Abstract
The taxonomy and phylogeny of Asian Meconopsis (Himalayan blue poppy) remain largely unresolved. We used the internal transcribed spacer (ITS) region of nuclear ribosomal DNA (nrDNA) and the chloroplast DNA (cpDNA) trnL-F region for phylogenetic reconstruction of Meconopsis and its close relatives Papaver, Roemeria, and Stylomecon. We identified five main clades, which were well-supported in the gene trees reconstructed with the nrDNA ITS and cpDNA trnL-F sequences. We found that 41 species of Asian Meconopsis did not constitute a monophyletic clade, but formed two solid clades (I and V) separated in the phylogenetic tree by three clades (II, III and IV) of Papaver and its allies. Clade V includes only four Asian Meconopsis species, with the remaining 90 percent of Asian species included in clade I. In this core Asian Meconopsis clade, five subclades (Ia–Ie) were recognized in the nrDNA ITS tree. Three species (Meconopsis discigera, M. pinnatifolia, and M. torquata) of subgenus Discogyne were imbedded in subclade Ia, indicating that the present definition of subgenera in Meconopsis should be rejected. These subclades are inconsistent with any series or sections of the present classifications, suggesting that classifications of the genus should be completely revised. Finally, proposals for further revision of the genus Meconopsis were put forward based on molecular, morphological, and biogeographical evidences.
Introduction
The genus Meconopsis Vig. includes about 50–60 species that are distributed mainly in the Qinghai-Tibetan plateau (QTP) [1]–[4] and is a symbol of the Himalayan alpine flowers. The only European species, Meconopsis cambrica is distributed in the humid and shady deciduous forests of Ireland and from south-west England to Northern Spain. The genus is well known as the ‘Himalayan blue poppy’ and has fascinated the Western world because of its attractive flowers [1]–[5]. Species of this genus can also be found in some European gardens, since they were introduced in these regions about two centuries ago. However, the taxonomy and phylogeny of the genus of this famous garden plant species remain largely unresolved [2], [3], [6].
The genus Meconopsis was founded in 1814 on the basis of the single European species Papaver cambrica L. [1]. This species is different from typical Papaver species because of the presence of a short style and the complete absence of a sessile stigmatic disc surmounting the ovary. With the description of more species of Meconopsis, the generic limits between Meconopsis and its close relatives have become more unclear. For example, nine species of Meconopsis have been incorrectly included in the closely related genera such as Papaver, Cathcartia, and Stylophorum [1], [7], [8]. The monotypic Stylomecon was described originally as Meconopsis heterophylla on the basis of its obvious style; subsequently, Kadereit and Baldwin [7] suggested that it should be included in Papaver. The taxonomic status of Meconopsis villosa (Cathcartia villosa) is also yet unknown [7], [9]. In fact, parallel evolution of the style in the subfamily Papaveroideae was detected by conducting phylogenetic and ontogenetic analyses on the plant group [10]–[11].
Since the first detailed taxonomic study of Meconopsis [12], several influential but controversial classification systems for the genus have been proposed [1]–[2], [13]–[15]. Much of the differences between systems can be attributed to the definition of primary classification of the genus. For example, in the system of Prain [13], two sections were recognized on the base of the pubescence characters. This treatment was substantially accepted by Fedde [16], but the sections were treated as subgenera and the minor groups were raised to the rank of sections. The monotypic section Cambricae included the only European Meconopsis (M. cambrica). Subsequent authors emphasized the characters of style. For example, in the system of Taylor [1], the genus Meconopsis was treated as two subgenera (Eumeconopsis and Discogyne) on the based of the shape of the style. In the subgenus Eumeconopsis, three sections were determined primarily by habit, flower-colour and pubescence characters, and the monotypic section Cambricae of Fedde [16] was retained. Also, the monotypic genus Cathcartia, firstly described in 1851 based on the unique capsule-valves extending beyond the base of the style, was referred to Meconopsis [1]. Except for the definition of subgenera, Taylor’s classification system was substantially re-organized by Wu and Chuang in 1980 [2]. In this system, five sections and nine series were recognized based on characters of inflorescence, stem, leaf and root, with section Cambricae including M. cambrica and nine species of Asian Meconopsis.
