presented in relation to control Renilla luciferase levels, normalized against the empty control vector. Stars in the graph indicate reporter activity significance levels in pair-wise comparisons; N.S. = Non Significant, * P<0.05, ** P<0.01, *** P<0.001. Error bars represent standard error of the mean. RLU = Relative Luciferase Units.
Highly significant differences in transcriptional activity were observed to be associated with the SINE and Lp. In particular, LpWhite was associated with a lower promoter activity in all three matched comparisons with the LpSolid variant (SINE_LpWhite vs. SINE_LpSolid; noSINE_LpWhite vs. no SINE_LpSolid; LpWhite vs. LpSolid; Figure 3B). Constructs containing the SINE insertion were associated with lower luciferase activities in the two matched comparisons with constructs lacking the SINE insertion, but only one reached statistical significance (noSINE_LpSolid vs. SINE_LpSolid; Figure 3B). The two polymorphisms appeared to influence transcriptional activity in an additive manner in this assay, which led to a highly significant, three-fold difference in transcriptional activity between the SINE_LpWhite reporter (mimicking the Extreme white haplotype) and the noSINE_LpS reporter (mimicking the Solid haplotype).
These constructs were confounded by the relationship between SNP#21 and the Lp since the SNP#21*A allele was always associated with LpWhite and the SNP#21*G with LpSolid (Figure 3B). In order to assess the functional significance of SNP#21, we performed luciferase assays with the constructs SINE_LpWhite, noSINE_LpWhite and LpWhite in which the candidate SNP position was mutated to a G (S allele). These constructs were then compared with the originals, which harboured an A at this position. No significant difference in luciferase activity was observed between constructs with the same SINE and Lp alleles differing only with regard to SNP#21 (Figure S1).
MITF-M promoter activity in dogs requires a 128 bp sequence located upstream of the Lp region
The region less than 400 bp upstream of the MITF-M promoter harbours eleven known transcription factor binding sites and the corresponding region in humans has an extremely high predicted regulatory potential (Figure 3C). Six different luciferase reporter constructs (Figure 3C) were designed in order to define the elements that constitute the essential parts of the canine MITF-M promoter and interact with the Lp variants associated with solid and white coat colour. The results demonstrated that the 128 bp sequence only present in Promoter construct 1, containing four SOX10, one PAX3 and three LEF1 binding sites (Figure 3C), is crucial for MITF-M promoter activity. Moreover, the results confirmed that the LpWhite variant is associated with significantly lower activity compared to the solid variant, but only when it occurs in the context of the entire minimal promoter (Promoter 1_LpWhite vs. Promoter 1_LpSolid; Figure 3C).
Further support for the functional significance of the MITF-M length polymorphism
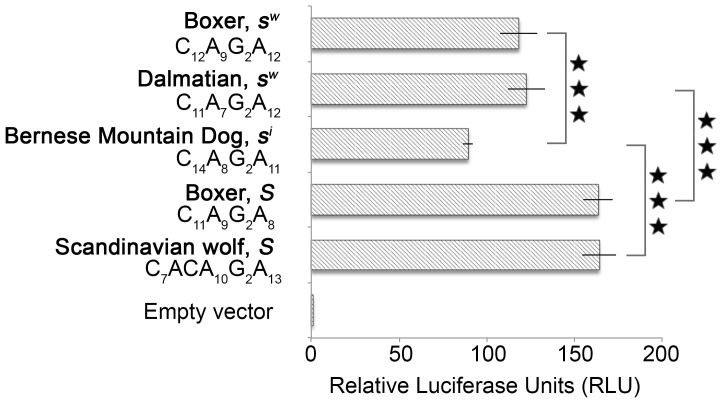
Encouraged by the very robust difference in promoter activity between the Solid and Extreme white Lp variants in the context of four different constructs (Figures 3B and C), we decided to characterize promoter activity with three additional Lp variants using the minimal MITF promoter construct described in Figure 3C. Dalmatian dogs are white with black spots and the breeding experiments Little carried out indicated that this spectacular coat colour pattern is due to homozygosity for Extreme white and Ticking [4]; Ticking is a dominant modifier of white spotting that causes spots of pigmented hair in white areas [2]. The MITF alleles found in Dalmatians had the shortest Lp (32 bp) of all alleles present in spotted dogs but had a C mononucleotide repeat (n = 11) that was longer than those detected in Solid Lp variants, n = 9–10 (Table 2). Interestingly, the Dalmatian Lp construct resulted in a reporter activity indistinguishable from the Boxer sw variant and significantly lower than the reporter activity associated with the Boxer S variant (Figure 4). The Bernese Mountain Dogs exhibit Irish white spotting (si/si) and carried an Lp variant denoted 35D that was also found in a wolf from Belarus (Table 2). The si reporter construct showed even lower promoter activity than the two sw constructs (Figure 4). Finally, we investigated one representative allele (34A, Table 2) of the Lp motif, characterized by a disrupted C mononucleotide repeat common in wolves but not yet observed among dogs. This construct (C7ACAG2A13) resulted in a reporter activity very similar to the one observed for the solid Boxer variant (Figure 4). These experiments strengthen the association between the Lp and MITF-M promoter activity, and show that three different promoter variants associated with a white spotting phenotype are consistently associated with a lower reporter activity than those obtained with constructs mimicking Solid alleles.
