Abstract
Premature newborns are frequently exposed to hyperoxic conditions and experimental data indicate modulation of liver metabolism by hyperoxia in the first postnatal period. Conversely, nothing is known about possible modulation of growth factors and signaling molecules involved in other hyperoxic responses and no data are available about the effects of hyperoxia in postnatal liver haematopoiesis. The aim of the study was to analyse the effects of hyperoxia in the liver tissue (hepatocytes and haemopoietic cells) and to investigate possible changes in the expression of Vascular Endothelial Growth Factor (VEGF), Matrix Metalloproteinase 9 (MMP-9), Hypoxia-Inducible Factor-1α (HIF-1α), endothelial Nitric Oxide Synthase (eNOS), and Nuclear Factor-kB (NF-kB). Experimental design of the study involved exposure of newborn rats to room air (controls), 60% O2 (moderate hyperoxia), or 95% O2 (severe hyperoxia) for the first two postnatal weeks. Immunohistochemical and Western blot analyses were performed. Severe hyperoxia increased hepatocyte apoptosis and MMP-9 expression and decreased VEGF expression. Reduced content in reticular fibers was found in moderate and severe hyperoxia. Some other changes were specifically produced in hepatocytes by moderate hyperoxia, i.e., upregulation of HIF-1α and downregulation of eNOS and NF-kB. Postnatal severe hyperoxia exposure increased liver haemopoiesis and upregulated the expression of VEGF (both moderate and severe hyperoxia) and eNOS (severe hyperoxia) in haemopoietic cells. In conclusion, our study showed different effects of hyperoxia on hepatocytes and haemopoietic cells and differential involvement of the above factors. The involvement of VEGF and eNOS in the liver haemopoietic response to hyperoxia may be hypothesized.
Introduction
Hyperoxic ventilation is frequently involved in neonatal intensive care units for treatment of respiratory distress syndrome and pulmonary hypertension. However, many authors recommend limitation of hyperoxia exposure in the newborn period due to increased awareness about its noxious effects [1]–[3]. Premature newborns, in particular, are known to be more susceptible to oxidative stress due to immaturity of the antioxidant system [4]–[5] and due to deficiency of antioxidant precursors in parenteral nutrition [6]. The role of hyperoxia in the pathogenesis of bronchopulmonary dysplasia and retinopathy of prematurity has been widely studied but the effects of neonatal hyperoxia on the other organs have not yet been fully considered.
Some authors suggested that hyperoxic effects on liver may contribute to lung injury due to hyperoxia exposure [7]–[10] and that the liver may possibly represent a therapeutic target for patients with hyperoxia-induced lung injury [11]. In particular, it has been suggested that molecules released by the liver in response to hyperoxia may enter the bloodstream and produce responses in the lung [10]. In the literature, some reports are present about the effects of hyperoxia on the expression and activities of hepatic proteins in rats and mice, with particular reference to metabolic activities. Increased expressions of Cyp1a1/Cyp1a2, arginase I, 5-lipoxygenase, cyclooxygenases-2, iNOS, mitochondrial enoyl-CoA hydratase I and heme-oxygenase-1 have been reported in rodent liver in different types of hyperoxia exposure [7], [9]–[15]. Conversely, hyperoxia exposure has been reported to reduce the expression of 10-formyltetrahydrafolate dehydrogenase, alpha-tochopherol transfer protein and other cytochrome isoforms [15]–[17]. There are no data, instead, about hyperoxia-induced modulation in liver of growth factors and signaling molecules which are known to be involved in other tissues, such as Vascular Endothelial Growth Factor (VEGF), Matrix Metalloproteinases (MMPs), Hypoxia-Inducible Factor-1α (HIF-1α), endothelial Nitric Oxide Synthase (eNOS), and Nuclear Factor-kB (NF-kB).
In rodents, the bone marrow becomes active in haemopoiesis after birth but liver and spleen maintain this function at least in the first postnatal period. It is known that in early embryo the yolk sac represents the primary site of haemopoiesis. Haematopoietic stem cells then colonize the umbilical cord, the aorta-gonad-mesonephros region and later the embryonic liver. The liver is usually considered to cease its action in haematopoiesis soon after birth but it maintains haematopoiesis at low levels also during adulthood [18]. Hypoxia is a known inducer of medullary and extramedullary erythropoiesis but no data are available about the effects of hyperoxia in liver haematopoiesis.
The aim of the present study was to evaluate the hepatic effects of neonatal exposure to chronic moderate (60% O2) and severe (95% O2) hyperoxia, with particular reference to the main factors involved in response to hyperoxia. Hyperoxia exposure has been reported to induce proliferative responses in foci containing stem cells, such as neurogenic sites [19], so that particular attention was also put on liver haemopoiesis and in possible mechanisms involved.
Materials and Methods
Animals and experimental procedure of hyperoxia exposure
Female wild-type Sprague-Dawley rats (Harlan, Udine, Italy) and their offspring were housed and handled in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study was approved by the Italian Ministry of Health (Permit Number: 220/2009-B). Experimental procedures of hyperoxia exposure have been detailed in previous works [19]–[25]. Briefly, the study was conducted on male or female rat pups kept together with their nursing mother in conventional facilities. Mothers and litters were placed in clear polished acrylic chambers provided with software enabling a continuous monitoring of O2 and CO2 (BioSpherix, OxyCycler model A84XOV, Redfield, NY). Animals were maintained under standardized conditions of light (12∶12 h light-dark cycle, illumination onset 07.00 a.m.) at 24°C. After term gestation, the newborn rats were randomly distributed between the following experimental groups: a) newborn rats (n = 6; 3 male, 3 female) raised in ambient air for 2 weeks; b) newborn rats (n = 6; 3 male, 3 female) continuously exposed to 60% oxygen for 2 weeks; c) newborn rats (n = 6; 3 male, 3 female) continuously exposed to 95% oxygen for 2 weeks. Each experimental group comprised a balanced sample of rats across litters, i.e., two rats (one male and one female) from each of three litters, in order to limit possible interfering effects due to litter differences. According to the literature on matter [26]–[27], the nursing dams of each group were rotated every one day to prevent any negative effect of hyperoxia on nursing. The offspring were well nursed and the dam rotation did not exert any negative influence on the pups. The above O2 percentages were considered as they are the most frequently used models of hyperoxia exposure in the literature. In particular, 60–65% and 95% O2 at sea level are usually defined as moderate and severe hyperoxia, respectively [26]–[28]. At the experimental endpoints animals were sacrificed with Nembutal (40 mg/kg). Direct analyses of tissue oxygen partial pressure were not performed in the present study but increased levels of oxygen and hemoglobin oxygen saturation have already been demonstrated in previous analogous experimental protocols [29]–[31]. Moreover, hyperoxic breathing has been reported to increase portal pO2 and portal venous oxygen saturation [32]. Liver was excised from each rat, washed in phosphate-buffered saline (PBS) and cut into two blocks which have been fixed in 10% phosphate-buffered formalin or frozen at −80°C.
Light microscopy analysis and immunohistochemistry
Tissues were fixed in 10% phosphate-buffered formalin for 72 h. Each tissue block was dehydrated in a series of alcohol solutions of 50%, 70%, 80%, 95%, and 99% and then in xylene. Samples were then paraffin-embedded and cut into 5 µm-thick sections. Sections were de-waxed (xylene and alcohol in progressively lower concentrations), rehydrated and processed for haematoxylin-eosin and Silver staining (Bio Optica, Milano, Italy), according to manufacturer protocols, and for anti- Ki-67, CD34, CD45, VEGF, eNOS, NF-kB and HIF-1α immunohistochemical analyses.