Molecular phylogenetic analysis showed that Meconopsis, Papaver, Roemeria, and Stylomecon were included in ‘Old World Papaveroideae’ (OWP) in the subfamily Papaveroideae [17], and neither Meconopsis nor Papaver formed a monophyletic clade. Further, the phylogenetic relationships of the main clades in the OWP were unresloved. A subsequent phylogenetic analysis (an RFLP analysis of chloroplast DNA (cpDNA) trnK) of the OWP indicated that 14 species of Asian Meconopsis formed two clades, but one clade was weakly supported (bootstrap support value, <50). Meconopsis cambrica, the only European species of the genus, was placed in the third clade that included all sampled species of Papaver, Stylomecon, and Roemeria [18], with a bootstrap support lower than 50%. Interestingly, if the European Meconopsis was excluded from the phylogenetic analysis, the topology of the main clades in the OWP would have changed remarkably [18]. When the basalmost Asian Meconopsis clade was not included in the phylogenetic analysis [10], the topology of the main clades in the OWP was also considerably different from that reported previously [17]–[18]. Although the phylogenetic position of the western European endemic M. cambrica was determined recently [19], the relationships of the main clades of OWP remain unresolved. Also, the Asian Meconopsis accessions in the analyses were obtained from Genbank, for which getting information concerned is difficult; therefore, the phylogenetic relationships in the clades of Asian Meconopsis were seldom discussed [19]. The above-mentioned phylogenetic analyses suggest the importance of field sampling and sampling strategy in phylogenetic analysis of Meconopsis. Many species of Meconopsis are rare and endangered species that are distributed in the geographically challenged area (QTP); therefore, collecting samples from the wild is a challenging task. Nevertheless, sufficient sampling should be necessary to reconstruct a reliable phylogeny of Meconopsis, considering the complicated relationships between the genus and its close relatives.
In this study, 42 species representing all series of Meconopsis [1], [2] and representatives of all sections in Papaver [19]–[26] were sampled to reconstruct a phylogeny of the OWP. One species each of the oligotypic genus Roemeria and the monotypic genus Stylomecon were also included in the analysis. This study aimed to (1) elucidate the phylogenetic relationships between Meconopsis and its close relatives and (2) provide a proposal for the taxonomic treatment for Asian Meconopsis.
Materials and Methods
Plant materials
Our sample included 42 species of Meconopsis (of the approximately 50–60 species) representing all sections and series in the genus [1], [2] and 30 species of Papaver (of the approximately 80 species) representing all the 11 sections of the genus (Table S1) [19]–[26]. Of the 112 individuals from the 42 species of Meconopsis, 88 were newly sequenced using internal transcribed spacer (ITS) of nuclear ribosomal DNA (nrDNA) and cpDNA trnL-F regions, and the sequences of the remaining 24 individuals were obtained from Genbank (Table S1). Over half of the Meconopsis species were sampled by including more than one individual from one or several populations. Sequences of Papaver were mainly obtained from Genbank. Previous studies [10], [17]–[18] indicated that insufficient sampling can influence the topologies of the OWP phylogenetic trees; therefore, 19 species representing 12 genera of Papaveraceae s.s. were included in the phylogenetic analysis. Fumaria densiflora and Discocapnos mundtii from family Fumariaceae s.s. were sampled as outgroups, on the basis of recent studies on the phylogeny of family Papaveraceae s.l.
DNA extraction, PCR amplification, and sequencing
Total genomic DNA was isolated from silica gel-dried leaves by using a modified cetyltrimethylammonium bromide (CTAB) method [27]. Amplification of the nrDNA ITS and the trnL-F regions followed Yang et al. [28]. The PCR products were purified using Gel Band Purification Kit (Tiangen Biotech, Beijing, China), and then sequenced using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) on an ABI PRISM 3730xl analyzer (Applied Biosystems). Direct sequencing of ITS region produced double peaks in chromatograms of six individuals. Molecular cloning and sequence analyses of the ITS region detected sequence polymorphisms in these individuals. Further phylogenetic analyses combined with morphological evidence suggest that all intra-individual polymorphisms can be attributed to inter-species hybridization other than to ITS paralogs. Phylogenetic analyses of ITS region in the present study mainly aim to resolve general relationships in the genus; therefore, the hybrids were excluded from the phylogenetic analyses.
Phylogenetic analysis
The DNA sequences were aligned using the default parameters in Clustal X [29], and then refined manually in BioEdit. To reconstruct the phylogeny of Meconopsis and its close relatives, Maximum parsimony (MP) and Bayesian inference (BI) analyses for the nrDNA ITS and cpDNA trnL-F regions were implemented in software packages PAUP* 4.0b10 [30] and MrBayes version 3.1.2 [31], respectively. For the MP analysis, all characters were weighted equally and treated as unordered, with gaps considered as missing. Heuristic searches were conducted using 1,000 replicates of random addition, tree-bisection-reconnection branch swapping, the MULTREES option, and a maximum of 1,000 trees saved per round. The confidence of clades in the MP trees was estimated by performing bootstrap analysis with 1,000 replicates by using the heuristic search. Before performing the BI analysis, the best evolutionary models of the two regions were determined using the Akaike Information Criteria implemented in MrModeltest 2.3 [32]. The GTR+I+G model was chosen for the nrDNA ITS and trnL-F regions. Two separate runs of four Markov chains for 10,000,000 generations were applied for each data set, sampling one tree per 1000 generations. The 50% majority-rule consensus tree was constructed after removing the first 2,000 trees. MCMC convergence was explored by examining the Potential Scale Reduction Factor (PSRF) convergence diagnostics for all parameters in the model. Posterior probabilities were calculated for sampled trees.