Figure 4. Luciferase activity associated with different alleles of the MITF length polymorphism in dogs and wolves.
The luciferase constructs were designed according to MITF-M minimal promoter activity (fragment 1 in Figure 3C). Firefly luciferase reporter levels are presented in relation to control Renilla luciferase levels, normalized against the empty control vector. Stars in the graph indicate reporter activity significance levels in pair-wise comparisons; N.S. = Non Significant, * P<0.05, ** P<0.01, *** P<0.001. Error bars represent standard error of the mean. RLU = Relative Luciferase Units.
Discussion
Coat colour was probably one of the first traits altered during dog domestication. White spotting in dogs can be traced back as long as there are written records or dog portraits, and have been present for thousands of years. Changes in coat colour occur during the domestication due to selection against wild-type colour; as a measure to facilitate animal husbandry (less camouflage), to facilitate the identification of undesired domestic/wild crossbred animals or for fashion and because of relaxed purifying selection [19]. In fact, Columella, the Roman authority on agriculture, wrote already in the first century AD that shepherds prefer white sheep dogs “because it is unlike a wild beast, and sometimes a plain means of distinction is required in the dogs when one is driving off wolves in the obscurity of early morning or even at dusk” [20]. Our previous study demonstrated that the Extreme white (sw) allele in Boxers is not associated with coding changes in MITF [6]. This conclusion was achieved by taking advantage of the fact that the reference individual (Tasha) for the dog genome assembly showed the flash phenotype (S/sw). This allowed us to sequence BACs representing each of the two alleles. A careful examination of the entire non-coding region showing the strongest association to white spotting, combined with a screen across a diverse panel of dogs, revealed four candidate mutations that showed the strongest association to white spotting. The present study demonstrated that none of these four candidates could be the sole causal mutation for Extreme white. This is because the four candidate mutations either occur at a relatively high frequency in the wild ancestor of dogs or do not perfectly co-segregate with phenotype among dog breeds. It is possible that these candidate mutations have an effect on pigmentation in wolves and they may even cause some subtle forms of white spotting, as this could be difficult to notice in a wolf with its long fur, basic grey colour and greying with age effect. However, we are convinced that the Extreme white allele is not common in wolves, as to the best of our knowledge Extreme white has never been reported in wolves. This colour morph would also be strongly selected against in a natural population due to both the lack of camouflage (except in arctic environments) and the well established association with deafness [14].
Our results combined with the previous characterization of the canine MITF locus strongly suggest that the different white spotting alleles (sw, sp and si) are not caused by three independent mutation events, but rather reflect haplotype effects due to different combinations of multiple causative regulatory mutations selected during dog domestication and breed formation. This interpretation is supported by the observation that there is more extensive sequence similarity among the sw, sp and si haplotypes than between any of these and haplotypes associated with solid colour [6]. For instance, Extreme white and Piebald alleles may share all four candidate mutations examined in this study. Thus, MITF in dogs is another example of ‘evolution of alleles’ by consecutive accumulation of multiple causal mutations that occurs in domestic animals at loci under strong selection [21].
