Abstract
Reforestation of riparian zones is increasingly practiced in many regions for purposes of biodiversity conservation, bank stabilisation, and improvement in water quality. This is in spite of the actual benefits of reforestation for recovering underlying soil properties and function remaining poorly understood. Here we compare remnant riparian rainforest, pasture and reforestation plantings aged 2–20 years in an Australian subtropical catchment on ferrosols to determine the extent to which reforestation restores key soil properties. Of the nine soil attributes measured (total nitrogen, nitrate and ammonium concentrations, net nitrification and ammonification rates, organic carbon, bulk density, fine root biomass and water infiltration rates), only infiltration rates were significantly lower in pasture than remnant riparian rainforest. Within reforestation plantings, bulk density decreased up to 1.4-fold and infiltration rates increased up to 60-fold with time post-reforestation. Our results suggest that the main outcome of belowground processes of early reforestation is the recovery of the soils' physical structure, with potential beneficial ecosystem services including reduced runoff, erosion and associated sediment and nutrient loads in waterways. We also demonstrate differential impacts of two commonly planted tree species on a subset of soil properties suggesting that preferential planting of select species could accelerate progress on specific restoration objectives.
Introduction
Globally, riparian zones are widely recognized for their critical role in water regulation [1]–[5], and conservation of biodiversity [6]–[9]. Clearing of vegetation from stream banks can cause increased soil erosion and decreased water quality through the loss of filtration services, in addition to the loss of critical species habitat [10]. In direct response, degraded stream-banks are increasingly being restored to reinstate ecological functions [11]. Riparian zones are also regarded as foci of restoration opportunity as they are often preferentially renounced from agricultural production over agronomically more valuable areas [9].
Soil physical, chemical and functional properties change with succession from pasture to secondary forest including reforestation plantings in the tropics, subtropics and temperate regions [12]–[16]. Soil properties such as bulk density, soil water content, fine root biomass, porosity, stocks of nitrogen, carbon and phosphorus, nitrogen cycling, availability of macro- and micronutrients, as well as toxins (metals, salts), have been studied in context of land use change [17]–[19]. However, most studies focus on mid- to long-term effects of reforestation with less consideration of the short-term effects (<10–20 years) of reforestation plantings, and associated reinstatement of soil functions that might motivate landholders to become actively engaged in restoration. Another knowledge gap is the relative contribution of tree species to outcomes of riparian restoration plantings, and such information could inform decisions on species selection and reforestation goals.
In Australia, riparian reforestation is a major focus of habitat restoration in the tropics and subtropics; over 70% of rainforest restoration projects have targeted banks of rivers and streams [20]. However, much evaluation of the success of reforestation plantings has focused on aboveground attributes [21]–[23], with less regard to belowground processes including soil function (but see Paul [14] for the recovery of soils via different rainforest restoration pathways). Understanding belowground processes is important to quantify the capacity of reforestation to recover ecosystem function.
Soil properties and functions would intuitively be expected to change over time following reforestation towards a state convergent on intact rainforest ecosystems. Studying riparian soil under remnant rainforest, pasture, and within 20 years post reforestation, we addressed three questions (i) how do soil attributes differ between pasture and remnant riparian rainforest, (ii) how do soils change with time following reforestation, and (iii) do tree species affect soils differentially following reforestation?
The study was motivated by the need for restoration of forest cover in water catchments due to the improvements in water quality that can be achieved [24]. The region targeted for our study is located in the Australian subtropics and has experienced large-scale clearing of rainforest since the mid 19th century. The region is an important water catchment that supplies drinking water to more than 100000 people in the region [25], and numerous land owners and stakeholders are committed to ongoing restoration of riparian zones. Our research aimed to generate knowledge of soil properties to direct future reforestation activities.
Materials and Methods
2.1. Study site
This study focused on riparian areas in the Maleny region in southeastern Queensland, Australia (26°45′S, 152°48′E). The climate is classified as subtropical with an annual rainfall of 1900 mm and annual daily mean temperature maximum and minimum of 23.2°C and 14.2°C, respectively. The soils in the region are classified as permeable red clay-loam ferrosols [26], [27] or Nitisol in the US soil taxonomy. Prior to European settlement in the mid-1800s and subsequent land clearing for timber production and agriculture (especially dairy production), the region was dominated by cool-subtropical rainforest classified as complex notophyll vine forest [28], [29].
2.2. Sampling design
Field measurements and soil sampling were carried out between May and June 2013. The sampling design was based around riparian reforestation projects established in the region by the Lake Baroon Catchment Care Group (LBCCG). Reforestation sites were grouped into five spatial clusters across the Maleny Plateau (range of elevation 196 to 441 m a.s.l.) to capture broad variation in landform and land use history. A subset of one to three reforestation sites ranging in age between 2 and 20 years were then selected from each cluster for a total of 10 sites (Table 1). Sites were also established in remnant riparian rainforest where available (2 of 5 clusters) and samples were collected from pasture (adjacent to plantings or remnant forest) at all sites in each cluster.