Results
Sequence characterization and phylogenetic analyses
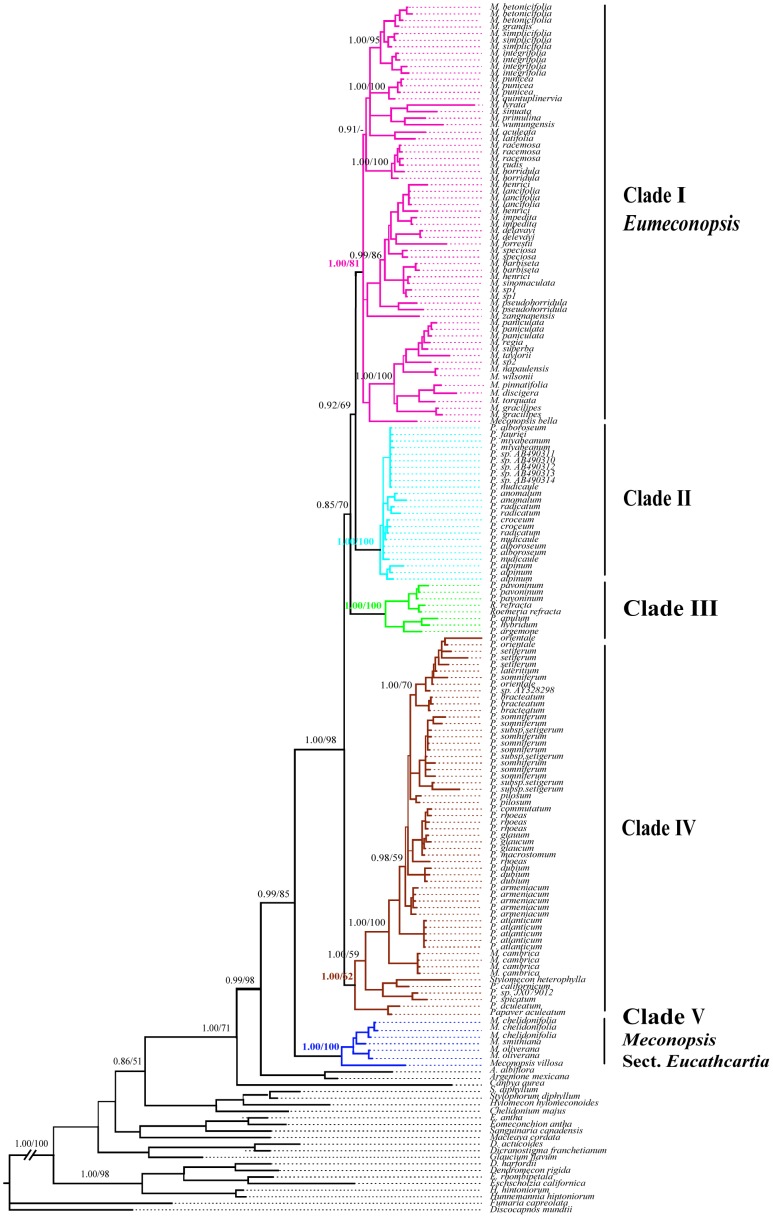
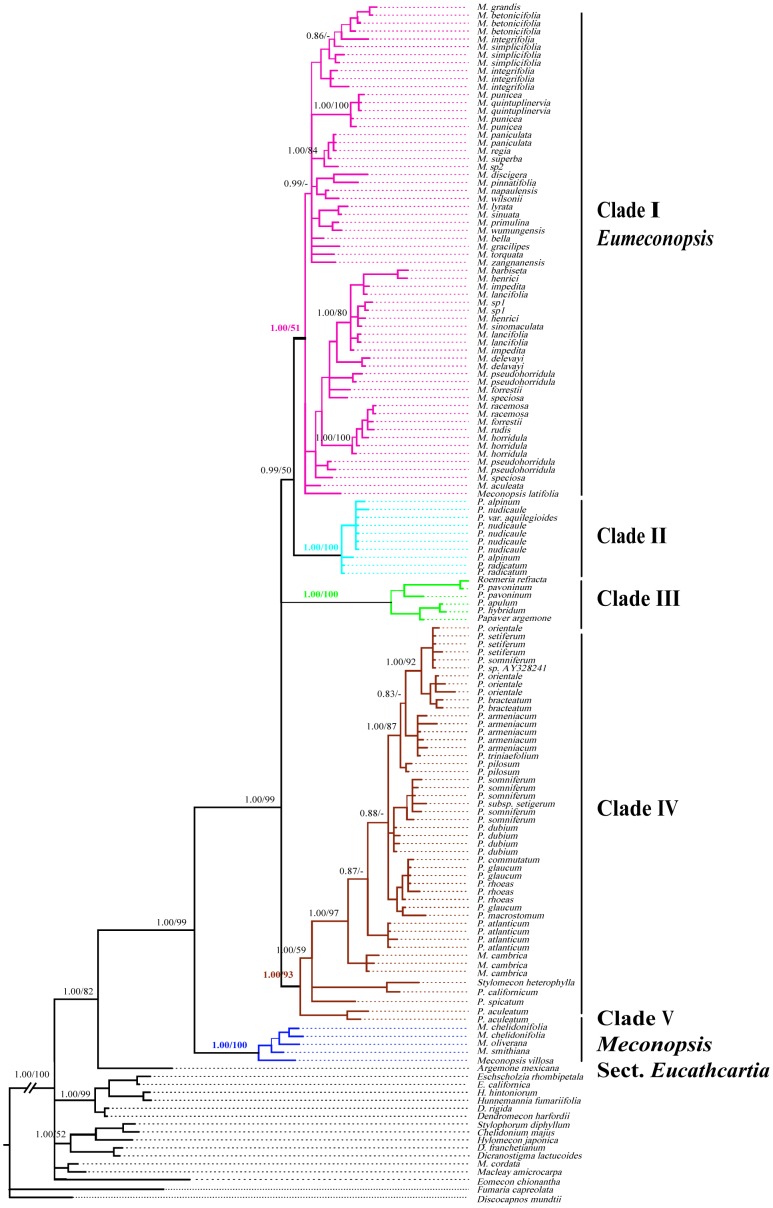
The aligned nrDNA ITS sequence data matrix was 866 bp in length, with 360 potentially parsimony-informative and 87 uninformative variable characters. The aligned cpDNA trnL-F matrix consisted of 964 characters, of which 280 were variable and 194 were potentially parsimony informative. The BI consensus trees of the nrDNA ITS and cpDNA trnL-F matrices are shown in Figures 1 and 2, respectively. The topologies of most parsimony trees (not shown) reconstructed with the two matrices were nearly identical to those shown in Figures 1 and 2 except for some terminal branches. The monophyly of the Old World clade (including Meconopsis, Papaver, Stylomecon, and Roemeria) in subfamily Papaveroideae was strongly supported in Figure 1 (Bootstrap support (BS) = 85; Bayesian posterior probability (PP) = 0.99) and Figure 2 (BS = 99; PP = 1.00), with New World clade (Argemone) as a sister group. Five main clades (I–V) recognized in the nrDNA ITS gene tree were well supported by the cpDNA trnL-F gene tree, and neither Meconopsis nor Papaver was supported as monophyly. The monotypic genera Stylomecon and oligotypic genera Roemeria were nested in clades of Papaver or Meconopsis.