This study has provided strong genetic and functional evidence that the length polymorphism (Lp) in the MITF-M promoter is one of the causal polymorphisms. It is probably the most important regulatory mutation causing decreased MITF-M transcription, and consequently affecting white spotting in dogs. Firstly, we have not yet found any overlap between the Lp alleles associated with white-spotting or solid colour in dogs (Table 2). The white-spotting alleles had a longer C mononucleotide repeat and all, except the one found in Dalmatians, had a longer total repeat region. We detected an extensive genetic diversity of the Lp among wolves. We found as many as 15 different alleles when only examining 17 wolves. A very striking and unexpected observation was the considerable Lp allele-sharing between white-spotting alleles in dogs and wolves, but no allele-sharing between solid alleles and wolf alleles. This is unexpected because Solid is assumed to be the wild-type allele at this locus. We propose that the explanation for this pattern is that there has been selection both for and against white spotting (thus for solid colour in the latter case) during dog domestication and breed formation. In the early history of domestication there was selection against wild-type colours, while during breed formation there was selection for breed-specific colour such as white spotting in Boxers and solid black colour in Labradors. A repeat polymorphism like this is expected to have a very dynamic evolution, where new alleles are generated by slippage during DNA replication, consistent with the rich Lp diversity among wolves. Thus, the very tight Lp distribution observed in solid dogs and the lack of overlap with alleles found in wolves is hardly in agreement with selective neutrality.
The extensive Lp diversity may have no obvious phenotypic effects in wolves, because such effects may depend on epistatic interaction with other sequence polymorphisms not present in wolves at MITF or at other loci in the genome. However, it is also possible that the MITF polymorphisms contribute to coat colour diversity in wolves, ranging from dark grey to almost white colour. This is worth exploring using samples from phenotypically well-characterized wolves.
This study also provided functional data supporting the conclusion that the Lp directly affects white spotting. Luciferase reporter constructs containing the longer Lp variants, associated with Extreme white (sw) in Boxers and Dalmatians and with the Irish spotting (si) allele in Bernese Mountain Dogs, had all significantly lower activities compared with constructs containing the short Lp Solid variant, indicating that the Lp affects MITF-M transcription (Figures 3B and 4). The Extreme white (sw) and Solid Lp variants in Boxers differ in size by four nucleotides, which corresponds to approximately one half turn of the DNA helix. This may have important consequences for the functional interaction between transcription factors binding to either flanking side of the Lp (Figure 3C). Transcription factors that face the same side of the DNA helix in the context of the short Lp Solid variant will face opposite sides of the DNA helix in the Lp White variant, which is expected to result in reduced capacity to establish functional interaction. Previous studies have shown that transcription is markedly affected when crucial promoter elements are separated by half a helical turn [22]–[23]. In favour of such a scenario is the cluster of LEF1, SOX10 and PAX3 binding sites which are located approximately 200 bp upstream of the Lp. Importantly, the PAX3 and SOX10 transcription factors have been shown to physically interact and to be critical for MITF-M transcription [24]. Furthermore, a 10 bp insertion that disrupts a PAX3 binding site in the MITF-M promoter is causing the “splashed white” phenotype in horses [25]. The corresponding PAX3 site in the dog promoter is the one located between the Lp and the TATA-box (Figure 3C). LEF1 is known to physically interact with MITF and to be involved in transcriptional self-activation of the MITF-M promoter [26]. Our data support previous findings from other species that the cluster of LEF1, SOX10 and PAX3 binding sites are crucial for MITF-M expression (Figure 3C). Our data also demonstrate that it is unlikely that the Lp region has a direct effect on transcription factor binding, as we observed no effect of the Lp variants unless the upstream 200 bp region containing the cluster of LEF1, SOX10 and PAX3 sites were included in the construct.
The SINEC-Cf insertion located about three kb upstream of the MITF-M transcription start site had a widespread distribution among wolf populations, implying that this must be an ancestral polymorphism rather than a recent derived mutation. However, the SINE insertion shows a very strong association to the Extreme white and Piebald haplotypes but is rare or absent among Solid and Irish spotting haplotypes (those associated with no spotting or the least white spotting). Our reporter assay data suggested that the SINE-region acts as a weak silencer element and that it acts in an additive mode together with the long Lp variants to reduce MITF-M promoter activity associated with sw and sp. The mechanism underlying the repressive function of the region containing the SINE insertion remains to be defined, but for example, SINE elements are known to be targets for DNA methylation [27]. It is possible that differential methylation within this region is involved in controlling MITF-M transcription. Unfortunately, we have not been able to analyse the MITF-M expression or methylation status during different stages of canine melanocyte development due to the implicit difficulties in sampling dog embryos.