Table 1. GPS coordinates and ages of reforestation and remnant sites.
| Site type | Age (years) | Site location |
| Reforestation | 3 | 26°46′02 S, 152°49′38 E |
| Reforestation | 3 | 26°46′17 S, 152°49′51 E |
| Reforestation | 4 | 26°45′06 S, 152°50′46 E |
| Reforestation | 6 | 26°42′01 S, 152°53′58 E |
| Reforestation | 7 | 26°46′10 S, 152°49′57 E |
| Reforestation | 8 | 26°44′05 S, 152°50′54 E |
| Reforestation | 11 | 26°44′46 S, 152°51′13 E |
| Reforestation | 11 | 26°46′12 S, 152°49′39 E |
| Reforestation | 12 | 26°46′08 S, 152°49′43 E |
| Reforestation | 20 | 26°42′01 S, 152°53′58 E |
| Remnant | - | 26°45′07 S, 152°51′02 E |
| Remnant | - | 26°44′50 S, 152°51′11 E |
All sites were located on private land in the Maleny region. Pasture sites were located immediately adjacent to each forested site. Ages are based on the year that they were planted relative to 2013.
At each reforestation or remnant site, five subplots were established. Each subplot was deliberately located under a different tree species. The five tree species targeted here were derived from a pool of species commonly used in local rainforest plantings, which in turn were selected to include early to late successional species, nitrogen fixing and/or waterlogging tolerant species. The tree species chosen to investigate species-specific effects on soil properties included Acacia melanoxylon R.Br. (Fabaceae, N2 fixer, early succession species), Ficus coronata Spin (Moraceae, nitrophile, waterlogging tolerant, early succession species), Flindersia schottiana F. Muell. (Rutaceae, mid-late succession), Homalanthus nutans (G.Forst.) Guill. (Euphorbiaceae, early succession species) and Podocarpus elatus R.Br. ex Endl (Podocarpaceae, mid-late succession, gymnosperm). In the event that one of the chosen tree species was absent from a site, a closely related species was substituted or, if not available, subplots were located under a random tree within the site. Subplots were positioned under the canopy of each selected tree (mean distance from stem 89.5 cm, SD 45.2 cm).
At each reforestation or remnant forest site, we performed the following procedures: (1) collected a single 8 cm diameter core to a depth of 30 cm (hereafter “textural core”) and divided it into 0–15 cm and 15–30 cm fractions for textural analysis; (2) performed an infiltration test; and, (3) collected two samples of the surface soil using a 12.5 cm diameter, 8.5 cm tall, 1043 cm3 volume bulk density ring (hereafter “bulk density ring”) from each of five subplots for analysis of physical and chemical properties of the soil. In addition, a single texture core and two to four bulk density ring subplots were collected from adjacent pasture and infiltration measurements performed. For the purposes of analysis, samples and infiltration measures from pasture were calculated as means for each cluster.
Overall, we sampled 10 reforestation sites, 2 remnant riparian rainforest sites (Table 1) and 5 pasture clusters comprising 24 cores for soil texture analysis, 150 cores for soil chemical and physical analyses and 24 infiltration tests. All sites were private land holdings and owners approval was obtained by Mark Amos (Lake Baroon Catchment Care Group, Maleny, info@lbccg.org.au). Upon returning from the field, all soil samples were stored at 4 °C in sealed plastic bags prior to respective analyses.
2.3. Texture analysis
Changes in soil texture mostly occur due to weathering over very long time periods and as demonstrated by Maloney [13]. Unless there is erosion or deposition of soil material, texture is unlikely to vary over time since reforestation. For this reason, the purpose of conducting a soil texture analysis at each site was not to determine changes due to reforestation, but to characterize and evaluate background variation in soil types among sites that might warrant differential treatments of sites in the analysis. Soil samples were not dispersed prior to analysis and as such, the calculated percentages represent the relative size of secondary particles (aggregates) present rather than primary particle size sensu stricto. This was considered a more relevant measure of soil function than particle size determined using standard methods, as it gives a relative measure of soil aggregation and structure. Furthermore, the ferrosols in this study are recognized as being subplastic, with clay-sized particles being primarily composed of sesquioxides that do not readily break down into primary particles [30]. These analyses of soil aggregation were conducted on air-dried soil from 0–15 and 15–30 cm depth cores according to the hydrometer method for soil texture analysis [31], [32]. For each sample, 40 g of air-dried soil was ground and mixed with 100 ml 50 g l−1 Sodium hexametaphosphate solution and filled to 500 ml with deionized water. Samples were shaken overnight and then emptied into a 1 l cylinder for analysis. Cylinders were filled to 1 l with deionized water, mixed with a plunger and left to settle. Temperature and specific gravity readings were taken 40 seconds and 7 hours after plunging [32]. Using temperature and specific gravity readings, the fraction of sand, silt and clay sized particles in each soil sample was then calculated as a percentage.
2.4. Soil physical properties: bulk density and infiltration rates
Soil bulk density was determined from the second bulk density ring in each subplot. Fresh soil was oven dried to a constant weight at 105 °C. Soil bulk density is expressed as grams of dry soil per volume. To quantify fine root biomass, soil from one bulk density ring per subplot was passed through a sieve (8 mm mesh) and roots were manually removed. Roots were then oven dried at 65 °C to a constant weight, and results expressed as grams of fine roots per kilogram of dry soil.