As it regards immunohistochemical protocols, sections were incubated in 0.3% hydrogen peroxide for 10 min at room temperature, to remove endogenous peroxidase activity. To block non-specific background staining slides were incubated with UltraVision Protein Block (Lab Vision Thermo, CA, USA) for 10 minutes at room temperature.
Anti-CD34 immunohistochemical staining was preceded by permeabilization with proteinase K (DakoCytomation, S3020) for 5 min at room temperature. Anti-CD45 immunohistochemical reaction was preceded by antigen unmasking by heating with 10 mM sodium citrate buffer, pH 6.0, at 90°C for 10 min. For the other immunohistochemical stainings antigen unmasking was not necessary.
The primary antibodies used were the following: rabbit monoclonal anti-ki-67 (M3062, Spring Bioscience, Pleasanton, CA, USA); rabbit polyclonal anti-VEGF (sc-152, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-NF-kB (sc-109, Santa Cruz Biotechnology), and anti-eNOS (sc-654, Santa Cruz Biotechnology); mouse monoclonal anti-CD34 (sc-7324, Santa Cruz Biotechnology), anti-CD45 (Biocare Medical, clone FL3A11, purified 5450-P) and anti-HIF-1α (sc-53546, Santa Cruz Biotechnology). Primary antibodies were applied for 90 minutes at room temperature. Anti-Ki-67 antibody was diluted 1∶200 in PBS; all the other antibodies were diluted 1∶100 in PBS. Slides were incubated with Primary Antibody Enhancer (Lab Vision Thermo, CA, USA) for 10 minutes at room temperature, then with HRP Polymer (Lab Vision Thermo, CA, USA) for 30 minutes at room temperature. The immunohistochemical reactions were revealed with DAB Plus Chromogen (Lab Vision Thermo, CA, USA). Peroxidase reaction was developed using diaminobenzidine (DAB) chromogen and nuclei were counterstained with haematoxylin. Lastly, sections were dehydrated, cleared with xylene (J. T. Baker, Deventer, Holland), and mounted in Eukitt (O. Kindler GmBH, Freiburg, Germany). Negative controls were performed by omitting the primary antibody. Samples were then observed by means of Leica DM 4000 light microscopy (Leica Cambridge Ltd., Cambridge, UK) equipped with a Leica DFC 320 camera (Leica Cambridge Ltd.) for computerized images.
Terminal-deoxinucleotidyl-transferase-mediated dUTP nick end-labeling analysis
Terminal-deoxinucleotidyl-transferase-mediated dUTP nick end-labeling (TUNEL) is a method of choice for a rapid identification and quantification of apoptotic cells. DNA strand breaks, yielded during apoptosis, can be identified by labeling free 3′-OH termini with modified nucleotides in an enzymatic reaction. Paraffin embedded tissue sections were de-waxed and rehydrated. All steps were realized with FragEL DNA fragmentation Detection Kit according to the manufacturer’s instructions (Calbiochem Merck, Cambridge, MA, USA). After two rinses in PBS, tissue slides were dehydrated, mounted by using a permanent media and analyzed by light microscope. Three slides from each animal were assessed, apoptotic cells count was performed on ten fields per slide. Negative controls were performed by omitting the incubation in the presence of the enzymatic mixture, positive control was performed by treating one slide with DNAse I.
Computerized morphometry measurements and image analysis
After digitizing the images deriving from immunohistochemically and Silver stained sections, morphometric softwares were used for image analysis as follows. The percentage area of haemopoiesis was calculated with ImageJ software, a public domain Java image processing program created at the Research Services Branch, National Institute of Health, Bethesda, MD, USA (http://rsb.info.nih.gov/ij). Analyses were performed on 1132 µm×841 µm fields. The haemopoietic foci were manually delineated and the ratio between their areas and the area exhibited by the whole section was calculated and expressed in form of percentage.
The amount of reticular fibers (Silver staining) and eNOS expression were evaluated in terms of percentage area on 271 µm×185 µm fields with QWin Plus 3.5 software. In each field, the areas stained in brown, with immunohistochemistry, and black, with Silver staining, were automatically measured after definition of the color interval at the histograms of distribution of hue, saturation and brightness. The ratio between the positive area and the area exhibited by the whole section was calculated and expressed in form of percentage.
Anti-VEGF, NF-kB, and HIF-1α immunostainings, together with TUNEL reaction, were morphometrically evaluated in terms of percentages of positive nuclei on 271 µm×185 µm fields. In each field, the total number of hepatocyte nuclei and the number of positive nuclei were directly counted. The percentages of positive nuclei were derived by dividing the number of positive nuclei by the total number of nuclei and multiplying by 100.
Mean morphometric values for each parameter and each case were derived from ten fields taken from each of three slides (total number of fields examined = thirty). Fields were chosen in each section through systematic and uniformly random sampling of the fields of vision through a pre-defined XY step, i.e., randomly choosing the first field in a section and then identifying the other fields by constant predefined movements along the X and Y axis. This procedure is usually followed in stereology to allows unbiased, efficient and precise estimates [19].
Western blotting analysis
Liver lysates (20 µg) were electrophoresed and transferred to nitrocellulose membrane. Nitrocellulose membranes, blocked in 5% non-fat milk, 10 mmol/L Tris pH 7.5, 100 mmol/L NaCl, 0.1% Tween-20, were probed with mouse monoclonal anti-β-tubulin antibody (T4026, Sigma-Aldrich, St Louis, MO, USA) (primary antibody dilution 1∶1000), mouse monoclonal anti-MMP-9 (catalog number: IM37, Calbiochem Merck, Cambridge, MA, USA) (primary antibody dilution 1 mg/ml) and rabbit polyclonal anti-e-NOS antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (primary antibody dilution 1∶200) and then incubated in the presence of specific enzyme conjugated IgG horseradish peroxidase. Immunoreactive bands were detected by ECL detection system (Amersham Int, Buckinghamshire, UK) and analysed by densitometry. Densitometric values, expressed as Integrated Optical Intensity (IOI), were estimated in a CHEMIDOC XRS system by the QuantiOne 1-D analysis software (BIORAD, Richmond, CA, USA). Values obtained were normalized basing on densitometric values of internal β-tubulin.
Statistics
Statistical analysis was performed with GraphPad Prism 5 software applying the analysis of variance (one-way ANOVA and Bonferroni test). Results are expressed as mean ± SD. Values of p<0.05 were considered statistically significant.
