Abstract
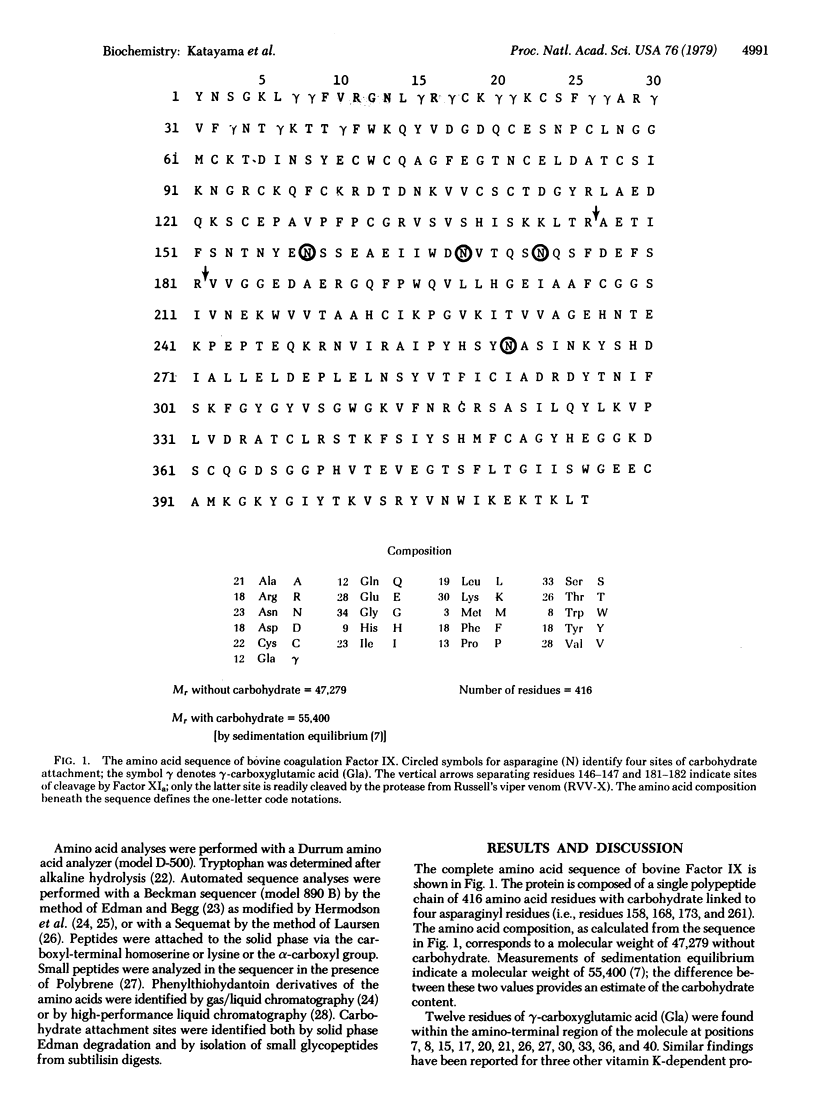
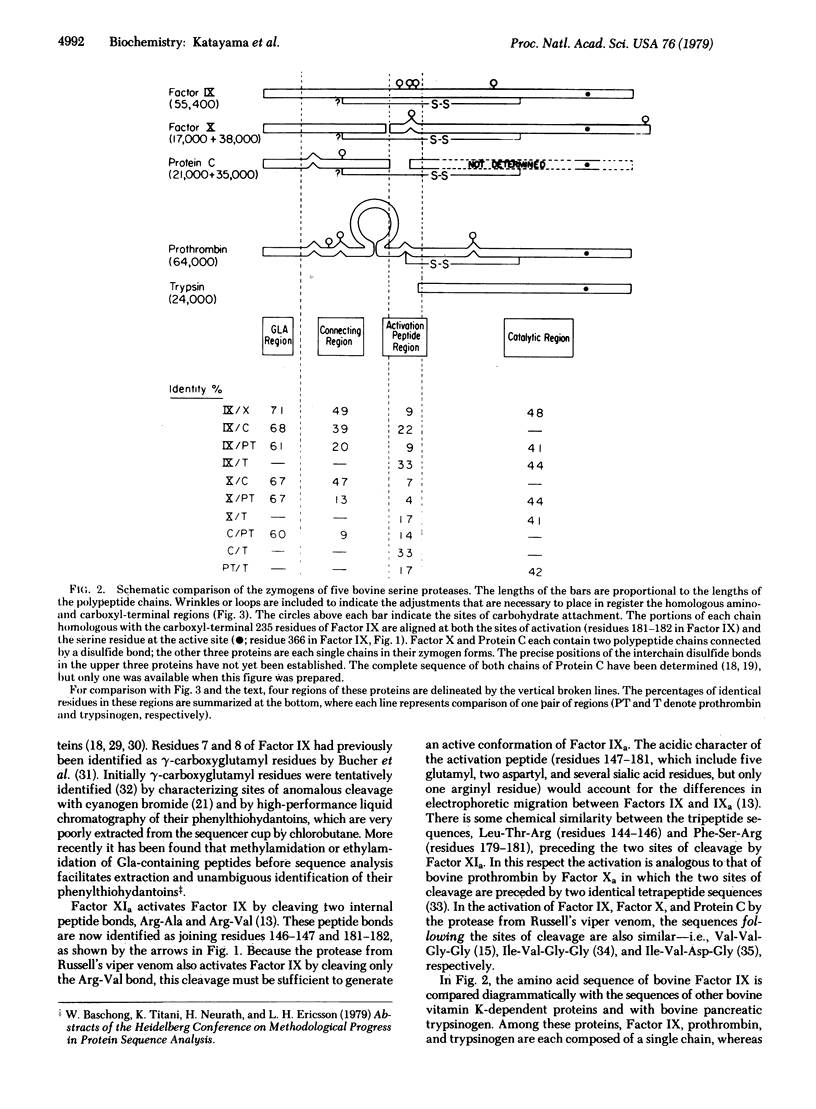
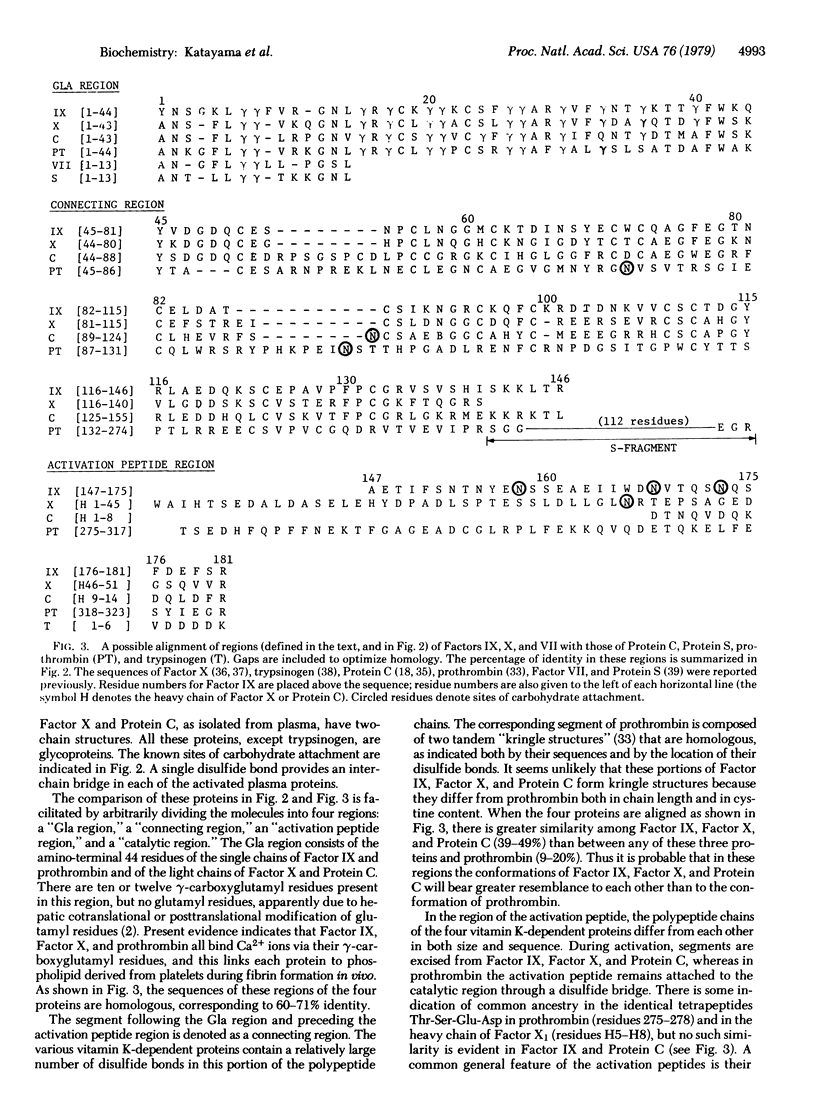
The amino acid sequence of bovine blood coagulation Factor IX (Christmas Factor) is presented and compared with the sequences of other vitamin K-dependent plasma proteins and pancreatic trypsinogen. The 416-residue sequence of Factor IX was determined largely by automated Edman degradation of two large segments, containing 181 and 235 residues, isolated after activating Factor IX with a protease from Russell's viper venom. Subfragments of the two segments were produced by enzymatic digestion and by chemical cleavage of methionyl, tryptophyl, and asparaginyl-glycyl bonds. Comparison of the amino acid sequences of Factor IX, Factor X, and Protein C demonstrates that they are homologous throughout. Their homology with prothrombin, however, is restricted to the amino-terminal region, which is rich in gamma-carboxyglutamic acid, and the carboxyl-terminal region, which represents the catalytic domain of these proteins and corresponds to that of pancreatic serine proteases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGGELER P. M., WHITE S. G., GLENDENING M. B., PAGE E. W., LEAKE T. B., BATES G. Plasma thromboplastin component (PTC) deficiency; a new disease resembling hemophilia. Proc Soc Exp Biol Med. 1952 Apr;79(4):692–694. doi: 10.3181/00379727-79-19488. [DOI] [PubMed] [Google Scholar]
- Andersson L. O., Borg H., Miller-Andersson M. Purification and characterization of human factor IX. Thromb Res. 1975 Sep;7(3):451–459. doi: 10.1016/0049-3848(75)90039-0. [DOI] [PubMed] [Google Scholar]
- BIGGS R., DOUGLAS A. S., MACFARLANE R. G., DACIE J. V., PITNEY W. R., MERSKEY Christmas disease: a condition previously mistaken for haemophilia. Br Med J. 1952 Dec 27;2(4799):1378–1382. doi: 10.1136/bmj.2.4799.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen P. J., Cross G. A., Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976 Oct 14;263(5578):613–614. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- Bucher D., Nebelin E., Thomsen J., Stenflo J. Identification of gamma-carboxyglutamic acid residues in bovine factors IX and X, and in a new vitamin K-dependent protein. FEBS Lett. 1976 Oct 1;68(2):293–296. doi: 10.1016/0014-5793(76)80456-5. [DOI] [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K. Basic mechanisms in blood coagulation. Annu Rev Biochem. 