Infiltration rates were measured using a ponded disk permeameter [33]. Soils were assumed to be at field capacity as the region had received in excess of 30 mm in the week preceding the first set of measurements and over 100 mm in the week prior to the second sampling visit. Infiltration tests were performed at a random, relatively flat area representative of the site. Tests in the adjacent pasture were performed at a random point beyond the farthest extent of the neighbouring forest canopy. Upon setting up of the permeameter, grass and other ground covers were clipped to just above the soil surface and the soil pre-moistened with rainwater to reduce the time needed to reach steady state infiltration. The permeameter was filled with rainwater collected from water tanks on local landholder properties to ensure that water quality was similar to that occurring under natural condition. Once the disk permeameter was in place, measurements of water level changes were recorded until the time interval between measurements was stable. As soils were close to field capacity at the start of measurements, steady state was reached quickly. Infiltration rate was calculated as the average volume change per second and then converted into millimeters per hour.
2.5. Soil chemical properties: nitrogen and organic carbon
Total concentrations of soil nitrogen (TN) and organic carbon (SOC) were determined using a TruSpec CHN analyzer (LECO Australia Pty. Ltd., Castle Hill, NSW 2154, Australia), using dried, ground and sieved soil (2 mm sieve). Nitrate (NO3 -) and ammonium (NH4 +) were extracted with potassium chloride (KCl) and quantified via colourimetric assays. To remove NO3 - and NH4 + from soil exchange sites, 15 g of fresh sieved soil was mixed with 1 M KCl in a 15 ml falcon tube and shaken on an orbital shaker for 1 hour. Samples were centrifuged at 4000 rpm for 4 minutes and 1 ml of the supernatant was frozen until analysis via colourimetric assays according to standard procedures [34], [35]. Net nitrification and ammonification rates were calculated from the difference in concentration between fresh soil samples and those incubated for 20 days at 28°C. Briefly, 15 g of soil were weighed into 50 ml falcon tubes and incubated for 20 days. Soils were kept at field moisture by watering daily with deionized water. Seeds that germinated during the incubation period were removed. Concentrations of NO3 - and NH4 + in the incubated soils following KCl extraction were quantified using colourimetric assays as described above. Net nitrification and ammonification rates are expressed as mg nitrate or ammonium generated per kg dry soil per day.
2.6. Statistical analysis
Statistical analyses were performed in R version 3.0.1 [36]. To avoid non-independence among subsamples, we derived the aggregated mean of soil attributes for each site (reforestation or remnant rainforest) or cluster (pasture) and used these values as inputs in all analyses, excepting those determining species-specific effects which used values from a single subplot within each site. Pairwise correlation tests (Spearman's) were performed to examine associations between tested soil attributes. Soil attributes under both pasture and remnant riparian rainforest were directly compared using a one-way analysis of variance (ANOVA). Developmental trajectories of soil attributes within reforested sites (site level and separately for specific tree species within sites) were examined using linear regressions between either age or log-transformed age and individual soil attributes. The age term was log-transformed to account for potential non-linear developmental trajectory of reforestation plantings; often showing a rapid change immediately post planting followed by diminishing returns as the system reaches natural equilibrium. For the purposes of quantifying response trajectories with age, we assume that pasture represents 0 years post reforestation.
Results
Of the 56 subplots within sites, 47 (84%) were associated with one of the five target trees. Homalanthus nutans was the species most commonly absent, being present in 50% of reforested sites. Soil aggregation showed only minor variation among sites and land uses so was not considered further in subsequent analyses (sand sized aggregates: mean = 60%, range = 46–76%, clay sized aggregates: mean = 30%, range = 14–46%).
3.1. Correlations between variables
Infiltration rates were inversely correlated with bulk density (P = 0.005) but were not correlated with soil particle size (percentage sand, silt and clay) at either 0–15 cm or 15–30 cm depth (P>0.5, not shown). Bulk density was correlated with both SOC and TN (P = 0.02 and 0.01 respectively) (Table 2). Pairwise correlations revealed strong associations between soil nitrogen-related variables.
Table 2. Pairwise correlations (Spearman's) between soil attributes across pasture, remnant rainforest and reforestation sites (n = 5, 2, 10 respectively).
| Variable | Variable | Spearman's correlation coefficient | P value |
| Bulk density | SOC | −0.554 | 0.023 |
| Bulk density | TN | −0.615 | 0.010 |
| Infiltration | Bulk density | −0.654 | 0.005 |
| TN | SOC | 0.784 | <0.001 |
| Ammonification rate | Nitrification rate | −0.664 | 0.005 |
| Ammonification rate | Fine root biomass | 0.662 | 0.005 |
| Nitrate | Ammonification rate | −0.529 | 0.031 |
| Nitrate | TN | 0.495 | 0.045 |
| Nitrate | SOC | 0.630 | 0.008 |
Only significant pairwise correlations (P<0.05) are shown.
3.2. Differences between pasture and remnant rainforest
There was no apparent difference between pasture and remnant riparian rainforest in the tested soil attributes except infiltration rates (Table 3). Infiltration rates were significantly greater in the remnant rainforest (1421±995 mm h−1) than in pasture sites (220±151 mm h−1) (F = 9.526, DF = 5, P = 0.027, Table 3).