Results
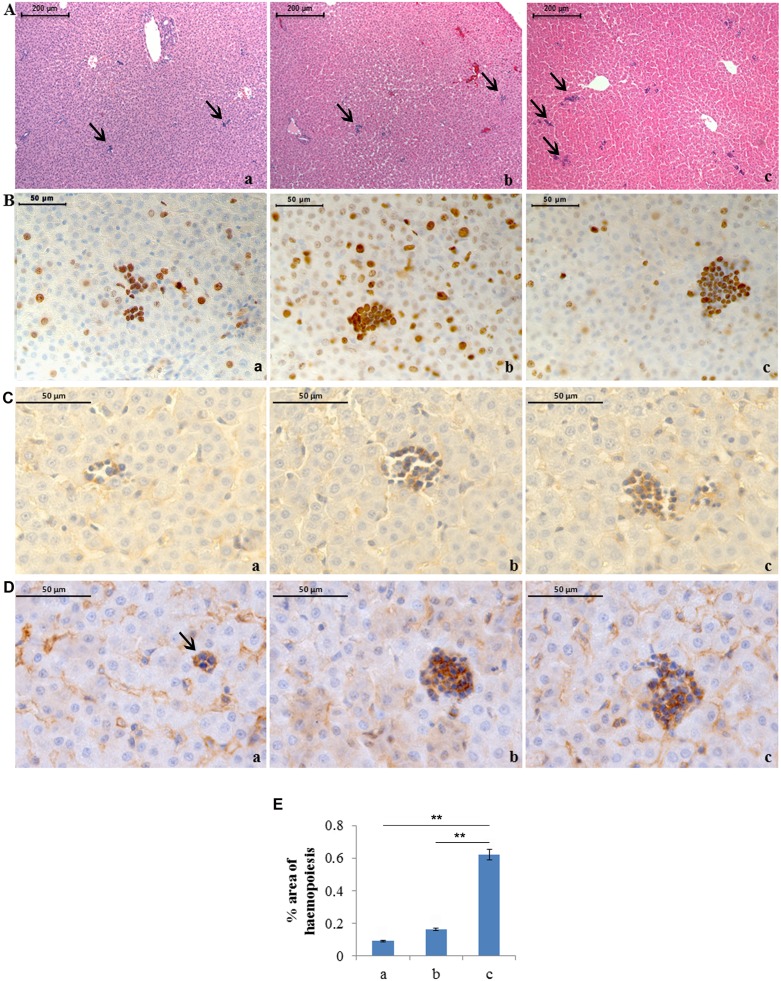
Sex differences were not found in the analysis of the various parameters. Significantly increased areas of haemopoiesis were found through morphometry in livers of rats exposed to severe hyperoxia with respect to normoxia and moderate hyperoxia (Fig. 1). Anti-Ki67 immunohistochemistry showed diffuse positivity of haemopoietic cells in all groups; haemopoietic cells in samples exposed to hyperoxia appeared larger. Positivity of the above foci for anti-CD34 and -CD45 immunohistochemistries confirmed the haemopoietic nature of the cells (Fig. 1).
Figure 1. Severe hyperoxia increases liver haemopoiesis.
A) Haematoxylin-eosin staining of neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia. Arrows indicate haemopoietic foci. Scale bar: 200 µm, magnification 10x. B) Anti-ki67 immunohistochemistry showing diffuse positivity of haemopoietic cells. Scale bar: 50 µm, magnification 40x. C) Anti-CD34 immunohistochemistry showing diffuse positivity of haemopoietic cells. Scale bar: 50 µm, magnification 63x. D) Anti-CD45 immunohistochemistry showing diffuse positivity of haemopoietic cells. Scale bar: 50 µm, magnification 63x. E) Graphic representation of the mean values of the percentage area of liver haemopoietic foci (±SD) determined in ten fields for each of three slides per sample. ** = p<0.01.
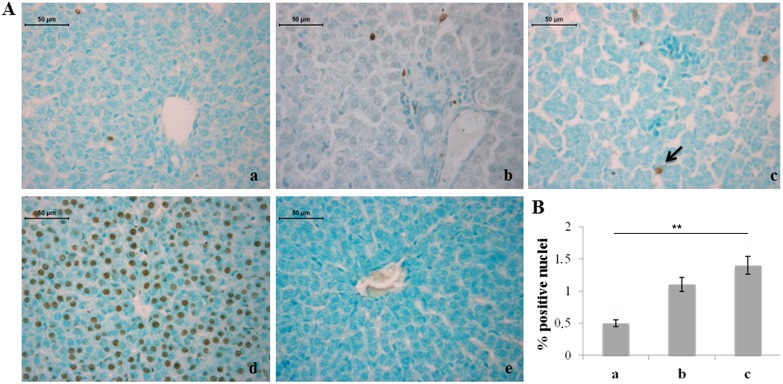
As it regards possible noxious effects of hyperoxia on the liver tissue, TUNEL analysis showed statistically significant increase in the percentage of apoptotic hepatocytes in rats exposed to 95% oxygen compared to rats exposed to normoxia (Fig. 2). Different percentages of TUNEL positivity were not found in haemopoietic foci of the three experimental groups (data not shown).
Figure 2. Severe hyperoxia increases hepatocyte apoptosis.
A) TUNEL detection of apoptotic nuclei (arrow) in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) positive control; e) negative control. Scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentage of TUNEL positive nuclei (±SD) determined by direct visual counting of ten fields for each of three slides per sample. ** = p<0.01.
Silver staining, which selectively stains reticular fibers in black, showed a reduced amount of reticular fibers in rats exposed to both moderate and severe hyperoxia with respect to unexposed ones (Fig. 3).
Figure 3. Moderate and severe hyperoxia reduces the amount of reticular fibers in rat liver.
A) Silver staining of neonatal rat livers exposed to moderate and severe hyperoxia. Reticular fibers are labeled by black stain. In livers of rats exposed to moderate and severe hyperoxia a lower amount of reticular fibers is visible. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia. Scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentage area of reticular fibers (±SD); densitometric analysis determined by quantifying thresholded area for black color in ten fields for each of three slides per sample. * = p<0.05.
Due to the above changes in components of the extracellular matrix, we then focused our attention on MMP-9, analysed through Western blot. Increased liver expression of this molecule was observed in rats exposed to severe hyperoxia compared to moderate hyperoxia-exposed and unexposed ones (Fig. 4).
Figure 4. Severe hyperoxia increases liver expression of MMP-9.
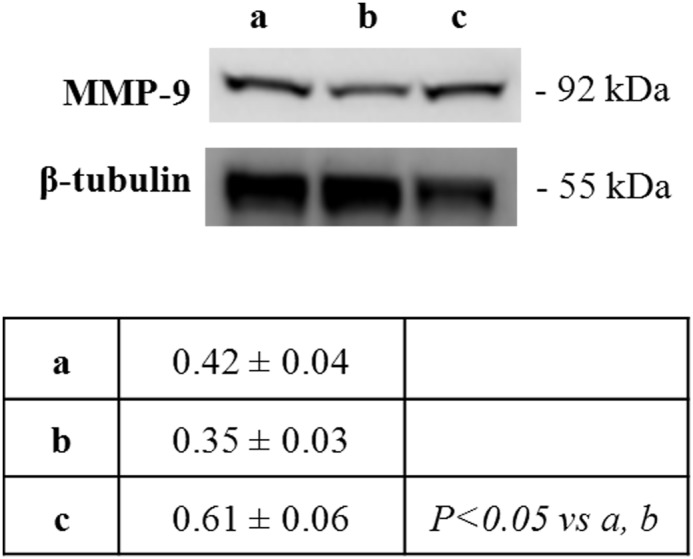
Western blotting analysis of MMP-9 expression in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia. Each membrane has been probed with anti-tubulin antibody to verify loading evenness. Data are the densitometric measurements of protein bands expressed as mean values of Integrated Optical Intensity (IOI) (±SD).