Figure 1. The Bayesian tree of Meconopsis constructed using the internal transcribed spacer region of nuclear ribosomal DNA (nrDNA ITS).
Numbers on the branches denote the Bayesian posterior probabilities and the bootstrap values for maximum parsimony (MP) for the main clades.
Figure 2. The Bayesian tree of Meconopsis inferred from the trnL-F fragment.
Numbers on the branches denote the Bayesian posterior probabilities and the bootstrap values for maximum parsimony (MP) for the main clades.
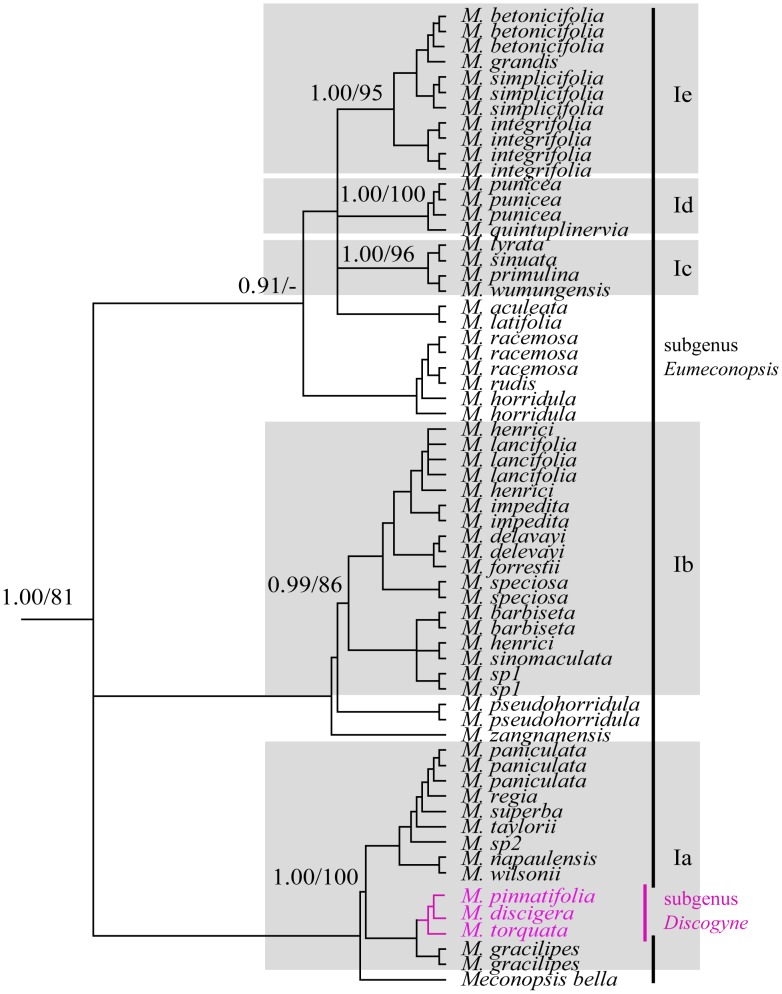
Clade I comprised 37 species of Meconopsis, and only five species (M. cambrica and four species of clade V) of the genus were placed outside. Plants of this clade are only found in the areas from the Himalayas to the Hengduan Mountains. Clade I of the ITS tree revealed five well supported subclades (Ia–Ie; Figure 3), which were not well resolved in the trnL-F gene tree (Figure 2). Seven species of the subsection Eupolychaetia [1] and three species of the subgenus Discogyne formed subclade Ia (Figures 1 and 3), and the remaining four subclades were not consistent with any sections or series of the present classifications [1], [2] (Table S2).