SNP#21 occurs in the vicinity of an evolutionary conserved element but this actual nucleotide position is not strongly conserved. It could be a derived mutation since it is rare in wolves. The association between SNP#21*A and white spotting across breeds is far from perfect and the reporter assay did not reveal any effect on transcriptional regulation. However, this does not exclude the possibility that this substitution could have a minor effect on white spotting, as a reporter assay will never perfectly replicate transcriptional regulation in vivo.
We also provided support for the possibility that the 12 bp deletion in exon 1B is a derived allele that promotes white spotting. This variant was only found in the highly inbred Scandinavian wolves, and was thought unlikely to be causative in the previous study because it was found at a low frequency in some solid dogs [6]. However, the present study has shown that no MITF polymorphism shows complete agreement with phenotype and in fact, the insertion in exon 1B, together with the Lp, shows the best concordance with white spotting. The deletion appears to be fixed in dogs with the extreme white and piebald phenotypes, but rare or absent in wolves, solid dogs and dogs with the Irish spotting phenotype. No functional assays were attempted for this polymorphism since it occurs in an exon and in our previous study we concluded that it was unclear if this exon is functional in carnivores [6]. However, recently released RNAseq data (January 2104; Broad CanFam3.1/canFam3; http://genome.ucsc.edu) show that MITF exon 1B is transcribed in dogs and was found in one transcript from blood (CUFF.25976.1) and one from lung (CUFF.27047.1) Both transcripts originated from the derived allele associated with Extreme white and Piebald. This finding suggests that the 12 bp deletion in exon 1B may very well affect MITF function during the development of melanocytes. Thus, the extensive white spotting in Extreme white and Piebald dogs may be due to the combined effect of mutations affecting MITF-M transcription and a coding mutation in exon 1B.
The luciferase assay indicated that the Lp variant associated with Irish spotting had an even lower MITF-M promoter activity than the variants associated with Extreme white and Piebald, although the two latter alleles cause more extensive white spotting (Figure 4). However, this is consistent with our interpretation that canine MITF alleles are not due to single mutations but the combined effect of multiple mutations in the MITF region. An important difference between Irish spotting and Extreme white/Piebald is that only the two latter alleles carry the SINE insertion upstream of MITF-1M and the 12 bp deletion in MITF-1B. Another interesting difference between these two groups of alleles is that si/si homozygotes show a high degree of symmetric white spotting whereas sp/sp and sw/sw homozygotes show asymmetric white spotting (Figure 1). This is probably not caused by the nature of the underlying mutations but rather a dosage effect, i.e. to which extent MITF function is affected during melanocyte development, because sw/S heterozygotes also show symmetric white spotting.
MITF must be one of the loci in the dog genome that has been under strongest positive selection during domestication and breed formation. It is likely that Little's [4] original classification of three white spotting alleles, Extreme white, Piebald and Irish spotting, is an underestimate and that a more extensive allelic diversity is created by combining the variability at the Lp with other sequence variants in the MITF region. For instance, Beagles are considered to be homozygous Piebald but as Little described, they vary extensively from almost solid coloured to a phenotype mimicking extreme white. It is still an open question whether this variability is caused by genetic heterogeneity at MITF or genetic variation at other loci affecting melanocyte development. In order to advance our knowledge about MITF it will be essential to make further genetic studies of dogs that are very well characterized for the white spotting phenotype. We recommend that coat colour is carefully registered when performing genome-wide associations studies of other traits or disorders in breeds with a variable white spotting phenotype, like the Beagle. Such studies will reveal the extent to which the variability in white spotting is caused by genetic heterogeneity at MITF or if the influence of yet unidentified modifying loci is at play. Furthermore, it is essential to resequence the entire MITF region from Piebald and Irish spotting chromosomes since they may harbour sequence variants in addition to the ones evaluated in the current study, which were detected in a sequence comparison of the Extreme white and Solid alleles from a single heterozygous dog [6].