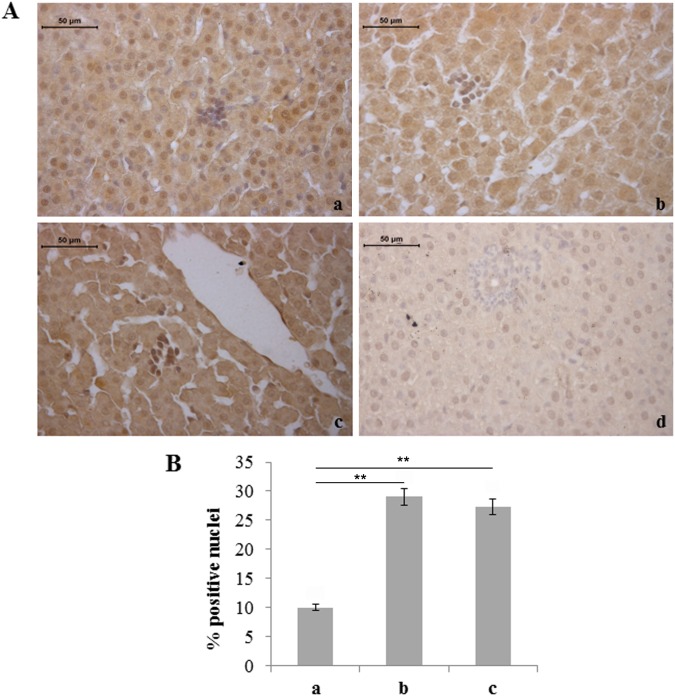
We also considered molecules involved in haemopoiesis and remodeling of blood vessels. The number of hepatocytes immunostained for VEGF was found to be reduced in rats exposed to 95% hyperoxia with respect to the other two experimental groups (Fig. 5). Conversely, increased expression of VEGF, in terms of staining intensity and number of positive cells, was observed in the liver haemopoietic foci of rats exposed to moderate and severe hyperoxia (Fig. 6).
Figure 5. Severe hyperoxia decreases the number of hepatocytes immunostained for VEGF.
A) Immunohistochemical detection of VEGF expression in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentage of VEGF positive nuclei (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. * = p<0.05; ** = p<0.01.
Figure 6. Moderate and severe hyperoxia increases VEGF expression in liver haemopoietic foci.
A) Immunohistochemical detection of VEGF-positive haemopoietic cells in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of VEGF-positive haemopoietic cells percentages (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. ** = p<0.01.
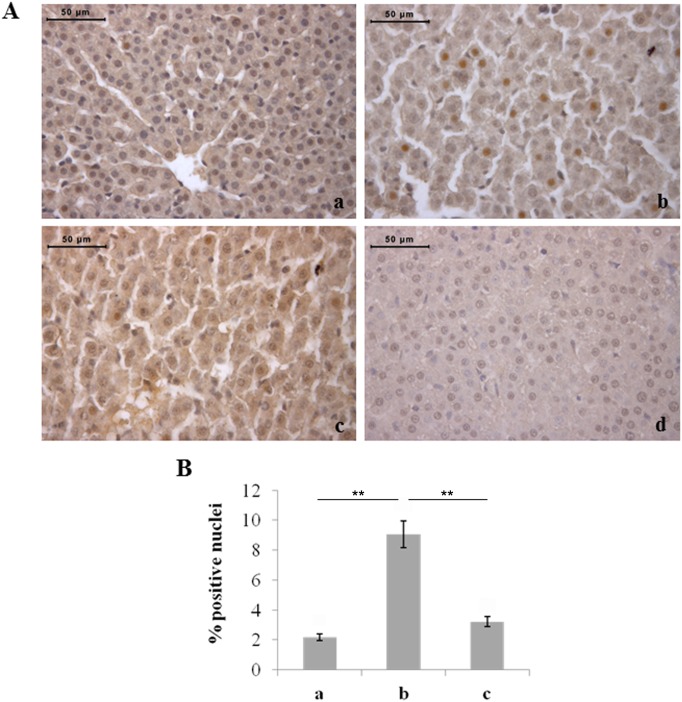
The expression of HIF-1α involved in oxygen homeostasis maintenance was also investigated. A statistically significant increase in the percentage of positive hepatocyte nuclei was found in rats exposed to 60% oxygen compared to the other two experimental groups (Fig. 7). Conversely, significant changes were not observed in the liver haemopoietic cells of the different experimental groups (data not shown).
Figure 7. Moderate hyperoxia increases the number of HIF-1α-positive hepatocytes with respect to normoxia and severe hyperoxia.
A) Immunohistochemical detection of HIF-1α expression in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentages of HIF-1α positive nuclei (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. ** = p<0.01.
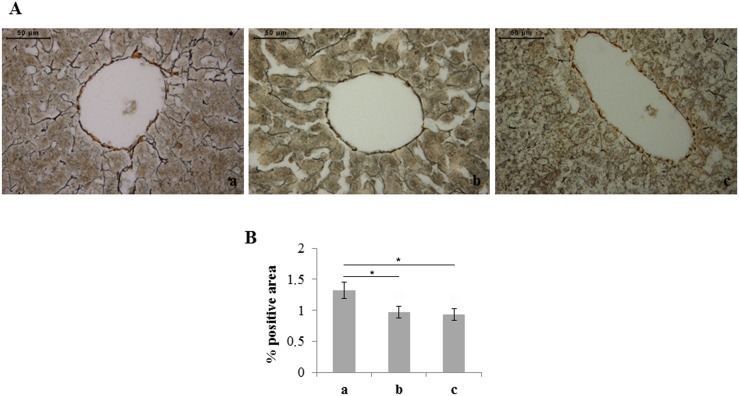
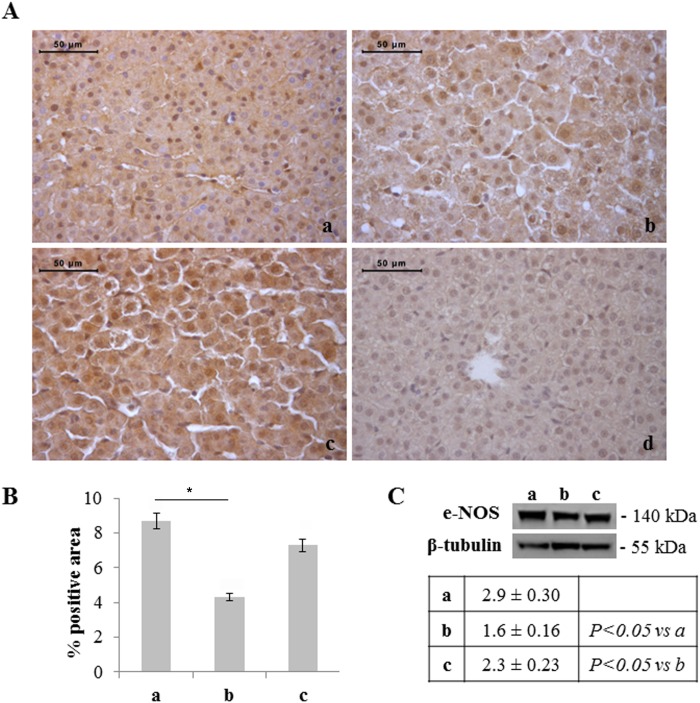
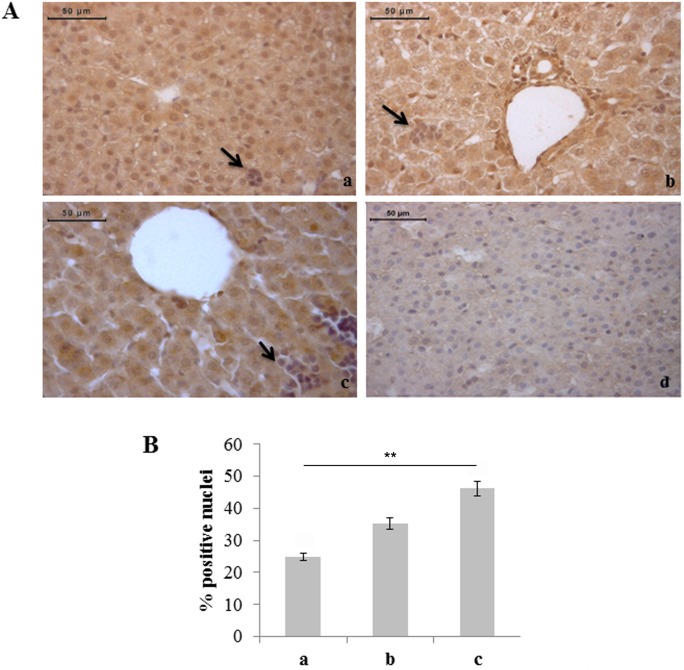
As it regards the expression of eNOS, a decrease in its positivity was found in hepatocytes of rats exposed to moderate hyperoxia with respect to normoxia (Western blot and immunohistochemistry) and severe hyperoxia (Western blot) (Fig. 8). Specific evaluation of haemopoietic foci, instead, showed a progressive increase in the number of positive cells with increased severity of hyperoxia, the percentage of immunostained cells being significantly higher in 95% hyperoxia than in normoxia (Fig. 9).