Table 3. ANOVA results from a comparison of soil attributes between remnant riparian rainforest (n = 2) and pasture (n = 5).
| Variable | ANOVA P value | Mean pasture ±1 SD | Mean remnant ±1 SD | Contrast remnant (R) vs. pasture (P) |
| Fine root biomass (g kg−1) | 0.09 | 6.64±4.05 | 13.00±0.38 | NS |
| Bulk density (g cm−3) | 0.36 | 0.69±0.08 | 0.61±0.12 | NS |
| SOC (%) | 0.60 | 6.15±1.83 | 7.31±4.15 | NS |
| TN (%) | 0.85 | 0.71±0.14 | 0.74±0.26 | NS |
| Nitrate (mg kg−1) | 0.26 | 11.8±7.24 | 22.7±17.9 | NS |
| Nitrification rate (mg kg−1 soil d−1) | 0.65 | 0.67±0.75 | 0.39±0.03 | NS |
| Ammonium (mg kg−1) | 0.46 | 4.36±4.27 | 7.85±8.10 | NS |
| Ammonification rate (mg kg−1 soil d−1) | 0.73 | 0.65±0.98 | 0.65±0.98 | NS |
| Infiltration rate (mm h−1) | 0.03 | 220±151 | 1421±995 | R>P |
Bold text indicates significance at 0.05 level, NS not significant if P>0.05, SD standard deviation.
3.3. Recovery of soil properties under reforestation plantings
Only bulk density and infiltration rates showed a relationship with age within 20 years post reforestation (Table 4). In each case, models with a log-transformed age term explained more of the variation than models with an un-transformed age term. Bulk density of the soils ranged from 0.312 to 0.991 g cm-3, and decreased significantly with time post reforestation (F = 6.312, DF = 13, P = 0.026, Figure 1). Conversely, infiltration rates significantly increased (F = 10.56, DF = 13, P = 0.006) within 20 years post reforestation (Figure 1). Infiltration rates in reforestation sites ranged from 149 mm h-1 at a 7-year-old reforestation site to over 1800 mm h−1 at an 11-year-old reforestation site. Infiltration rates of pasture sites varied from 3 mm h−1 to over 650 mm h−1, although this variation was slightly less when data was aggregated by cluster.
Table 4. Relationship between tested soil attributes and the age of the reforestation sites (n = 15).
| Variable | Age | Log Age | ||||||
| Slope | R2 value | F statistic | P value | Slope | R2 value | F statistic | P value | |
| Fine root biomass (g kg−1 soil) | 0.02 | 0.001 | 0.02 | 0.90 | 1.14 | 0.03 | 0.34 | 0.57 |
| Bulk density (g cm−3) | −0.009 | 0.28 | 5.16 | 0.04 | −0.12 | 0.32 | 6.31 | 0.03 |
| SOC (%) | −0.03 | 0.01 | 0.15 | 0.70 | −0.03 | <0.001 | 0.002 | 0.97 |
| TN (%) | −0.001 | 0.004 | 0.05 | 0.83 | 0.005 | <0.001 | 0.004 | 0.95 |
| Nitrate (mg kg−1) | 0.09 | 0.002 | 0.03 | 0.86 | 3.58 | 0.03 | 0.38 | 0.55 |
| Nitrification rate (mg kg−1 d−1) | 0.03 | 0.13 | 1.93 | 0.19 | 0.30 | 0.07 | 0.95 | 0.35 |
| Ammonium (mg kg−1) | −0.09 | 0.04 | 0.51 | 0.49 | −1.72 | 0.11 | 1.55 | 0.24 |
| Ammonification rate (mg kg−1 d−1) | −0.03 | 0.11 | 1.54 | 0.24 | −0.47 | 0.15 | 2.25 | 0.16 |
| Infiltration rate (mm h−1) | 54.9 | 0.39 | 9.77 | 0.008 | 678 | 0.45 | 10.6 | 0.006 |
Pasture is assumed to be 0 years post reforestation. Results from linear models with both age and log-transformed age are shown. P values in bold are significant at the 0.05 level.
Figure 1. Differences in bulk density (left panel) and infiltration rates (right panel) among pasture (P), reforestation sites aged 2 to 20 years, and remnant rainforest (R) (n = 5, 10 and 2 respectively).
Lines show fitted values of the modeled relationship between soil properties and log-transformed age of reforestation. Open circles show reforestation plantings, filled circles indicate pasture clusters and remnant riparian rainforest.
3.4. Species specific effects on the recovery of soils under reforestation plantings
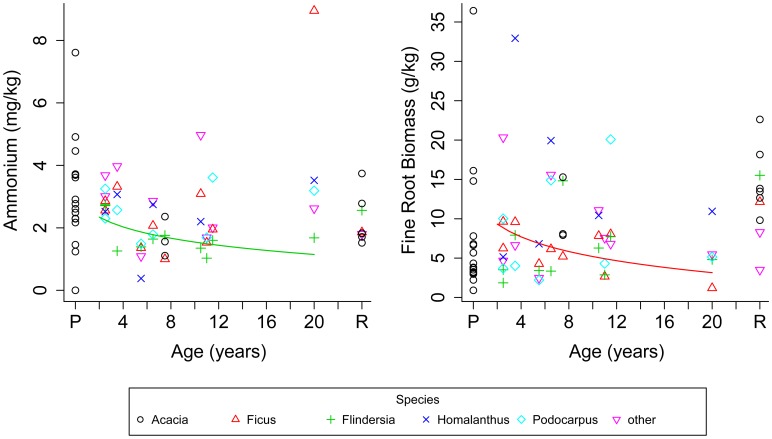
Species-specific effects were apparent for two of five tree species when tested soil attributes were modeled against log-transformed age (Figure 2). Ammonium concentrations in soil associated with Flindersia schottiana decreased with time post reforestation (F = 4.97, DF = 8, P = 0.056). Fine root biomass in soil associated with Ficus coronata decreased through time within 20 years post reforestation (F = 4.921, DF = 8, P = 0.057).