Materials and Methods
Design of luciferase constructs
The exact borders for the two genomic regions containing SINE and Lp were chosen based on the 7X regulatory potential and mammalian conservation in the corresponding human regions [28] (Figure 3A). These were identified by performing a BLAT search against the human sequence in the UCSC [29] Genome Browser (http://genome.ucsc.edu/), since the regulatory potential track was only available in the Human Mar. 2006 assembly (NCBI36/hg18). Luciferase reporter constructs were designed as follows (Figure 3B; Table 3): SINE+Lp White (corresponding to the sw allele), no SINE+LpWhite (similar to si), SINE+LpSolid and no SINE+LpSolid (corresponding to S allele). In order to investigate the transcriptional activities of the two Lp's in the absence of the SINE-region, two additional constructs were included, with only the long or short Lp, respectively.
Table 3. Polymorphisms included in the Luciferase reporter design defined in Figure 3.
| Position | ||||
| Sequence polymorphism | Genome assembly (bp)1 | Position relative to TSS | Phenotype correlation2 | Designation2 |
| Simple repeat (12/14 bases) | 21,835,941–21,835,953 | −3450 bp | No | 25 |
| SINE insertion | 21,836,232–21,836,429 | −3150 bp | Yes | 24 |
| Candidate SNP | 21,838,204 | −1200 bp | Yes | 21 |
| Simple repeat (9 bases) | 21,838,718–21,838,745 | −700 bp | No | 20 |
| SNP | 21,838,840 | −600 bp | No | 19 |
| Length polymorphism | 21,839,331–21,839,366 | −100 bp | Yes | 18 |
| Indel (2 bases) | 21,839,397 | −60 bp | No | 17 |
Inserts containing SINE/no SINE and LpSolid or White (LpSolid: C10A8G2A11, LpWhite: C12A9G2A12) (Figure 3B) were PCR-amplified from DNA samples from a solid and a white Boxer using KOD Hot Start DNA Polymerase (Novagen, Merck KGaA, Darmstadt, Germany). Sequences containing the Lp were cloned, transformed and grown according to manufacturer's instructions (pCR®-Blunt II-TOPO® vector, One Shot® MAX Efficiency® DH10B-T1® Competent Cells, Invitrogen, Carlsbad, CA, USA). Purified Lp clones were both sequenced and fragment analysed (MegaBACE, GE Healthcare, Uppsala, Sweden) in order to isolate clones with a correct composition of the Lp. Lp inserts of desired size were subsequently restricted from pCR®-Blunt II-TOPO® vector (Invitrogen), ligated into pGL3 Basic vector (Promega, Madison, WI, USA) and cultured according to manufacturer's instructions (One Shot® MAX Efficiency® DH10B-T1® Competent Cells, Invitrogen), followed by sequencing and verification by fragment analysis to ensure fidelity. PCR products containing the SINE/no SINE were then ligated into the pGL3 Basic+Lp vector. The ready vectors were sequenced and the Lp again verified by fragment analysis. In total, six different constructs were designed (Figure 3B).
The constructs used to identify the canine MITF minimal promoter were designed based on the predicted regulatory elements in the region. These were identified by using TransFac Professional and the UCSC Genome Browser 7X regulatory potential and mammalian conservation tracks in the corresponding human region (hg18 assembly). Three different constructs (Promoter 1–3) were designed for both LpSolid and LpWhite (Figure 3C). Promoter 1 was approximately 470 bp, Promoter 2 was approximately 340 bp and Promoter 3 approximately 260 bp. Genomic DNA from S and sw boxers was PCR-amplified, ligated into pGL3 Basic vector (Promega) and cultured according to manufacturer's instructions (One Shot® MAX Efficiency® DH10B-T1® Competent Cells, Invitrogen).
The constructs used to test the different Lp variants were amplified using the same primers as for the Promoter 1 construct. All primers used in PCR and sequencing are listed in Table S3.
Site-directed mutagenesis
The nucleotide at the candidate SNP#21 located 1.2 kb upstream of the MITF-M TSS was altered by site-directed mutagenesis (GENEART Site-Directed Mutagenesis System, Invitrogen) in the following constructs: SINE+LpWhite, no SINE+LpWhite and LpWhite (Figure S1). All constructs were verified by sequencing. Oligonucleotides used in mutagenesis are listed in Table S3.