Figure 8. Moderate hyperoxia reduces hepatocyte eNOS expression with respect to normoxia and severe hyperoxia.
A) Immunohistochemical detection of eNOS expression in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentage of eNOS positive area (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. * = p<0.05. C) Western blotting analysis of eNOS expression in neonatal rats. Each membrane has been probed with anti β-tubulin antibody to verify loading evenness. The most representative out of three separate experiments is shown. Data are the densitometric measurements of protein bands expressed as mean values of Integrated Optical Intensity (IOI) (±SD).
Figure 9. Severe hyperoxia increases eNOS expression in haemopoietic foci.
A) Immunohistochemical detection of eNOS-positive haemopoietic cells (arrows) in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentages of eNOS-positive haemopoietic cells (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. ** = p<0.01.
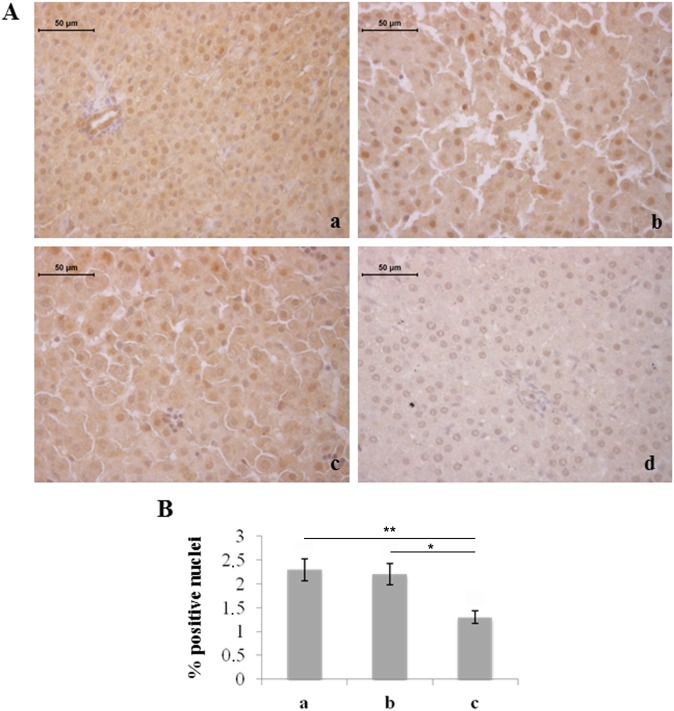
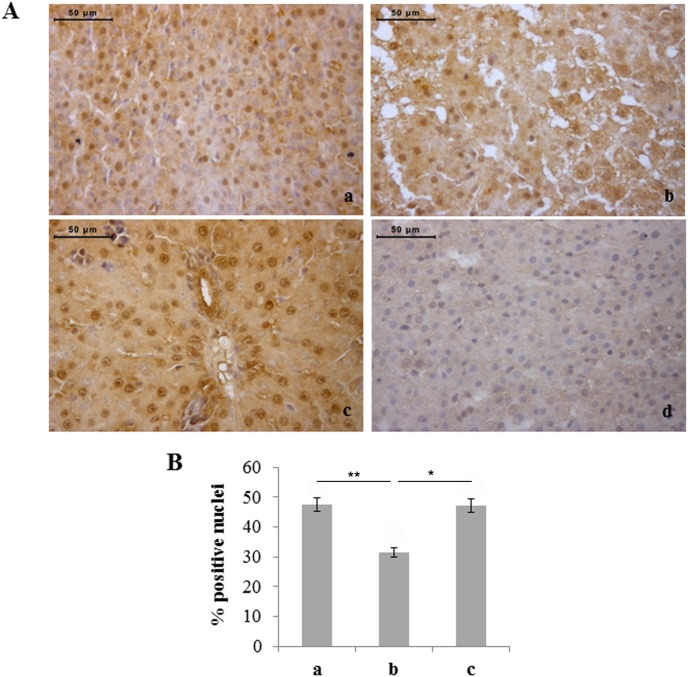
Finally, NF-kB nuclear translocation was also studied through immunohistochemistry due to its role in the response to oxidative stress and development/differentiation of haemopoietic cells. The percentage of NF-kB positive hepatocyte nuclei was significantly lower in rats exposed to 60% hyperoxia than in controls and rats exposed to 95% hyperoxia (Fig. 10). Significant changes were not observed in the percentages of NF-kB positive liver haemopoietic cells of the different experimental groups (data not shown).
Figure 10. Moderate hyperoxia reduces the number of NF-kB positive hepatocytes with respect to normoxia and severe hyperoxia.
A) Immunohistochemical detection of Nfκb expression in neonatal rat livers exposed to moderate and severe hyperoxia. a) ambient air; b) 60% hyperoxia; c) 95% hyperoxia; d) negative control; scale bar: 50 µm, magnification 40x. B) Graphic representation of the percentages of NF-kB positive nuclei (±SD); densitometric analysis determined by direct visual counting of ten fields for each of three slides per sample. * = p<0.05; ** = p<0.01.
Discussion
In the present work we evaluated the effects of postnatal hyperoxia exposure on rat liver with reference to both hepatocytes and hepatic haemopoietic cells, reporting differential effects on the two components. Markers of apoptosis and proliferation were considered, together with a series of factors previously found in other tissues to be involved in hyperoxic response.
There are papers in the literature reporting increased apoptotic cell index due to hyperoxia exposure in different tissues, such as lungs [33], brain [6], [34]–[37] and heart [25]. In the present work, statistically significant increase of apoptotic hepatocyte percentage was found in the livers of rats exposed to severe hyperoxia compared to unexposed ones. This finding indicates that hyperoxia may exert a noxious effect in the liver tissue of newborn rats, although only as a consequence of exposure to severe hyperoxia. Preceding works of our group involving analogous experimental conditions have also reported increased apoptosis in the heart [25] and dentate gyrus [19] in response to severe, but not moderate, hyperoxia.
As it regards haemopoietic foci, instead, a significant increase in the percentage of apoptotic cells was not found. Conversely, we observed a paradoxical response of liver hemopoiesis to hyperoxia, foci of liver haemopoiesis being larger and more numerous in rats exposed to severe hyperoxia with respect to controls. This finding may be interpreted in different ways. For instance, it could be a response to hyperoxia-induced haemolytic reactions, as exposure of rats to 80% hyperoxia for 11 days has been reported to increase haemolysis and decrease haemoglobin concentration [38]. Otherwise, it could be intriguing to hypothesize a direct effect of hyperoxia on haemopoietic stem cells. We have previously found, for instance, that both moderate and severe hyperoxia may induce a proliferative response in the main sites of postnatal neurogenesis, the subventricular zone and dentate gyrus [19]. Some mechanisms involved in hypoxia-induced increase in haemopoiesis could be paradoxically in common with hyperoxia-induced changes.
In the present paper, we also evaluated the extracellular matrix in order to find possible signs/mechanisms of liver damage and consider a possible role of its remodeling in the haemopoietic response. A decrease in the reticular fiber content was found in the liver of rats exposed both to moderate and severe hyperoxia, suggesting a possible role of changes in the organization of the local extracellular matrix.