1975;44:799–829. doi: 10.1146/annurev.bi.44.070175.004055. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Yates S. G., Davie E. W. A comparison of human prothrombin, factor IX (Christmas factor), factor X (Stuart factor), and protein S. Biochemistry. 1977 Feb 22;16(4):698–706. doi: 10.1021/bi00623a022. [DOI] [PubMed] [Google Scholar]
- Di Scipio R. G., Kurachi K., Davie E. W. Activation of human factor IX (Christmas factor). J Clin Invest. 1978 Jun;61(6):1528–1538. doi: 10.1172/JCI109073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScipio R. G., Davie E. W. Characterization of protein S, a gamma-carboxyglutamic acid containing protein from bovine and human plasma. Biochemistry. 1979 Mar 6;18(5):899–904. doi: 10.1021/bi00572a026. [DOI] [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Fujikawa K., Titani K., Walsh K. A., Neurath H. Bovine factor IX (Christmas factor). Further evidence of homology with factor X (Stuart factor) and prothrombin. FEBS Lett. 1974 Oct 1;47(1):132–135. doi: 10.1016/0014-5793(74)80442-4. [DOI] [PubMed] [Google Scholar]
- Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H., Titani K. Bovine factor X1 (Stuart factor). Primary structure of the light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):16–19. doi: 10.1073/pnas.72.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernlund P., Stenflo J., Tufvesson A. Bovine protein C: amino acid sequence of the light chain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5889–5892. doi: 10.1073/pnas.75.12.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Enfield D. L., Titani K., Ericsson L. H., Davie E. W. A comparison of bovine prothrombin, factor IX (Christmas factor), and factor X (Stuart factor). Proc Natl Acad Sci U S A. 1974 Feb;71(2):427–430. doi: 10.1073/pnas.71.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell's viper venom. Biochemistry. 1972 Dec 19;11(26):4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Kato H., Davie E. W. The mechanism of activation of bovine factor IX (Christmas factor) by bovine factor XIa (activated plasma thromboplastin antecedent). Biochemistry. 