Figure 3. The Bayesian tree of clade I from Figure 1 .
Numbers on the branches denote the Bayesian posterior probabilities and the bootstrap values for maximum parsimony (MP) and the Bayesian posterior probabilities for the main clades.
All sampled species of Papaver sect. Meconella formed a well-supported clade II, and species of this section were distributed in the arctic and subarctic mountains (outside the QTP). Clade III consisted of Roemeria refracta and all the four species in Papaver section Argemonidium. Clade IV comprised Meconopsis cambrica, Stylomecon heterophylla, and 16 species of Papaver. The 16 species of Papaver represented nine of the 11 sections except sections Meconella and Argemonidium. Papaver aculeatum, the only poppy native to Eastern South Africa, and two species (Stylomecon heterophylla and Papaver californicum) endemic to California occupied the basal positions in this clade. Meconopsis cambrica from Western Europe was included in a separate branch. The remaining 13 species of Papaver were distributed in North Africa and Europe to South-West and Central Asia, with species diversity centre located in the Mediterranean area where 32 species of Papaver were distributed [33]. Clade V consisted of four Meconopsis species that were sporadically distributed in the narrow area from south Himalayas to east Hengduan Mountains, extending to the mountains of Central Asia.
Discussion
Phylogeny of Asian Meconopsis
Previous studies have indicated that Meconopsis, Papaver, Roemeria, and Stylomecon formed a well-supported clade, the Old World clade of the subfamily Papaveroideae. This group was divided into four parallel clades, but only a small clade, including species of Papaver section Argemonidium and Roemeria, was well supported (Figure 1 in [11]). In this study, the phylogenetic analyses of the nrDNA and cpDNA sequences combined with extensive sampling showed that neither Meconopsis nor Papaver is monophyletic (Figures 1 and 2), supporting the results of Kadereit et al. [18] and Carolan et al. [10]. Most importantly, our results identified five well-supported OWP clades, which were well resolved in the gene trees reconstructed with the nrDNA ITS and cpDNA trnL-F sequences (Figures 1 and 2). The two Asian Meconopsis clades (I and V) were separated in the phylogenetic trees by three clades of Papaver and its allies, with clades I and V occupying the shallow-most and basal-most positions in the OWP. The only European Meconopsis species (M. cambrica) were nested in clade IV comprising 16 species of Papaver. In addition, in clade I, which contained about 90% species of Asian Meconopsis, five well-supported subclades were recognized in the nrDNA ITS tree. The three clades of Meconopsis are discussed below according to morphology and biogeography.
Clade I, Meconopsis section Eumeconopsis. This core Meconopsis clade comprised 37 species of Asian Meconopsis, including 34 species of subgenus Eumeconopsis and three species of subgenus Discogyne (Figures 1–3). Only five species of subgenus Eumeconopsis were placed outside this clade: Meconopsis cambrica in clade IV and the four species in clade V. Species of Meconopsis in these two clades are characterised by four-petalled yellow flowers with uniform colour of petals and stamens, whereas the two organs in species of clade I often display different colour. Further, species of Meconopsis in clade I can be differentiated from typical Papaver on the basis of the presence of a typical style and lack of a stigmatic disc.
Our phylogenetic results indicated that three species (M. discigera, M. pinnatifolia, and M. torquata) of subgenus Discogyne were imbedded in clade I that included 34 species of subgenus Eumeconopsis (Figures 1–3), which are inconsistent with recent classifications [1], [2] in which Meconopsis was divided into two subgenera on the basis of ovary characters. The subgenus Discogyne was first recognised by Taylor [1] on the basis of the distinct stylar disc surmounting the ovary (Figure 4). It includes four species distributed in the eastern Himalayas [34], and two new species (M. manasluensis and M. bhutanica) described recently should be included in this subgenus because of the presence of a stylar disc [3], [35]. Considering the unique structure, Taylor thought that this group could be treated as a separate genus; however, the habit was similar to some more typical Meconopsis species found in the same distribution area.
Figure 4. The illustrations show representative species from subclade Ia.
(A, B) Plant and fruit of Meconopsis pinnatifolia (subgenus Discogyne); (C, D) M. paniculata (subgenus Eumeconopsis); (E, F) M. wilsonii (subgenus Eumeconopsis).