Figure 2. Differences in ammonium (left panel) and fine root biomass (right panel) over time under five tree species in pasture (P), reforestation sites aged 2 to 20 years, and remnant rainforest (R).
Genus names refer to the selected tree species under which the soil samples were taken. “Other” refers to soil samples taken from non-specified tree species or pastoral sites. Lines show fitted values of the modeled relationship between individual soil properties and log-transformed age for species with significant models - Flindersia schottiana (a) and Ficus coronata (b).
Discussion
Replanting riparian areas can be driven by a variety of objectives including increasing landscape connectivity for wildlife movements and biogeochemical functions such as trapping nutrients, sediment, pesticides, bank stabilisation, improved water quality and flood mitigation [10]. Here, we studied the trajectory of riparian reforestation to identify key changes in soil attributes through the transition from pasture to riparian tree plantings of increasing age and remnant rainforest. We showed that the major belowground outcomes within 3 to 20 years since planting are physical and associated functional attributes of the soil.
4.1. Differences in soil properties between pasture and remnant rainforest
Infiltration rate was the only soil variable studied here that differed significantly between pasture and remnant riparian rainforest. Pasture infiltration rates were ≈15% of those of riparian rainforest, consistent with the finding of an infiltration rate in pasture 20% of that in remnant rainforest by Peñuela and Drew [15]. We detected no statistically significant differences in other soil properties between pasture and remnant rainforest soils. Limited effects of land use on selected chemical properties are consistent with previous findings in Australian on ferrosol soils [14]. However, it was expected that attributes such as bulk density and fine roots would differ between land use types [14]. It is possible that these attributes differ at a scale inscrutable beyond background variability between sites, possibly necessitating longitudinal study of permanent plots following reforestation. Limited availability of remnant forest within sampling clusters, a consequence of extensive clearing of riparian rainforest in the region, was also a contributing factor in the comparatively low statistical power for detecting changes in measured attributes. Nevertheless, the data also indicate that there is little degradation of soil with respect to N and C stock in the studied systems.
4.2. Recovery of soils under reforestation plantings
Bulk density and infiltration rates both changed significantly with time under the reforestation plantings. Bulk density showed a negative trend with age whereas infiltration increased with time in the reforestation plantings. The observed trend for decreasing bulk density is consistent with previous studies globally [9], [14], [37], [38]. The higher bulk density and lower infiltration rates of the pastures may be partially attributed to compaction by livestock and/or machinery [39]. Lower bulk densities are often associated with soil of forests and reforestation plantings because greater presence of roots and soil biota and higher concentrations of organic matter enhance soil porosity. In remnant forest and reforestation plantings, the high turnover of roots results in more open soil pores as roots grow and subsequently die [40]. Additionally, pastures generally have more shallow and non-woody roots while forests have a broader range of fine to large woody roots including with larger, woody roots that leave conduits in the soil post death [41]–[43].
Higher densities and diversities of soil organisms in forests and reforestation plantings [44], [45] contribute to the aeration of soil and reduce bulk density. High soil organic matter is also associated with low bulk density [18], a trend seen in this study as bulk density was negatively correlated with SOC. This was expected as SOC is indicative of litter dynamics, turnover and decomposition rates and is responsible for providing low-density mass to the soil and improving soil structure [15], [18], [37], [46]. The bulk density values in our study (0.65 g cm−3) are low in comparison to average soils (>1.2 g cm−3). Paul [14] also reported low bulk densities (0.6–0.8 g cm−3) for similar ferrosols in reforestation plantings and remnant rainforest, confirming the low bulk density of this soil type. We attribute these low bulk densities to the friable nature of the ferrosols in the study region, high porosity and the high organic matter content in the topsoil. We also propose that the low bulk density contributes to the trend of increasing infiltration rates with time following reforestation as the two variables were inversely correlated.
Similar to prior studies, we found a significant increase in infiltration rates with reforestation age, with infiltration rates of the pastures being always lower than those of adjacent reforestation sites. We note a strong positive pattern with age within the first 5–10 years post reforestation, indicating that even young tree plantings are able to promote increased infiltration rates [37], [47]. The trajectory in infiltration rates from the plantings (up to 20 years old) indicate that increases of 182 mm h−1 can be expected for each year in the first decade since planting, with most gains seen within the first 10 years post-reforestation. This trend is consistent with findings of Bharati [48] of short term changes in infiltration rates under riparian buffers when compared to adjacent cultivated fields and pastures, noting a 5-fold increase in infiltration rates under riparian buffers after 6 growing seasons. Our finding that infiltration rates are strongly enhanced soon after reforestation has meaningful consequences for decision making on land use, water quality and conservation with reduced run-off, slowed surface erosion and reduced non-point source pollution as beneficial outcomes [49].
Here, two of the tested nine soil attributes varied between pasture and reforestation sites. One possible explanation is the recentness of the plantings. Reforestation sites were relatively recent compared to studies that focused on plantings of much greater age (up to 60 years) [13], [38], [43] and this may have contributed to the uniformity between sites in our study. However, the common lack of differences between the studied pasture and remnant riparian rainforest sites suggests that inherent high soil fertility may be a larger contributor to soil properties than land cover, as has been suggested previously [43], [50].