Cell culture
The human melanoma cell line 624mel [30] was grown in RPMI 1640 (Gibco, Invitrogen GmbH, Karlsruhe, Germany) supplemented with 10% heat inactivated foetal bovine serum (FBS) (Gibco) and 1X Penicillin, Streptomycin and Glutamine (PSG) (Gibco). Cells were split every two to three days and tested negative for mycoplasma (Venor GeM Mycoplasma Detection Kit, Minerva Biolabs, Berlin, Germany).
Luciferase assays
Human 624mel melanoma cells [30] were transiently transfected at approximately 90% confluency at passage three in 6-well plates. Triplicates for each construct were performed for each experiment. 2 µg of luciferase reporter plasmid (Promega) and 50 ng of control Renilla plasmid (Promega) were transfected into each well of a 6-well plate utilizing 4 µl of Lipofectamine 2000 CD reagent (Invitrogen) in Opti-MEM medium (Gibco). The cells were lysed 24 h post-transfection and Firefly and Renilla luciferase activities were measured using the Dual Luciferase Reporter Assay System (Promega) with an Infinite M200 Luminometer (Tecan Munich GmbH, Kirchheim, Germany). Firefly values were divided by Renilla values to normalize for fluctuations in plated cells and transfection efficiency. Expression values of all test constructs were compared to the expression of the empty vector. Transfections were independently repeated four times per experiment. Values were transformed utilizing a transformation selector [31] (LN BASE e (X+1)) followed by a one way analysis of variance (Holm-Sidak method) (SigmaPlot v12).
Genotyping of SINEC-Cf and Length Polymorphism (Lp) in wolves
The presence of the SINEC-Cf insertion was analysed by PCR followed by agarose gel electrophoresis. The Lp and unique flanking sequence from each wolf DNA sample was PCR-amplified and cloned according to manufacturer's instructions (pCR®-Blunt II-TOPO® vector, One Shot® MAX Efficiency® DH10B-T1® Competent Cells, Invitrogen). Approximately 10 colonies/sample were sequenced. Where multiple Lp variants were identified from the same individual, the most prevalent Lp clone was selected to be representative. All primers are specified in Table S3.
Genotyping of SNP#21 in wolves and dogs
Samples were genotyped on an ABI7900HT instrument using the TaqMan Allelic Discrimination Assay according to the manufacturer's instructions (Applied Biosystems, Foster City, CA, USA). Sequences for primers and probes are available in Table S3.
AMY2B copy number assay
The AMY2B locus copy number assay was performed as described [18].
Haplotype visualisation
The relationship between Lp and SINE haplotypes was investigated with conservative (ε = 0) median network (Network version 4.6.1.1; http://www.fluxus-engineering.com). Haplotype components (Table 2) were weighted (w) as follows: [SINE/-], w = 20; CX, w = 10; [-/A], w = 20; [-/CN], w = 20; AY, w = 10; AZ, w = 10. This generated a priority order where the components with the lowest expected mutation rate (insertion/deletion events) were assigned the highest ranking [32].
Supporting Information
Luciferase activity of different combinations of the SINE, LpWhite and the two SNP#21 alleles. Firefly luciferase reporter levels are presented in relation to control Renilla luciferase levels, normalized against the empty control vector. Stars in the graph indicate reporter activity significance levels in pair-wise comparisons; N.S. = Non Significant, * P<0.05, ** P<0.01, *** P<0.001. Error bars represent standard error of the mean. RLU = Relative Luciferase Units.
(JPG)
Wolves genotyped for the SINE, Exon 1B deletion, SNP#21, Lp, and AMY2B copy number.
(PDF)
Allele frequency for SNP#21 in 17 dog breeds.
(PDF)
Primer sequences.
(PDF)
Acknowledgments
Sincere thanks are due to Steven Rosenberg who provided the 624mel cell line and to Anders Sundström for dog drawings.
Funding Statement
The study was supported by the Swedish Foundation for Strategic Research, the Knut and Alice Wallenberg Foundation, and the Swedish Research Council. JRSM was supported by the Swedish Research Council, FORMAS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Montoliu L, Oetting WS, Bennett DC (2011) Color Genes. European Society for Pigment Cell Research: WorldWideWeb(URL:http:—www.espcr.org-micemut).