In fact, except for the distinct stylar disc surmounting the ovary, species of subgenus Discogyne are morphologically quite similar to the others in subclade Ia (Figure 4). Both are monocarpic herbs up to 1.0–2.0 m in height, with indumentum sparsely to densely bristly throughout whole plant. Leaves are basal and cauline, lamina lanceolate or elliptic-oblong, pinnatilobate to pinnatisect. Inflorescence is racemose or paniculate (Figure 4). Recently, Kadereit and Erbar [11] confirmed that the obvious stylar disc initially used as a diagnostic trait to distinguish genera in the OWP or subgenera in Meconopsis has evolved several times independently. Also, our field investigations found that style length and the presence or absence of stylar disc varied remarkably at population levels in some species of Meconopsis, such as M. integrifolia (Figure 5). Thus, it is not appropriate to delimitate two subgenera of the genus Meconopsis on the basis of stylar disc, which has experienced parallel evolution in the lineages of the OWP.
Figure 5. The illustrations show morphological diversity of styles within Meconopsis integrifolia.
A and B, C and D, and E and F represent plants from the same population, respectively.
Although the present classifications [1], [2] of Meconopsis were not recognized by our molecular results, there are some consistencies between subclades of clade I and sections/series of Taylor [1] (Table S2). For instance, subsection Eupolychaetia is consistent with subclade Ia except for species of subgenus Discogyne. Subclade Ic includes M. lyrata, M. primulana, M. wumungensis, and M. sinuate, which is substantially congruent with series Primulinae. The only exception is M. sinuate, which was placed in series Aculeatae by Talyor [1], should be transferred to series Primulinae on the base of our molecular evidence (Figures 1–3). Series Simplicifoliae [1], [2] (Table S2) includes M. punicea, M. quintuplinervia, and M. simplicifolia. But our results suggest that the last species should be transferred to series Grandes. In fact, the semi-drooping flower and narrowly ellipsoid-oblong capsule (Figure 6d, 6e) of M. simplicifolia are quite similar to those of series Grandes (M. betonicifolia and M. grandis) (Figure 6b, 6c) rather than those of series Simplicifoliae (M. punicea and M. quintuplinernia) (Figure 6f–6i), which have drooping flower and ellipsoid or obovoid capsule.
Figure 6. The illustrations show plants of subclade Ie (a–e) and Id (f–i).

(a) Meconopsis integrifolia; (b) M. grandis; (c) M. betonicifolia; (d, e) M. simplicifolia; (f, g) M. punicea; (h, i) M. quintuplinervia.
Nearly all species in clade I are exclusively distributed in the unique habitats of the QTP, the roof of the world. The only exception is Meconopsis quintuplinervia, which is distributed eastwardly from the QTP to the adjacent Qinling Mountains. The distributions of these subclades showed clear geographic patterns. Subclade Ia included 10 species that mainly occurred in the rainy regions of the southern QTP (Himalayas), with only one species (Meconopsis wilsonii) extending eastwardly in the eastern QTP (Hengduan Mountains). Plants of this clade are often covered with villous or small much-branched hairs, with stem heights of 1.0 to 2.5 m. In contract, species of subclade Ib are mainly distributed in the eastern QTP, and the bristly plants are often shorter than 0.6 m. The shorter stem, thicker leaves, and covered bristles indicate the adaptation of plants to drier habitats. The small clade Ic mainly occurred in the Himalayas, and the remaining two subclades occurred in the eastern QTP. The correction between species of subclade Ie and the regional climate in the QTP has been detected by our previous phylogeographic analysis [28]; in this study, the lineage-specific distribution patterns suggest that climatic divergence driven by the rapid uplift of the QTP has led to lineage divergence in the genus Meconopsis.
Clade IV, Meconopsis cambrica plus Papaver. This clade consisted of M. cambrica, Stylomecon heterophylla, and representatives of nine sections in Papaver (i.e. sects. Carinatae, Meconidium, Pseudopilosa, Rhoeadium, Oxytona (Macrantha), Papaver, Pilosa, Californicum, and Horrida). The only European species of Meconopsis, M. cambrica, was placed within this core Papaver clade, which is congruent with previous molecular analysis [10]. In fact, Ernst in 1962 [36] had found that the gynaecium of M. cambrica has pseudodoral veins, a distinct character absent from all other species of Meconopsis examined but present in all sections of Papaver s.s. Recently, Kadereit and Erbar [11] found that gynaecium ontogeny in Papaver s.s is characterised by a unique “garland-like” stage, and remnants of this stage were observed in M. cambrica. These morphological and anatomical characters provide strong supports for a close relationship of M. cambrica and Papaver s.s [8]. The monotypic genus Stylomecon characterised by a distinct style considerably similar to that of Meconopsis was also placed within clade IV. Stylomecon heterophylla was originally described as Meconopsis heterophylla, but our results supported the findings of Kadereit and Baldwin [19] that it should be treated as Papaver.