4.3. Species specific effects on the recovery of soils under reforestation plantings
In previous studies, species-specific effects were not used to help elucidate changes in soil properties post-reforestation [14], [51], although it is known that species-specific effects can modify soil properties [52]. We detected two species-specific effects in the five tree species and eight variables examined. Ficus coronata and Flindersia schottiana showed negative relationships with time since reforestation for fine root biomass and soil ammonium levels respectively. We hypothesized that as reforested sites become more established, fine root biomass increases to access nutrients in the surface soil layer of rainforests [41], [43], [53]. The studied F. coronata specimens displayed the opposite trend to that expected, showing a negative fine root relationship with time post-reforestation. This decreasing trend exhibited by F. coronata may be explained by a need for greater structural support through large woody roots in which they invest carbon at the expense of fine surface roots, or the ability to access nutrients from the deeper soil, however greater sampling at depth would be needed to confirm these hypotheses. Nevertheless, if verified, an ability to shift root production into in deeper layers could be a useful trait providing physical impediment in the subsoil, particularly where greater soil stability is a desired goal of reforestation.
Another species-specific effect was observed with F. schottiana showing a decreasing relationship with ammonium concentrations and time post-reforestation. This result was not entirely unusual as ammonium is readily converted to nitrate in some soils and nitrate may be preferentially used by plants [54]. However, we did not detect higher nitrate concentration in soil under F. schottiana, which would be expected if nitrification is simulated. This may be due to high N uptake and demand of F. schottiana, which was not investigated here. However, this finding conforms broadly to the results of prior studies [14], [38], [55], which saw that ammonium levels were highest in pasture and lowest in forest.
It is noteworthy that the species-specific effects detected were not the same as those that were seen at a site level. When means at the site level were considered, only infiltration and bulk density were significant, however at a species level, both F. schottiana and F. coronata showed significant variations through time for ammonium levels and fine root biomass respectively. This suggests that while individual species may be able to preferentially alter their immediate surroundings, their influence is diminished at a site level. A possible explanation is that the diverse mix of native tree species, representing a variety of physiotypes, and the individual species-specific effects on soil are less influential than the effects of the overall plantings on soil properties. Had the reforestation planting been of a simpler species mix or the relative proportion of select species been greater, soil traits may have reflected more strongly particular species-specific effects. This indicates that particular reforestation outcomes may be facilitated by explicit selection of certain tree species. Further studies have to determine the efficacy and benefits of a less species-rich reforestation species mixture.
Conclusion
Reduced bulk density and enhanced infiltration rates were the key immediate outcomes resulting from riparian reforestation plantings. It is noteworthy that the most change occurred within just 5 to 10 years post-planting suggesting that this form of active restoration can reinstate important soil functions within a comparatively short time. Given the linkages between increased infiltration and ecosystem services such as nutrient buffering, filtration of runoff and sediments, we conclude that the reforestation is, by extension, achieving the objective of improving water quality. This finding has consequences for land managers and conservationists interested in lowering runoff, non-point source erosion and limiting loss of topsoil, all of which are major concerns and motivators for reforestation. Further, soil infiltration rates are intricately linked with several soil properties influenced by slight changes in soil structure well before changes are perceivable in other soil properties [56]. This signifies that the reforestation plantings are on a positive trajectory to restoring other soil properties and ecosystem function with infiltration rates as front-runner in these changes.
Supporting Information
(XLSX)
Acknowledgments
We thank the landholders that participated in the study for generously allowing access to their land. We also thank the Lake Baroon Catchment Care Group (LBCCG) for liaising with landholders, being so knowledgeable and motivated, and accommodating to our needs. We acknowledge the advice and thank Jessica Vogt and Richard Brackin on sample processing.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the corresponding author susanne.schmidt@uq.edu.au.
Funding Statement
LPS was supported by funding from the Australian Government's National Environmental Research Program (Tropical Ecosystems and Environmental Decisions Hubs). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Connor S, Nelson PN, Armour JD, Hénault C (2012) Hydrology of a forested riparian zone in an agricultural landscape of the humid tropics. Agr Ecosyst Environ. 180: 111–122. [Google Scholar]
- 2. Meynendonckx J, Heuvelmans G, Muys B, Feyen J (2006) Effects of watershed and riparian zone characteristics on nutrient concentrations in the River Scheldt Basin. Hydrol Earth Syst Sci Discuss 3: 653–679. [Google Scholar]
- 3. Orzetti LL, Jones RC, Murphy RF (2010) Stream condition in Piedmont streams with restored riparian buffers in the Chesapeake Bay watershed. J Am Water Resour As 46: 473–485. [Google Scholar]
- 4. Russo TA, Fisher AT, Roche JW (2012) Improving riparian wetland conditions based on infiltration and drainage behavior during and after controlled flooding. J Hydrol 432: 98–111. [Google Scholar]
- 5. Vidon P (2012) Towards a better understanding of riparian zone water table response to precipitation: surface water infiltration, hillslope contribution or pressure wave processes? Hydrol Processes 26: 3207–3215. [Google Scholar]
- 6. Charron I, Lalonde O, Roy AG, Boyer C, Turgeon S (2008) Changes in riparian habitats along five major tributaries of the saint Lawrence river, Québec, Canada: 1964–1997. River Res Appl 24: 617–631. [Google Scholar]
- 7. Griscom HP, Griscom BW, Ashton MS (2009) Forest Regeneration from Pasture in the Dry Tropics of Panama: Effects of Cattle, Exotic Grass, and Forested Riparia. Restor Ecol 17: 117–126. [Google Scholar]
- 8. Scott SJ, McLaren G, Jones G, Harris S (2010) The impact of riparian habitat quality on the foraging and activity of pipistrelle bats (Pipistrellus spp.). J Zool 280: 371–378. [Google Scholar]
- 9. Rodrigues Nogueira Jr L, de Moraes Goncalves JL, Lex Engel V, Parrotta J (2011) Soil dynamics and carbon stocks 10 years after reforestation of degraded land using Atlantic Forest tree species. Forest Syst 20: 536–545. [Google Scholar]
- 10.Naiman RJ, Décamps H, McClain ME (2005) Riparia: ecology, conservation and management of streamside communities. New York: Academic Press.