MMPs, collectively called matrixins, are a family of proteases which specifically participate in remodeling and turnover of extracellular matrix components [39]. Specifically, MMP-9 is a member of the gelatinase family, also including MMP-2, which is known to be highly expressed in damaged and regenerating livers [40]–[41]. MMP-9 expression has been found to be modified in lung following hyperoxia [42]–[43]. MMP-9 plays an important role in the structural changes consequent to oxygen-induced lung injury in newborn mice [42]. Increased expression of MMPs by macrophages has also been associated with lung emphysema [44]. On the basis of these data, we also evaluated MMP-9 expression in the liver of rats exposed to hyperoxia and we observed an increased expression of MMP-9 in livers exposed to severe hyperoxia compared to moderate hyperoxia-exposed and unexposed ones. Thus, it may be hypothesized a role for upregulation of MMP-9 expression in the hyperoxia-induced remodeling of extracellular matrix.
VEGF may also play a key role in hyperoxia, specifically as a survival factor in oxidant injury [45]. Both increases and decreases in VEGF content have been reported in lung due to hyperoxia exposure, as a consequence of different experimental conditions (degree of hyperoxia, age and species of the animals, recovery periods) [45]–[48]. For instance, we previously reported decrease of VEGF expression in the lung of newborn rats exposed to 60% hyperoxia for the first 14 postnatal days [22] but few experimental data are available about hyperoxia-induced changes in VEGF expression in other tissues than lungs. VEGF and VEGF receptor 2 have been reported to be decreased in the brain of adult mice exposed to 50% hyperoxia for up to 3 weeks [31]. In a previous study of our group addressing the effects of hyperoxia on the heart (same experimental conditions of the present work), we reported a decrease and an increase of VEGF protein content in moderate and severe hyperoxia, respectively [24]. In liver, we found a different kind of change in VEGF expression: a reduction in the number of VEGF-positive hepatocytes was found in rats exposed to 95% hyperoxia with respect to the other two experimental groups. Thus, hyperoxia exposure showed an additional noxious effect on hepatocytes consisting in impairment of VEGF expression.
Investigation of VEGF expression was also particularly intriguing regarding haemopoietic cells as VEGF controls hematopoietic stem cell survival and proliferation through an internal autocrine loop mechanism [49]. Consistently with a role of VEGF in liver haemopoiesis induction, in our study we observed an increased expression of VEGF, in terms of staining intensity and number of positive cells, in the liver haemopoietic foci of rats exposed to moderate and severe hyperoxia. In the present study, anti-VEGF immunostaining also involved the nuclear compartment. This is consistent with other reports [50]–[51] and may be particularly important for the haemopoietic cells which are internally activated by VEGF [49]. Thus, cell specific activation of VEGF synthesis may be considered one of the mechanisms involved in the hyperoxia-induced haemopoietic response.
The expression of VEGF may be transcriptionally activated by HIF-1α [52] and previous study have shown that HIF-1α, although the major transcriptional factor involved in response to hypoxia [53], may also be activated by hyperoxia [24], [31], [54]. In the present work, however, HIF-1α immunostaining was not correlated with anti-VEGF positivity in hepatocytes. An increase in the percentage of anti-HIF-1α stained hepatocytes was found only in rats exposed to 60% hyperoxia. An increased expression of HIF-1α in haemopoietic cells would have possibly explained the upregulation of VEGF synthesis but changes were not found between the different experimental conditions. However, it must be considered that VEGF expression may also be activated by other alternative signaling pathways and regulators, such as PGC-1α [55], which could be investigated in the future.
The expression of eNOS was also evaluated as hyperoxia effects on the NOS expression and function have been reported [22], [24], [56]–[60] and NO is widely interrelated with the other factors considered. eNOS may be activated by VEGF or by stimuli such as hemodynamic shear stress and modified oxygen supply. It mediates, through NO production, vasodilatation, angiogenesis and increased vascular permeability [61]. Differential changes in eNOS expression have previously been reported in response to hyperoxia in various tissues. For instance, in a previous study of our group, the eNOS protein level was increased in the lung of newborn rats exposed to 60% hyperoxia for the first 14 postnatal days [22]. Conversely, eNOS protein content was decreased in the heart tissue of newborn rats exposed to 60% and 95% hyperoxia for the first 14 postnatal days [24] and the protein levels of eNOS and iNOS have been reported not to be modified in the liver of six-week-old mice exposed to >95% O2 for 72 or 96 h [11]. In the present study we also found different responses in hepatocytes and haemopoietic cells. In hepatocytes, eNOS expression was selectively reduced in moderate hyperoxia. In the liver tissue, the changes in eNOS and MMP9 expressions are similar and may be partially explained by previously reported finding that eNOS is essential for the efficient early induction of MMP-9 in damaged liver [62]. In haemopoietic foci, eNOS expression was progressively increased with increased degree of hyperoxia, reaching significance in severe hyperoxia with respect to normoxia. Thus, eNOS expression, directly induced by hyperoxia or indirectly activated by VEGF, could also play a role in hyperoxia-induced liver haemopoietic response. The hyperoxia-induced increase in eNOS expression in haemopoietic cells may be correlated with VEGF expression, as autocrine VEGF in acute myeloid leukemia cells has been reported to produce NO, through eNOS activation [63]. NO is also known to regulate haemopoiesis and stimulate cell growth in acute myeloid leukemia cells [63].
In the present study we also evaluated NF-kB nuclear translocation through immunohistochemistry, as NF-kB is involved in the response to oxidative stress [64] and it is known to play a role in development and differentiation of hemopoietic cells [65]–[66]. The percentage of NF-kB positive hepatocyte nuclei was significantly lower in rats exposed to 60% hyperoxia than in controls and rats exposed to 95% O2, whereas statistically significant differences were not found between normoxia and severe hyperoxia. An analogous finding was previously found in heart samples from the same experimental conditions, with decrease and increase of the percentage of NF-kB positive myocardial cell nuclei in moderate and severe hyperoxia, respectively [25]. Conversely, in the present study, significant differences were not found in NF-kB nuclear immunostaining of hemopoietic cells of the different experimental groups, although further studies will be needed to exclude a role for this factor in hyperoxia-induced changes in liver haemopoiesis.