Clade V, Meconopsis section Eucathcartia. This clade included M. chelidonifolia, M. oliverana, M. smithiana, and M. villosa and was strongly supported in the nrDNA ITS and cpDNA trnL-F gene trees (Figures 1 and 2). A clade that comprised M. chelidonifolia and M. villosa was recognized by previous molecular phylogenetic analyses; however, its relationship with other clades was poorly supported [17]. In this study, besides the two species mentioned above, M. oliverana and M. smithiana were also included in this well-supported clade, which occupies the basal position of the OWP. Morphologically, the four species are characterised by a polycarpic habit and four-petalled yellow flowers with uniform colouration of the petals and stamens; because of these features, species of clade V can be easily distinguished from other Asian Meconopsis species (clade I). Species of clade V are also different from typical Papaver species by the presence of a short style and complete absence of a sessile stigmatic disc surmounting the ovary. Because of the ‘unique’ capsule valves extending beyond the base of the style, M. villosa was first described as belonging to a separate genus Cathcartia by Hooker. This treatment was not recognised by Taylor [1], in his monography Cathcartia villosa and three species of Meconopsis (M. chelidonifolia, M. oliverana, and M. simthiana) were included in section Eucathcartia, one of the three sections in Meconopsis subgenus Eumeconopsis. Our results confirmed that these four species formed a well supported monophyletic clade.
Four species of clade V occupy the easternmost and southernmost portions of the distributional range of the genus, and these species grew in thickets at altitudes ranging from 1,500 to 2,500 m, a habit considerably different from that of the Himalayan species of Asian Meconopsis.
Taxonomic implications for Asian Meconopsis
The present study confirmed that the Asian Meconopsis forms two well-supported clades, separated by two clades of Papaver and one clade of Papaver plus Roemeria (Figures 1 and 2). Considering the botanical and economical consequences, the generic name for the Asian Meconopsis species should be retained [8], and Clade V should be advanced to generic level and be named as Cathcartia. The well recognized five subclades in clade I suggest that series Primulinae, Grandes, and Simplicifoliae (Table S2) of Taylor [1] could be retained after minor revision. Series Superbae and Robustae should be united, with subgenus Discogyne included in this group. Series Delavayanae should be merged into series Aculeatae, in which the relationships need further investigation. Our results suggest that special attention should be paid on the texture of leaves, types of bristle covered and geographic distributions of species for future revision of the genus.
Supporting Information
Sources of materials.
(DOC)
Acknowledgments
We thank Dr. You-Sheng Chen from Institute of Botany (IB), Chinese Academy of Sciences (CAS), for their kind help in sample collection, and Mr. Wan-Qing Jin and other members from IB-CAS for help with sample treatment and DNA sequencing.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are are available from the GenBank database and accession numbers are provided in the Supporting Information file S2.
Funding Statement
This study was supported by the National Natural Science Foundation of China (Grant Nos. 31270263, 31330008, and 31110103911). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taylor G (1934) An account of the genus Meconopsis. London: New flora and silva Ltd.
- 2. Wu C, Chuang H (1980) A study on the taxonomic system of the genus Meconopsis . Acta Bot Yunnanica 2: 371–381. [Google Scholar]
- 3. Egan PA (2011) Meconopsis autumnalis and M. manasluensis (Papaveraceae), two new species of Himalayan poppy endemic to central Nepal with sympatric congeners. Phytotaxa 20: 47–56. [Google Scholar]
- 4. Yoshida T, Sun H, Boufford D (2012) New species of Meconopsis (Papaveraceae) from Laojun shan and Yao shan, Northern Yunnan, China. Plant Divers Resour. 34: 145–149. [Google Scholar]
- 5. Yoshida K, Kitahara S, Ito S, Kondo T (2006) Ferric ions involved in the flower color development of the Himalayan blue poppy, Meconopsis grandis . Phytochemistry 67: 992–998. [DOI] [PubMed] [Google Scholar]
- 6. Toshio Y, Sun H, Grey-Wilson C (2012) A revision of Meconopsis lyrata (Cummins & prain) Fedde ex Prain and its allies. Curtis’s Bot Mag 29: 194–216. [Google Scholar]
- 7. Kadereit JW, Baldwin BG (2011) Systematics, phylogeny, and evolution of Papaver californicum and Stylomecon heterophylla (Papaveraceae). Madroño 58: 92–100. [Google Scholar]
- 8. Grey–Wilson C (2012) Proposal to conserve the name Meconopsis (Papaveraceae) with a conserved type. Taxon 61: 473–474. [Google Scholar]
- 9.Egan PA, Shrestha S (2012) Papaveraceae: Webedition 1. In: Watson MF, Akiyama S, Ikeda H, et al.., editors. Flora of Nepal. Available: http://data.rbge.org.uk/publications/FloraofNepal/library/Papaveraceae/1.