1974 Oct 22;13(22):4508–4516. doi: 10.1021/bi00719a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Thompson A. R., Legaz M. E., Meyer R. G., Davie E. W. Isolation and characterization of bovine factor IX (Christmas factor). Biochemistry. 1973 Nov 20;12(24):4938–4945. doi: 10.1021/bi00748a019. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hermodson M., Schmer G., Kurachi K. Isolation, crystallization, and primary amino acid sequence of human platelet factor 4. J Biol Chem. 1977 Sep 25;252(18):6276–6279. [PubMed] [Google Scholar]
- Hugli T. E., Moore S. Determination of the tryptophan content of proteins by ion exchange chromatography of alkaline hydrolysates. J Biol Chem. 1972 May 10;247(9):2828–2834. [PubMed] [Google Scholar]
- Katayama K., Titani K. Cleavage of gamma-carboxyglutamyl peptide bonds by cyanogen bromide and by N-bromosuccinimide. FEBS Lett. 1978 Nov 1;95(1):157–160. doi: 10.1016/0014-5793(78)80073-8. [DOI] [PubMed] [Google Scholar]
- Kisiel W., Ericsson L. H., Davie E. W. Proteolytic activation of protein C from bovine plasma. Biochemistry. 1976 Nov 2;15(22):4893–4900. doi: 10.1021/bi00667a022. [DOI] [PubMed] [Google Scholar]
- Laursen R. A. Solid-phase Edman degradation. An automatic peptide sequencer. Eur J Biochem. 1971 May 11;20(1):89–102. doi: 10.1111/j.1432-1033.1971.tb01366.x. [DOI] [PubMed] [Google Scholar]
- Lindquist P. A., Fujikawa K., Davie E. W. Activation of bovine factor IX (Christmas factor) by factor XIa (activated plasma thromboplastin antecedent) and a protease from Russell's viper venom. J Biol Chem. 1978 Mar 25;253(6):1902–1909. [PubMed] [Google Scholar]
- Magnusson S., Sottrup-Jensen L., Petersen T. E., Morris H. R., Dell A. Primary structure of the vitamin K-dependent part of prothrombin. FEBS Lett. 1974 Aug 25;44(2):189–193. doi: 10.1016/0014-5793(74)80723-4. [DOI] [PubMed] [Google Scholar]
- Osterud B., Flengsrud R. Purification and some characteristics of the coagulation factor IX from human plasma. Biochem J. 1975 Mar;145(3):469–474. doi: 10.1042/bj1450469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg J. S., Beeler D. L., Rosenberg R. D. Activation of human prothrombin by highly purified human factors V and X-a in presence of human antithrombin. J Biol Chem. 1975 Mar 10;250(5):1607–1617. [PubMed] [Google Scholar]