In conclusion, our study showed different effects of hyperoxia on hepatocytes and haemopoietic cells, with growth factors and intracellular mechanisms being differently involved. Postnatal hyperoxia shows detrimental action on hepatic tissue, with increased hepatocyte apoptosis, increased MMP-9 expression and decreased reticular fiber content. Decreased VEGF expression may also play a role in severe hyperoxia whereas some other changes have been found to mainly involve response to moderate hyperoxia, such as increased HIF-1α expression, and decrease expression of eNOS and NF-kB. Conversely, postnatal hyperoxia exposure increases liver haemopoiesis and upregulates VEGF and eNOS expression. Thus, it may be hypothesized that hyperoxia stimulates proliferation of haemopoietic cells through VEGF and/or eNOS, and these findings may put further questions on the modulation of stem cell proliferation by changes in O2 concentrations.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Saugstad OD (2005) Oxidative stress in the newborn - a 30-year perspective. Biol Neonate 88: 228–236. [DOI] [PubMed] [Google Scholar]
- 2. Deulofeut R, Critz A, Adams-Chapman I, Sola A (2006) Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. J Perinatol 26: 700–705. [DOI] [PubMed] [Google Scholar]
- 3. Deuber C, Terhaar M (2011) Hyperoxia in very preterm infants: a systematic review of the literature. J Perinat Neonatal Nurs 25: 268–274. [DOI] [PubMed] [Google Scholar]
- 4. Sturman JA, Gaull G, Raiha NC (1970) Absence of cystathionase in human fetal liver: is cystine essential? Science 169: 74–76. [DOI] [PubMed] [Google Scholar]
- 5. Buonocore G, Perrone S, Bracci R (2001) Free radicals and brain damage in the newborn. Biol Neonate 79: 180–186. [DOI] [PubMed] [Google Scholar]
- 6. Taglialatela G, Perez-Polo JR, Rassin DK (1998) Induction of apoptosis in the CNS during development by the combination of hyperoxia and inhibition of glutathione synthesis. Free Radic Biol Med 25: 936–942. [DOI] [PubMed] [Google Scholar]
- 7. Moorthy B, Nguyen UT, Gupta S, Stewart KD, Welty SE, et al. (1997) Induction and decline of hepatic cytochromes P4501A1 and 1A2 in rats exposed to hyperoxia are not paralleled by changes in glutathione S-transferase-alpha. Toxicol Lett 90: 67–75. [DOI] [PubMed] [Google Scholar]
- 8. Moorthy B, Parker KM, Smith CV, Bend JR, Welty SE (2000) Potentiation of oxygen-induced lung injury in rats by the mechanism-based cytochrome P-450 inhibitor, 1-aminobenzotriazole. J Pharmacol Exp Ther 292: 553–560. [PubMed] [Google Scholar]
- 9. Couroucli XI, Welty SE, Geske RS, Moorthy B (2002) Regulation of pulmonary and hepatic cytochrome P4501A expression in the rat by hyperoxia: implications for hyperoxic lung injury. Mol Pharmacol 61: 507–515. [DOI] [PubMed] [Google Scholar]
- 10. Rogers LK, Tipple TE, Britt RD, Welty SE (2010) Hyperoxia exposure alters hepatic eicosanoid metabolism in newborn mice. Pediatr Res 67: 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Malleske DT, Rogers LK, Velluci SM, Young TL, Park MS, et al. (2006) Hyperoxia increases hepatic arginase expression and ornithine production in mice. Toxicol Appl Pharmacol 215: 109–117. [DOI] [PubMed] [Google Scholar]
- 12. Okamoto T, Mitsuhashi M, Fujita I, Sindhu RK, Kikkawa Y (1993) Induction of cytochrome P450 1A1 and 1A2 by hyperoxia. Biochem Biophys Res Commun 197: 878–885. [DOI] [PubMed] [Google Scholar]
- 13. Miralles C, Busquets X, Santos C, Togores B, Hussain S, et al. (2000) Regulation of iNOS expression and glutathione levels in rat liver by oxygen tension. FEBS Lett 476: 253–257. [DOI] [PubMed] [Google Scholar]
- 14. Miralles C, Agustí AG, Aubry C, Sanchez JC, Walzer C, et al. (2000) Changes induced by oxygen in rat liver proteins identified by high-resolution two-dimensional gel electrophoresis. Eur J Biochem 267: 5580–5584. [DOI] [PubMed] [Google Scholar]
- 15. Mačak-Šafranko Z, Sobočanec S, Sarić A, Balog T, Sverko V, et al. (2011) Cytochrome P450 gender-related differences in response to hyperoxia in young CBA mice. Exp Toxicol Pathol 63: 345–350. [DOI] [PubMed] [Google Scholar]
- 16. Wong YL, Smith CV, McMicken HW, Rogers LK, Welty SE (2001) Mitochondrial thiol status in the liver is altered by exposure to hyperoxia. Toxicol Lett 123: 179–193. [DOI] [PubMed] [Google Scholar]
- 17. Ban R, Takitani K, Kim HS, Murata T, Morinobu T, et al. (2002) alpha-Tocopherol transfer protein expression in rat liver exposed to hyperoxia. Free Radic Res 36: 933–938. [DOI] [PubMed] [Google Scholar]
- 18. Kim CH (2010) Homeostatic and pathogenic extramedullary hematopoiesis. J Blood Med 1: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porzionato A, Macchi V, Zaramella P, Sarasin G, Grisafi D, et al.. (2014) Effects of postnatal hyperoxia exposure on the rat dentate gyrus and subventricular zone. Brain Struct Funct In press. [DOI] [PubMed]
- 20. Porzionato A, Zaramella P, Macchi V, Grisafi D, Salmaso R, et al. (2012) Fluoxetine may worsen hyperoxia-induced lung damage in neonatal rats. Histol Histopathol 27: 1599–1610. [DOI] [PubMed] [Google Scholar]
- 21. Porzionato A, Zaramella P, Macchi V, Sarasin G, Di Giulio C, et al. (2013) Cyclosporine and hyperoxia-induced lung damage in neonatal rats. Respir Physiol Neurobiol 187: 41–46. [DOI] [PubMed] [Google Scholar]
- 22. Grisafi D, Tassone E, Dedja A, Oselladore B, Masola V, et al. (2012) L-citrulline prevents alveolar and vascular derangement in a rat model of moderate hyperoxia-induced lung injury. Lung 190: 419–430. [DOI] [PubMed] [Google Scholar]
- 23. Grisafi D, Pozzobon M, Dedja A, Vanzo V, Tomanin R, et al. (2013) Human amniotic fluid stem cells protect rat lungs exposed to moderate hyperoxia. Pediatr Pulmonol 48: 1070–1080. [DOI] [PubMed] [Google Scholar]
- 24. Zara S, Macchi V, De Caro R, Rapino M, Cataldi A, et al. (2012) pPKCα mediated-HIF-1α activation related to the morphological modifications occurring in neonatal myocardial tissue in response to severe and moderate hyperoxia. Eur J Histochem 56: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zara S, De Colli M, Rapino M, Di Valerio V, Marconi GD, et al. (2013) NF-κB involvement in hyperoxia-induced myocardial damage in newborn rat hearts. Histochem Cell Biol 140: 575–583. [DOI] [PubMed] [Google Scholar]
- 26. Chen CM, Wang LF, Chou HC, Lang YD, Lai YP (2007) Up-regulation of connective tissue growth factor in hyperoxia-induced lung fibrosis. Pediatr Res 62: 128–133. [DOI] [PubMed] [Google Scholar]
- 27. Tang JR, Seedorf GJ, Muehlethaler V, Walker DL, Markham NE, et al. (2010) Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 299: L735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Balasubramaniam V, Mervis CF, Maxey AM, Markham NE, Abman SH (2007) Hyperoxia reduces bone marrow, circulating, and lung endothelial progenitor cells in the developing lung: implications for the pathogenesis of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L1073–1084. [DOI] [PubMed] [Google Scholar]
- 29. Bulte DP, Chiarelli PA, Wise RG, Jezzard P (2007) Cerebral perfusion response to hyperoxia. J Cereb Blood Flow Metab 27: 69–75. [DOI] [PubMed] [Google Scholar]
- 30. Duong TQ, Iadecola C, Kim SG (2001) Effect of hyperoxia, hypercapnia, and hypoxia on cerebral interstitial oxygen tension and cerebral blood flow. Magn Reson Med 45: 61–70. [DOI] [PubMed] [Google Scholar]