- 11. Sweeney BW, Bott TL, Jackson JK, Kaplan LA, Newbold JD, et al. (2004) Riparian deforestation, stream narrowing, and loss of stream ecosystem services. Proc Nat Acad Sci USA 101: 14132–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo LB, Gifford M (2002) Soil carbon stocks and land use change: a meta analysis. Glob Change Biol 8: 345–360. [Google Scholar]
- 13. Maloney KO, Garten CT, Ashwood TL (2008) Changes in soil properties following 55 years of secondary forest succession at Fort Benning, Georgia, USA. Restor Ecol 16: 503–510. [Google Scholar]
- 14. Paul M, Catterall CP, Pollard PC, Kanowski J (2010) Recovery of soil properties and functions in different rainforest restoration pathways. Forest Ecol Manage 259: 2083–2092. [Google Scholar]
- 15. Peñuela MC, Drew AP (2004) A model to assess restoration of abandoned pasture in Costa Rica based on soil hydrologic features and forest structure. Restor Ecol 12: 516–524. [Google Scholar]
- 16. Zhu B, Li Z, Li P, Liu G, Xue S (2010) Soil erodibility, microbial biomass, and physical-chemical property changes during long-term natural vegetation restoration: a case study in the Loess Plateau, China. Ecol Res 25: 531–541. [Google Scholar]
- 17. Bronick CJ, Lal R (2005) Soil structure and management: a review. Geoderma 124: 3–22. [Google Scholar]
- 18. Murty D, Kirschbaum MUF, McMurtrie RE, McGilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? a review of the literature. Glob Change Biol 8: 105–123. [Google Scholar]
- 19. Schoenholtz SH, Van Miegroet H, Burger JA (2000) A review of chemical and physical properties as indicators of forest soil quality: challenges and opportunities. Forest Ecol Manag 138: 335–356. [Google Scholar]
- 20.Catterall C, Harrison DA (2006) Rainforest Restoration Activities in Australia's Tropics and Subtropics. Cairns, Australia: Cooperative Research Centre for Tropical Rainforest Ecology and Managemen, Rainforest CRC. 0864437692 0864437692.
- 21. Catterall CP, Kanowski J, Wardell-Johnson GW, Proctor H, Reis T, et al. (2004) Quantifying the biodiversity values of reforestation: perspectives, design issues and outcomes in Australian rainforest landscapes. In: Conservation of Australia's Forest Fauna (ed Lunney, D), Vol. 2. Royal Zoological Society of New South Wales, Sydney 2: 359–393. [Google Scholar]
- 22. Kanowski J, Catterall C, Wardell-Johnson G, Proctor H, Reis T (2003) Development of forest structure on cleared rainforest land in eastern Australia under different styles of reforestation. Forest Ecol Manage 183: 265–280. [Google Scholar]
- 23. Kanowski J, Catterall CP (2010) Carbon stocks in above-ground biomass of monoculture plantations, mixed species plantations and environmental restoration plantings in north-east Australia. Ecol Manage Restor 11: 119–126. [Google Scholar]
- 24. Wickham JD, Wade TG, Riitters KH (2011) An environmental assessment of United States drinking water watersheds. Landscape Ecol 26: 605–616. [Google Scholar]
- 25.Australian Bureau of Statistics (2011) Census.
- 26. Ellison L, Coaldrake JE (1954) Soil mantle movement in relation to forest clearing in Southeastern Queensland. Ecology 35: 380–388. [Google Scholar]
- 27.Willmott WF (2007) Rocks and landscapes of the Sunshine Coast. Brisbane: Geological Society of Australia, Queensland Division.
- 28. Nakamura A, Catterall CP, House APN, Kitching RL, Burwell CJ (2007) The use of ants and other soil and litter arthropods as bio-indicators of the inpacts of rainforest clearing and subsequent land use. J Insect Conserv 11: 177–186. [Google Scholar]
- 29.Smith I (2001) Juggling-balancing Tourism and Biodiversity in a Small, Valuable Reserve - Mary Cairncross Scenic Reserve. In: Boyes B, editor. Biodiversity Conservation “From Vision to Reality”. Gatton, Queensland: Lockyer Watershed Management Association (LWMA) Inc. - Lockyer Landcare Group. 81–84.