- 2. Kaelin CB, Barsh GS (2013) Genetics of Pigmentation in Dogs and Cats. Annual Review of Animal Biosciences 1: 125–156. [DOI] [PubMed] [Google Scholar]
- 3.Andersson L, Plastow G (2011) The Genetics of the Pig. In: Rothschild MF, Ruvinsky A, editors. Molecular genetics of coat colour variation. Oxon, UK: CAB International. pp. 38–50. [Google Scholar]
- 4.Little CC (1957) The inheritance of coat colour in the dogs. Comstock Publishing Associates, Cornell University Press, Ithaca, NY. [Google Scholar]
- 5. Rothschild MF, Van Cleave PS, Glenn KL, Carlstrom LP, Ellinwood NM (2006) Association of MITF with white spotting in Beagle crosses and Newfoundland dogs. Anim Genet 37: 606–607 10.1111/j.1365-2052.2006.01534.x [DOI] [PubMed] [Google Scholar]
- 6. Karlsson EK, Baranowska I, Wade CM, Salmon Hillbertz NHC, Zody MC, et al. (2007) Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet 39: 1321–1328 10.1038/ng.2007.10 [DOI] [PubMed] [Google Scholar]
- 7. Leegwater PA, van Hagen MA, van Oost BA (2007) Localization of White Spotting Locus in Boxer Dogs on CFA20 by Genome-Wide Linkage Analysis with 1500 SNPs. Journal of Heredity 98: 549–552 10.1093/jhered/esm022 [DOI] [PubMed] [Google Scholar]
- 8. Levy C, Khaled M, Fischer DE (2006) MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med 12: 406–414. [DOI] [PubMed] [Google Scholar]
- 9. Steingrímsson E, Copeland NG, Jenkins NA (2004) Melanocytes and the microphthalmia transcription factor network. Annu Rev Genet 38: 365–411 10.1146/annurev.genet.38.072902.092717 [DOI] [PubMed] [Google Scholar]
- 10. Tsuchida S, Takizawa T, Abe K, Okamoto M, Tagawa M (2009) Identification of microphthalmia-associated transcription factor isoforms in dogs. The Veterinary Journal 182: 283–293 10.1016/j.tvjl.2008.06.004 [DOI] [PubMed] [Google Scholar]
- 11. Steingrímsson E, Moore KJ, Lamoreux ML, Ferré-D'Amaré AR, Burley SK, et al. (1994) Molecular basis of mouse microphthalmia (mi) mutations helps explain their developmental and phenotypic consequences. Nat Genet 8: 256–263 10.1038/ng1194-256 [DOI] [PubMed] [Google Scholar]
- 12. Tassabehji M, Newton VE, Read AP (1994) Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet 8: 251–255 10.1038/ng1194-251 [DOI] [PubMed] [Google Scholar]
- 13. Smith SD, Kelley PM, Kenyon JB, Hoover D (2000) Tietz syndrome (hypopigmentation/deafness) caused by mutation of MITF. J Med Genet 37: 446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Strain GM (2004) Deafness prevalence and pigmentation and gender associations in dog breeds at risk. The Veterinary Journal 167: 23–32. [DOI] [PubMed] [Google Scholar]
- 15. Blake JA, Bult CJ, Kadin JA, Richardson JE, Eppig JT (2011) The Mouse Genome Database (MGD): premier model organism resource for mammalian genomics and genetics. Nucleic Acids Res 39: D842–D848 10.1093/nar/gkq1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Widlund HR, Fisher DE (2003) Microphthalamia-associated transcription factor: a critical regulator of pigment cell development and survival. Oncogene 22: 3035–3041 10.1038/sj.onc.1206443 [DOI] [PubMed] [Google Scholar]
- 17. Schmutz SM, Berryere TG, Dreger DL (2009) MITF and White Spotting in Dogs: A Population Study. Journal of Heredity 100: S66–S74 10.1093/jhered/esp029 [DOI] [Google Scholar]
- 18. Axelsson E, Ratnakumar A, Arendt M-L, Maqbool K, Webster MT, et al. (2013) The genomic signature of dog domestication reveals adaptation to a starch-rich diet. Nature 495: 360–364 10.1038/nature11837 [DOI] [PubMed] [Google Scholar]
- 19. Fang M, Larson G, Ribeiro HS, Li N, Andersson L (2009) Contrasting mode of evolution at a coat color locus in wild and domestic pigs. PLoS Genet 5: e1000341 10.1371/journal.pgen.1000341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forster ES, Heffner, EH (translators) (1968) Lucius Junius Moderatus Columella on agriculture. Vol. II. Loeb Classical Library No. 407. Heinemann, London.