- 10. Carolan JC, Hook IL, Chase MW, Kadereit JW, Hodkinson TR (2006) Phylogenetics of Papaver and related genera based on DNA sequences from ITS nuclear ribosomal DNA and plastid trnL intron and trnL-F intergenic spacers. Ann Bot 98: 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadereit JW, Erbar C (2011) Evolution of gynoecium morphology in Old World Papaveroideae: A combined phylogenetic/ontogenetic approach. Amer J Bot 98: 1243–1251. [DOI] [PubMed] [Google Scholar]
- 12. Prain D (1895) Noviciae Indicae IX. J Asiatic Soc Bengal 64: 303–27. [Google Scholar]
- 13. Prain D (1906) A review of the genera Meconopsis and Cathcartia . Ann Bot 20: 23–70. [Google Scholar]
- 14. Prain D (1915) Some additional species of Meconopsis . Bulletin of Miscellaneous Information (Royal Gardens, Kew) 1915: 129–177. [Google Scholar]
- 15. Kingdon-Ward F (1926) Notes on the genus Meconopsis, with some additional species from Tibet. Ann Bot 40: 535–546. [Google Scholar]
- 16.Fedde F (1909) Papaveraceae–Hypecoideae et Papaveraceae–Papaveroideae. In: Engler A, editor. Vol 4, 104. Das Pflanzenreich. Leipzig: Englemann. pp: 245–271.
- 17.Jork KB, Kadereit JW (1995) Molecular phylogeny of the Old World representatives of Papaveraceae subf. Papaveroideae with special emphasis on the genus Meconopsis Vig. In: Plant Syst Evol 9 (Suppl.) Jensen U, Kadereit JW, editors. Systematics and evolution of the Ranunculiflorae. 9: 171–180. [Google Scholar]
- 18. Kadereit JW, Schwarzbach AE, Jork KB (1997) The phylogeny of Papaver s. l. (Papaveraceae): Polyphyly or monophyly? Plant Syst Evol 204: 75–98. [Google Scholar]
- 19. Kadereit JW, Preston CD, Valtueña FJ (2011) Is Welsh Poppy, Meconopsis cambrica (L.) Vig. (Papaveraceae), truly a Meconopsis? New Journal of Botany 1: 80–88. [Google Scholar]
- 20. Kadereit JW (1986) A revision of Papaver section Argemonidium . Notes Roy Bot Gard Edinburgh 44: 25–43. [Google Scholar]
- 21. Kadereit JW (1988) Papaver L. sect Californicum Kadereit, a new section of the genus Papaver . Rodora 90: 7–13. [Google Scholar]
- 22. Kadereit JW (1989) A revision of Papaver section Rhoeadium Spach. Notes Roy Bot Gard Edinburgh 45: 225–286. [Google Scholar]
- 23. Kadereit JW (1990) Some suggestions on the geographical origin of the central, west and north European synantropic species of Papaver L. Bot J Linn Soc. 103: 221–231. [Google Scholar]
- 24. Kadereit JW (1993) A revision of Papaver sect. Meconidium . Edinb J Bot 50: 125–148. [Google Scholar]
- 25. Kadereit JW (1996) A revision of Papaver L. sects Pilosa Prantl and Pseudopilosa M. Popov ex Gunther (Papaveraceae). Edinb J Bot 53: 285–309. [Google Scholar]
- 26.Bernath J (1998) Poppy: the genus Papaver. Amsterdam: Harwood Academic Publishers.
- 27. Rogers SO, Bendich AJ (1988) Extraction of DNA from plant tissues. Plant Mol Biol Manual A6: 1–10. [DOI] [PubMed] [Google Scholar]
- 28. Yang FS, Qin AL, Li YF, Wang XQ (2012) Great genetic differentiation among populations of Meconopsis integrifolia and its implication for plant speciation in the Qinghai-Tibetan Plateau. PLoS ONE 7: e37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swofford DL (2002) PAUP*: phylogenetic analysis using parsimony (*and other methods), Version 4.0. (Sinauer Associates, Sunderland (MA).
- 31. Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- 32. Nylander J, Ronquist F, Huelsenbeck J, Nieves-Aldrey J (2004) Bayesian phylogenetic analysis of combined data. Syst Biol 53: 47–67. [DOI] [PubMed] [Google Scholar]
- 33. Kadereit JW, Baldwin BG (2012) Western Eurasian–western North American disjunct plant taxa: The dry-adapted ends of formerly widespread north temperate mesic lineages–and examples of long-distance dispersal. Taxon 61: 3–17. [Google Scholar]
- 34.Zhang ML, Grey–Wilson C (2008) Meconopsis Viguier. In: Wu ZY, Raven PH, editors. Flora of China, vol 7. Beijing/St Louis: Science Press/Missouri Botanical Garden Press. pp. 262–278.
- 35. Yoshida T, Grey–Wilson C (2012) A new species of blue poppy. The Plantsman 11: 96–101. [Google Scholar]
- 36.Ernst WR (1962) A comparative morphology of the Papaveraceae. Ph. D. Dissertation. Stanford University, Stanford, California.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sources of materials.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are are available from the GenBank database and accession numbers are provided in the Supporting Information file S2.