Abstract
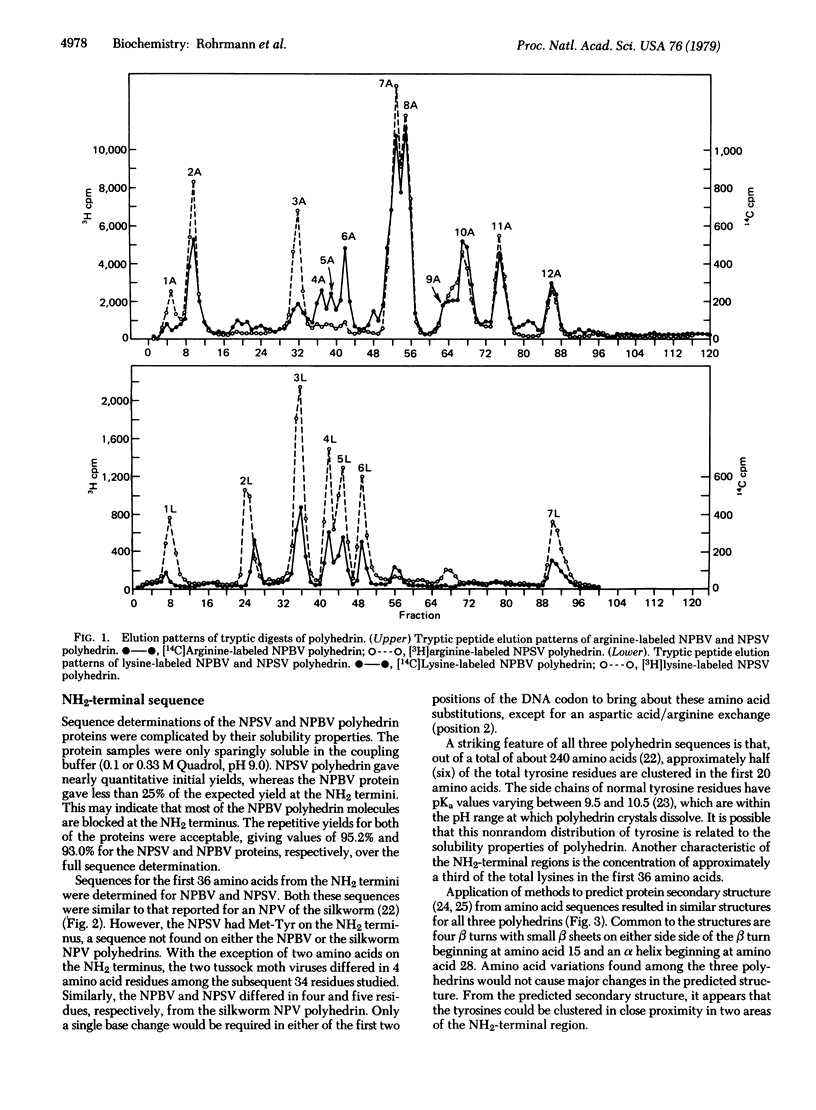
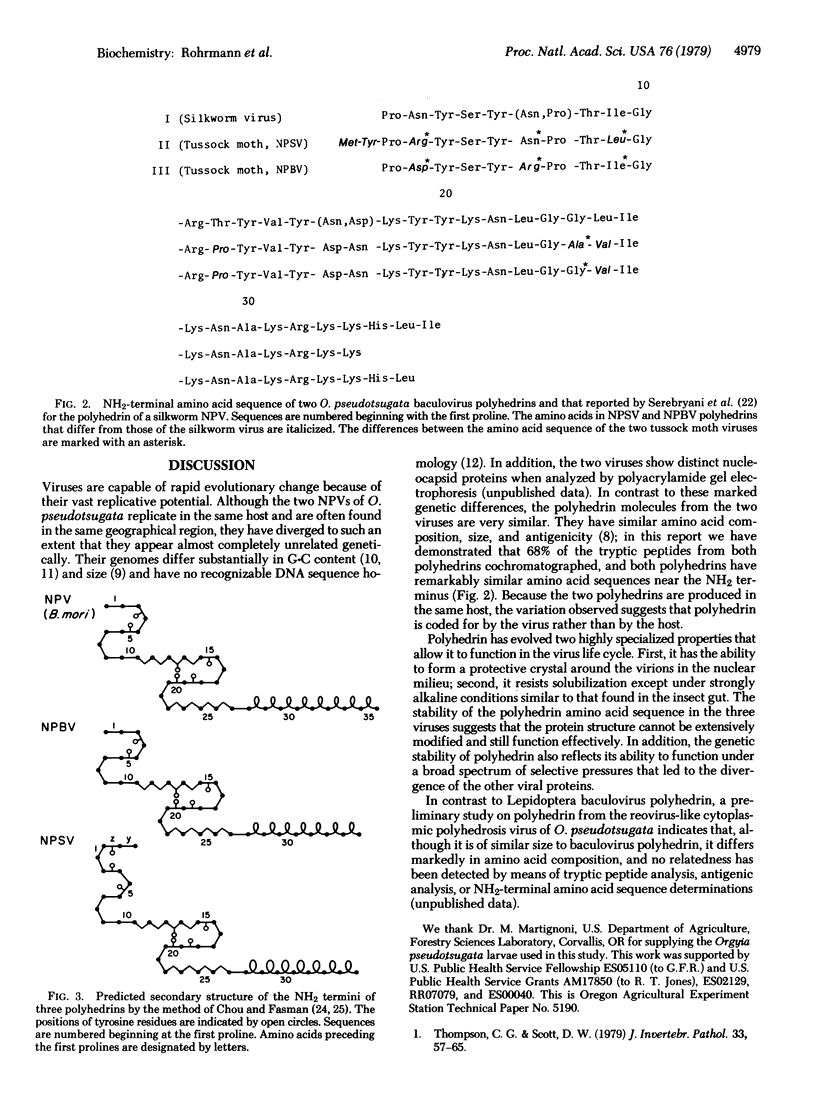
Comparative analysis of the tryptic peptides and terminal amino acid sequence was made on polyhedrins from two genetically different baculoviruses that are naturally pathogenic for the same insect host. Comparison of the tryptic peptides of the nucleopolyhedrosis bundle virus and nucleopolyhedrosis single-rod virus of Orgyia pseudotsugata by means of cation-exchange resins indicated that the proteins have a closely related amino acid sequence. The NH2-terminal amino acid sequence of polyhedrins from the two viruses differed in only 4 out of 34 amino acids. The nucleopolyhedrosis bundle virus and the nucleopolyhedrosis single-rod virus also differed in 4 and 5 out of 34 terminal amino acids, respectively, from the sequence reported for polyhedrin of a baculovirus of Bombyx mori [Serebryani, S. B., Levitina, T. L., Kautsman, M. L., Radavski, Y. L., Gusak, N. M., Ovander, M. N., Sucharenko, N. V. & Kozlov, E. A. (1977) J. Invertebr. Pathol. 30, 442-443]. In addition, the nucleopolyhedrosis single-rod virus had two amino acids (Met-Tyr) on the NH2 terminus that were not present on the terminus of nucleopolyhedrosis bundle virus or B. mori baculovirus polyhedrin. Approximately half (six) of the total tyrosine residues are clustered in the terminal 20 amino acids of the polyhedrins. Secondary structures predicted from the primary sequence suggest that the tyrosines are clustered in two areas. This nonrandom distribution and the pKa of about 10 for tyrosine may be related to the alkali solubility of the polyhedrin.
Keywords: baculovirus polyhedrin structure, genetic stability
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Beta-turns in proteins. J Mol Biol. 1977 Sep 15;115(2):135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Croizier G., Croizier L. Evaluation du poids moléculaire de la protéine des corps k'inclusion de divers Baculovirus d'insectes. Arch Virol. 1977;55(3):247–250. doi: 10.1007/BF01319910. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Direct microsequence analysis of polypeptides using an improved sequenator, a nonprotein carrier (polybrene), and high pressure liquid chromatography. Biochemistry. 1978 May 30;17(11):2124–2133. doi: 10.1021/bi00604a016. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Mak A., Smillie L. B., Bárány M. Specific phosphorylation at serine-283 of alpha tropomyosin from frog skeletal and rabbit skeletal and cardiac muscle. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3588–3592. doi: 10.1073/pnas.75.8.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niece R. L., Margoliash E., Fitch W. M. Complete amino acid sequence of guanaco (Lama guanicoe) cytochrome c. Biochemistry. 1977 Jan 11;16(1):68–72. doi: 10.1021/bi00620a011. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J., Brewer H. B., Jr Advances in the gas chromatographic analysis of amino acid phenyl- and methylthiohydantoins. Anal Biochem. 1972 Jan;45(1):43–59. doi: 10.1016/0003-2697(72)90006-1. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F., Beaudreau G. S. Characterization of DNA from polyhedral inclusion bodies of the nucleopolyhedrosis single-rod virus pathogenic for Orgyia pseudotsugata. Virology. 1977 Dec;83(2):474–478. doi: 10.1016/0042-6822(77)90198-2. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F., Carnegie J. W., Martignoni M. E., Beaudreau G. S. Characterization of the genome of the nucleopolyhedrosis bundle virus pathogenic for Orgyia pseudotsugata. Virology. 1977 Jul 15;80(2):421–425. doi: 10.1016/s0042-6822(77)80017-2. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F. Characterization of N-polyhedrin of two baculovirus strains pathogenic for Orgyia pseudotsugata. Biochemistry. 1977 Apr 19;16(8):1631–1634. doi: 10.1021/bi00627a017. [DOI] [PubMed] [Google Scholar]
- Rohrmann G. F., McParland R. H., Martignoni M. E., Beaudreau G. S. Genetic relatedness of two nucleopolyhedrosis viruses pathogenic for Orgyia pseudotsugata. Virology. 1978 Jan;84(1):213–217. doi: 10.1016/0042-6822(78)90237-4. [DOI] [PubMed] [Google Scholar]
- Schafer M. P., Rohrmann G., Heine U., Beaudreau G. S. DNA from two Orgyia pseudotsugata baculoviruses: molecular weight determination by means of electron microscopy and restriction endonuclease analysis. Virology. 1979 May;95(1):176–184. doi: 10.1016/0042-6822(79)90412-4. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Smith G. E. Comparative studies of baculovirus granulins and polyhedrins. Intervirology. 1975;6(3):168–180. doi: 10.1159/000149469. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]