- 30. McIntyre D (1976) Subplasticity in Australian soils. I. Description, occurrence, and some properties. Aust J Soil Res 14: 227–236. [Google Scholar]
- 31.DeForest JL (2011) Soil texture - Hydrometer method. Lab protocols for testing soils Ohio University, Athens, Ohio: The Department of Environmental and Plant Biology.
- 32.Robertson GP, Coleman DC, Bledsoe CS, Sollins P (1999) Standard soil methods for long-term ecological research. New York: Oxford University Press.
- 33. Perroux KM, White I (1988) Designs for Disc Permeameters. Soil Sci Soc Am J 52: 1205–1215. [Google Scholar]
- 34. Kandeler E, Gerber H (1988) Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol Fert Soils 6: 68–72. [Google Scholar]
- 35. Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5: 62–71. [DOI] [PubMed] [Google Scholar]
- 36.R Core Team (2013) R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- 37. Neris J, Jiménez C, Fuentes J, Morillas G, Tejedor M (2012) Vegetation and land-use effects on soil properties and water infiltration of Andisols in Tenerife (Canary Islands, Spain). Catena 98: 55–62. [Google Scholar]
- 38. Reiners WA, Bouwman AF, Parsons WFJ, Keller M (1994) Tropical rain forest conversion to pasture: changes in vegetation and soil properties. Ecol Appl 4: 363–377. [Google Scholar]
- 39. Bell LW, Kirkegaard JA, Swan A, Hunt JR, Huth NI, et al. (2011) Impacts of soil damage by grazing livestock on crop productivity. Soil Tillage Res 113: 19–29. [Google Scholar]
- 40. Beven K, Germann P (1982) Macropores and water flow in soils. Water Resour Res 18: 1311–1325. [Google Scholar]
- 41. Finér L, Ohashi M, Moguchi K, Hirano Y (2011) Factors causing variation in fine root biomass in forest ecosytems. Forest Ecol Manage 261: 265–277. [Google Scholar]
- 42. Jiménez EM, Moreno FH, Peñuela MC, Patiño S, Lloyd J (2009) Fine root dynamics for forests on contrasting soils in the Colombian Amazon. Biogeoscience 6: 2809–2827. [Google Scholar]
- 43. Powers JS, Perez-Aviles D (2012) Edaphic factors are a more important control on surface fine roots than stand age in secondary tropical dry forests. Biotropica 45: 1–9. [Google Scholar]
- 44. Colloff MJ, Pullen KR, Cunningham SA (2010) Restoration of an ecosystem function to revegetation communities: The role of invertebrate mscropores in enhancing soil water infiltration. Restor Ecol 18: 65–72. [Google Scholar]
- 45. Nakamura A, Proctor H, Catterall CP (2003) Using soil and litter arthropods to assess the state of rainforest restoration. Ecol Manage Restor 4: S20–S28. [Google Scholar]
- 46.Laub BG, McDonough OT, Needelman BA, Palmer MA (2013) Comparison of designed channel restoration and riparian buffer restoration effects on riparian soils. Restor Ecol: 1–9.
- 47. Wood HB (1977) Hydrologic differences between selected forested and agricultural soils in Hawaii. Soil Sci Soc Am J 41: 132–136. [Google Scholar]
- 48. Bharati L, Lee K-H, Isenhart TM, Schultz RC (2002) Soil-water infiltration under crops, pasture, and established riparian buffer in Midwestern USA. Agrofor Syst 56: 249–257. [Google Scholar]
- 49. Schultz R, Isenhart T, Simpkins W, Colletti J (2004) Riparian forest buffers in agroecosystems–lessons learned from the Bear Creek Watershed, central Iowa, USA. Agrofor Syst 61: 35–50. [Google Scholar]
- 50. Lamb D (1980) Soil nitrogen mineralisation in a secondary rainforest succession. Oecologia 47: 257–263. [DOI] [PubMed] [Google Scholar]
- 51. Paul M, Catterall CP, Pollard PC, Kanowski J (2010) Does soil variation between rainforest, pasture and different reforestation pathways affect the early growth of rainforest pioneer species? Forest Ecol Manage 260: 370–377. [Google Scholar]
- 52. Bezemer TM, Lawson CS, Hedlund K, Edwards AR, Brook AJ, et al. (2006) Plant species and functional group effects on abiotic and microbial soil properties and plant–soil feedback responses in two grasslands. J Ecol 94: 893–904. [Google Scholar]
- 53. Claus A, George E (2005) Effect of stand age on fine-root biomass and biomass distribution in three European forest chronosequences. Can J Forest Res 35: 1617–1625. [Google Scholar]
- 54. Piccolo MC, Neill C, Cerri CC (1994) Net nitrogen mineralization and net nitrification along a topical forest-to-pasture chronosequence. Plant Soil 162: 61–70. [Google Scholar]
- 55. Rasiah V, Florentine SK, Williams BL, Westbrooke ME (2004) The impact of deforestation and pasture abandonment on soil properties in the wet tropics of Australia. Geoderma 120: 35–45. [Google Scholar]
- 56. Radke JK, Berry EC (1993) Infiltration as a tool for detecting soil changes due to cropping, tillage, and grazing livestock. Am J Alternative Agr 8: 164–174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data are available from the corresponding author susanne.schmidt@uq.edu.au.