- 31. Benderro GF, Sun X, Kuang Y, Lamanna JC (2012) Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res 1471: 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hughes SJ, Yang W, Juszczak M, Jones GL, Powis SH, et al. (2004) Effect of inspired oxygen on portal and hepatic oxygenation: effective arterialization of portal blood by hyperoxia. Cell Transplant 13: 801–808. [DOI] [PubMed] [Google Scholar]
- 33. Wen ST, Chen W, Chen HL, Lai CW, Yen CC, et al. (2013) Amniotic fluid stem cells from EGFP transgenic mice attenuate hyperoxia-induced acute lung injury. PLoS One 8: e75383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hu X, Qiu J, Grafe MR, Rea HC, Rassin DK, et al. (2003) Bcl-2 family members make different contributions to cell death in hypoxia and/or hyperoxia in rat cerebral cortex. Int J Dev Neurosci 21: 371–377. [DOI] [PubMed] [Google Scholar]
- 35. Felderhoff-Mueser U, Bittigau P, Sifringer M, Jarosz B, Korobowicz E, et al. (2004) Oxygen causes cell death in the developing brain. Neurobiol Dis 17: 273–282. [DOI] [PubMed] [Google Scholar]
- 36. Felderhoff-Mueser U, Sifringer M, Polley O, Dzietko M, Leineweber B, et al. (2005) Caspase-1-processed interleukins in hyperoxia-induced cell death in the developing brain. Ann Neurol 57: 50–59. [DOI] [PubMed] [Google Scholar]
- 37. Zaghloul N, Nasim M, Patel H, Codipilly C, Marambaud P, et al. (2012) Overexpression of extracellular superoxide dismutase has a protective role against hyperoxia-induced brain injury in neonatal mice. FEBS J 279: 871–881. [DOI] [PubMed] [Google Scholar]
- 38. Webster NR, Toothill C, Cowen PN (1987) Tissue responses to hyperoxia. Biochemistry and pathology. Br J Anaesth 59: 760–771. [DOI] [PubMed] [Google Scholar]
- 39. Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ram M, Sherer Y, Shoenfeld Y (2006) Matrix metalloproteinase-9 and autoimmune diseases J Clin Immunol. 26: 299–307. [DOI] [PubMed] [Google Scholar]
- 41. Olle EW, Ren X, McClintock SD, Warner RL, Deogracias MP, et al. (2006) Matrix metalloproteinase-9 is an important factor in hepatic regeneration after partial hepatectomy in mice. Hepatology 44: 540–549. [DOI] [PubMed] [Google Scholar]
- 42. Sweet DG, Curley AE, Chesshyre E, Pizzotti J, Wilbourn MS, et al. (2004) The role of matrix metalloproteinases -9 and -2 in development of neonatal chronic lung disease. Acta Paediatr 93: 791–796. [DOI] [PubMed] [Google Scholar]
- 43. Kawada S, Fukaya K, Ohtani M, Kobayashi K, Fukusaki C (2008) Effects of pre-exposure to hyperbaric hyperoxia on high-intensity exercise performance. J Strength Cond Res 22: 66–74. [DOI] [PubMed] [Google Scholar]
- 44. Shapiro SD (1994) Elastolytic metalloproteinases produced by human mononuclear phagocytes. Potential roles in destructive lung disease. Am J Respir Crit Care Med 150: S160–164. [DOI] [PubMed] [Google Scholar]
- 45. Klekamp JG, Jarzecka K, Perkett EA (1999) Exposure to hyperoxia decreases the expression of vascular endothelial growth factor and its receptors in adult rat lungs. Am J Pathol 154: 823–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Corne J, Chupp G, Lee CG, Homer RJ, Zhu Z, et al. (2000) IL-13 stimulates vascular endothelial cell growth factor and protects against hyperoxic acute lung injury. J Clin Invest 106: 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. D’Angio CT, Maniscalco WM (2002) The role of vascular growth factors in hyperoxia-induced injury to the developing lung. Front Biosci 7: d1609–1623. [DOI] [PubMed] [Google Scholar]
- 48. Pogach MS, Cao Y, Millien G, Ramirez MI, Williams MC (2007) Key developmental regulators change during hyperoxia-induced injury and recovery in adult mouse lung. J Cell Biochem 100: 1415–1429. [DOI] [PubMed] [Google Scholar]
- 49. Gerber HP, Malik AK, Solar GP, Sherman D, Liang XH, et al. (2002) VEGF regulates haematopoietic stem cell survival by an internal autocrine loop mechanism. Nature 417: 954–958. [DOI] [PubMed] [Google Scholar]
- 50. Li W, Keller G (2000) VEGF nuclear accumulation correlates with phenotypical changes in endothelial cells. J Cell Sci 113: 1525–1534. [DOI] [PubMed] [Google Scholar]
- 51. Lejbkowicz F, Goldberg-Cohen I, Levy AP (2005) New horizons for VEGF. Is there a role for nuclear localization? Acta Histochem 106: 405–411. [DOI] [PubMed] [Google Scholar]
- 52. Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, et al. (2005) HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene 24: 3110–3120. [DOI] [PubMed] [Google Scholar]
- 53. Semenza GL (2012) Hypoxia-inducible factors in physiology and medicine. Cell 148: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wikenheiser J, Wolfram JA, Gargesha M, Yang K, Karunamuni G, et al. (2009) Altered hypoxia-inducible factor-1 alpha expression levels correlate with coronary vessel anomalies. Dev Dyn 238: 2688–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, et al. (2008) HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature 451: 1008–1012. [DOI] [PubMed] [Google Scholar]
- 56. Potter CF, Kuo NT, Farver CF, McMahon JT, Chang CH, et al. (1999) Effects of hyperoxia on nitric oxide synthase expression, nitric oxide activity, and lung injury in rat pups. Pediatr Res 45: 8–13. [DOI] [PubMed] [Google Scholar]
- 57. Thom SR, Bhopale V, Fisher D, Manevich Y, Huang PL, et al. (2002) Stimulation of nitric oxide synthase in cerebral cortex due to elevated partial pressures of oxygen: an oxidative stress response. J Neurobiol 51: 85–100. [DOI] [PubMed] [Google Scholar]
- 58. Atochin DN, Demchenko IT, Astern J, Boso AE, Piantadosi CA, et al. (2003) Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab 23: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 59. Curatola AM, Xu J, Hendricks-Munoz KD (2011) Cyclic GMP protects endothelial progenitors from oxidative stress. Angiogenesis 14: 267–279. [DOI] [PubMed] [Google Scholar]
- 60. Rubini A, Porzionato A, Zara S, Cataldi A, Garetto G, et al. (2013) The effect of acute exposure to hyperbaric oxygen on respiratory system mechanics in the rat. Lung 191: 459–466. [DOI] [PubMed] [Google Scholar]
- 61. Shaul PW (2002) Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64: 749–774. [DOI] [PubMed] [Google Scholar]
- 62. Mei Y, Thevananther S (2011) Endothelial nitric oxide synthase is a key mediator of hepatocyte proliferation in response to partial hepatectomy in mice. Hepatology 54: 1777–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koistinen P, Siitonen T, Mäntymaa P, Säily M, Kinnula V, et al. (2001) Regulation of the acute myeloid leukemia cell line OCI/AML-2 by endothelial nitric oxide synthase under the control of a vascular endothelial growth factor signaling system. Leukemia 15: 1433–1441. [DOI] [PubMed] [Google Scholar]
- 64. Janssen-Heininger YM, Poynter ME, Baeuerle PA (2000) Recent advances towards understanding redox mechanisms in the activation of nuclear factor kappaB. Free Radic Biol Med 28: 1317–1327. [DOI] [PubMed] [Google Scholar]
- 65. Bottero V, Withoff S, Verma IM (2006) NF-kappaB and the regulation of hematopoiesis. Cell Death Differ 13: 785–797. [DOI] [PubMed] [Google Scholar]
- 66. Gerondakis S, Banerjee A, Grigoriadis G, Vasanthakumar A, Gugasyan R, et al. (2012) NF-κB subunit specificity in hemopoiesis. Immunol Rev 246: 272–285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.