- 21. Andersson L (2013) Molecular consequences of animal breeding. Curr Opin Genet Dev 23: 295–301 10.1016/j.gde.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 22. Su M, Lee D, Ganss B, Sodek J (2006) Stereochemical analysis of the functional significance of the conserved inverted CCAAT and TATA elements in the rat bone sialoprotein gene promoter. J Biol Chem 281: 9882–9890 10.1074/jbc.M508364200 [DOI] [PubMed] [Google Scholar]
- 23. Yu M, Yang XY, Schmidt T, Chinenov Y, Wang R, et al. (1997) GA-binding protein-dependent transcription initiator elements. Effect of helical spacing between polyomavirus enhancer a factor 3(PEA3)/Ets-binding sites on initiator activity. J Biol Chem 272: 29060–29067. [DOI] [PubMed] [Google Scholar]
- 24. Lang D, Epstein JA (2003) Sox10 and Pax3 physically interact to mediate activation of a conserved c-RET enhancer. Human Molecular Genetics 12: 937–945. [DOI] [PubMed] [Google Scholar]
- 25. Hauswirth R, Haase B, Blatter M, Brooks SA, Burger D, et al. (2012) Mutations in MITF and PAX3 cause “splashed white” and other white spotting phenotypes in horses. PLoS Genet 8: e1002653 10.1371/journal.pgen.1002653.s009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saito H, Yasumoto K, Takeda K, Takahashi K, Fukuzaki A, et al. (2002) Melanocyte-specific microphthalmia-associated transcription factor isoform activates its own gene promoter through physical interaction with lymphoid-enhancing factor 1. J Biol Chem 277: 28787–28794 10.1074/jbc.M203719200 [DOI] [PubMed] [Google Scholar]
- 27. Ponicsan SL, Kugel JF, Goodrich JA (2010) Genomic gems: SINE RNAs regulate mRNA production. Curr Opin Genet Dev 20: 149–155 10.1016/j.gde.2010.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kolbe D, Taylor J, Elnitski L, Eswara P, Li J, et al. (2004) Regulatory potential scores from genome-wide three-way alignments of human, mouse, and rat. Genome Research 14: 700–707 10.1101/gr.1976004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King DC, Taylor J, Elnitski L, Chiaromonte F, Miller W, et al. (2005) Evaluation of regulatory potential and conservation scores for detecting cis-regulatory modules in aligned mammalian genome sequences. Genome Research 15: 1051–1060 10.1101/gr.3642605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marincola FM, Shamamian P, Alexander RB, Gnarra JR, Turetskaya RL, et al. (1994) Loss of HLA haplotype and B locus down-regulation in melanoma cell lines. J Immunol 153: 1225–1237. [PubMed] [Google Scholar]
- 31. Kirk RE (1982) Experimental Design: Procedures for the Behavioral Sciences. Brooks/Cole 79–84. [Google Scholar]
- 32. Bandelt HJ, Macaulay V, Richards M (2000) Median networks: speedy construction and greedy reduction, one simulation, and two case studies from human mtDNA. Mol Phylogenet Evol 16: 8–28 10.1006/mpev.2000.0792 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Luciferase activity of different combinations of the SINE, LpWhite and the two SNP#21 alleles. Firefly luciferase reporter levels are presented in relation to control Renilla luciferase levels, normalized against the empty control vector. Stars in the graph indicate reporter activity significance levels in pair-wise comparisons; N.S. = Non Significant, * P<0.05, ** P<0.01, *** P<0.001. Error bars represent standard error of the mean. RLU = Relative Luciferase Units.
(JPG)
Wolves genotyped for the SINE, Exon 1B deletion, SNP#21, Lp, and AMY2B copy number.
(PDF)
Allele frequency for SNP#21 in 17 dog breeds.
(PDF)
Primer sequences.
(PDF)