- Stenflo J. A new vitamin K-dependent protein. Purification from bovine plasma and preliminary characterization. J Biol Chem. 1976 Jan 25;251(2):355–363. [PubMed] [Google Scholar]
- Stenflo J., Jönsson M. Protein S, a new vitamin K-dependent protein from bovine plasma. FEBS Lett. 1979 May 15;101(2):377–381. doi: 10.1016/0014-5793(79)81048-0. [DOI] [PubMed] [Google Scholar]
- Suomela H. Human coagulation factor IX. Isolation and characterization. Eur J Biochem. 1976 Dec;71(1):145–154. doi: 10.1111/j.1432-1033.1976.tb11100.x. [DOI] [PubMed] [Google Scholar]
- Suttie J. W. Metabolism and properties of a liver precursor to prothrombin. Vitam Horm. 1974;32:463–481. doi: 10.1016/s0083-6729(08)60023-0. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Thøgersen H. C., Petersen T. E., Sottrup-Jensen L., Magnusson S., Morris H. R. The N-terminal sequences of blood coagulation factor X1 and X2 light chains. Mass-spectrometric identification of twelve residues of gamma-carboxyglutamic acid in their vitamin K-dependent domains. Biochem J. 1978 Nov 1;175(2):613–627. doi: 10.1042/bj1750613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Fujikawa K., Enfield D. L., Ericsson L. H., Walsh K. A., Neurath H. Bovine factor X1 (Stuart factor): amino-acid sequence of heavey chain. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3082–3086. doi: 10.1073/pnas.72.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Koide A., Ericsson L. H., Kumar S., Hermann J., Wade R. D., Walsh K. A., Neurath H., Fischer E. H. Sequence of the carboxyl-terminal 492 residues of rabbit muscle glycogen phosphorylase including the pyridoxal 5'-phosphate binding site. Biochemistry. 1978 Dec 26;17(26):5680–5693. doi: 10.1021/bi00619a014. [DOI] [PubMed] [Google Scholar]
- de Haën C., Neurath H., Teller D. C. The phylogeny of trypsin-related serine proteases and their zymogens. New methods for the investigation of distant evolutionary relationships. J Mol Biol. 1975 Feb 25;92(2):225–259. doi: 10.1016/0022-2836(75)90225-9. [DOI] [PubMed] [Google Scholar]



