Abstract
The increased prevalence of thumb carpometacarpal (CMC) joint osteoarthritis (OA) in women has been previously linked to the articular morphology of the trapezium. However, studies report conflicting results on how the articular shapes of male and female trapezia compare to one another, mainly because their findings are based on data from older cadaver specimens. The purpose of this in vivo study was to dissociate the effect of sex from that of aging and early OA by using cohorts of healthy young and healthy older subjects, as well as patients with early stage OA. Computed tomography scans from 68 healthy subjects and 87 arthritic subjects were used to obtain 3-D bone models. The trapezial and metacarpal articular surfaces were manually delineated on scaled bone models, to remove the effect of size, and then were compared between sex, age, and health groups by using polar histograms of curvature and average curvature values. We found no sex differences, but significant age-group and health-group differences, in the articular surfaces of both bones. The older healthy subjects had higher curvature in the concave and lower curvature in the convex directions of both the trapezial and metacarpal saddles than the healthy young subjects. Subjects with early OA had significantly different metacarpal and trapezial articular shapes from healthy subjects. These findings suggest that aging and OA affect the articular shape of the CMC joint, but that, in contrast to previously held beliefs, inherent sex differences are not responsible for the higher incidence of CMC OA in women.
Keywords: thumb CMC joint, articular joint shape, osteoarthritis, computed tomography, in vivo
1. INTRODUCTION
Thumb carpometacarpal (CMC) joint osteoarthritis (OA) is a degenerative disease of cartilage and bone that affects women more frequently than men and whose incidence increases with aging (Dahaghin et al., 2005; Haara et al., 2004; Wilder et al., 2006). Its etiology remains unknown, but several factors, including joint biomechanics, are implicated in its pathogenesis. Less congruent joints are prone to high cartilage stress, which in turn is associated with an increased risk of OA (Andriacchi et al., 2004; Carter and Wong, 1988; Hunter and Wilson, 2009a, 2009b; Wilson et al., 2008). As congruence depends on both articular joint shape and physiological position, its assessment remains challenging. Alternatively, the study of joint shape alone can provide insight into joint congruence and its potential relation to pathomechanics.
Differences in the articular morphology of male and female trapezia have been reportedly associated with the high prevalence of CMC OA in women (Ateshian et al., 1992; Aune, 1955; Eaton and Littler, 1973; North and Rutledge, 1983; Smith and Kuczynski, 1978; Xu et al., 1998). Previous studies, however, have reported conflicting findings on how the articular shape of the trapezium compares between men and women. Based on clinical observations, it has been suggested that women have shallower trapezial articular surfaces than men (Aune, 1955; Eaton and Littler, 1973). Through anthropometric measurements of 77 trapezia from elderly cadaver hands, North and Rutlege reported that trapezial surfaces are flatter in women than in men, and concluded that this difference may predispose women to CMC OA (North and Rutledge, 1983). In contrast, quantitative studies of arthritic cadaveric specimens (sample sizes of less than 46 and mean ages of more than 60 years) found that women have more curved trapezial articular surfaces than men (Ateshian et al., 1992; Kovler et al., 2004; Xu et al., 1998). Another cadaver study, which analyzed over 120 specimens with minimal eburnation or ostephytes, but undisclosed ages, reported no sex differences (Marzke et al., 2012).
Previously reported differences between male and female joints have been based on data from cadaveric specimens that were marked by advanced age and arthritic changes. To our knowledge, the study by Marzke et al., which reported no sex differences, is the only study that included joints with no or minimal signs of pathology, although the results were not stratified by age group or likelihood of pathology (Marzke et al., 2012). In the CMC joint, the effect of aging has only been studied in older joints (Xu et al., 1998) and the effect of OA only at its end stage (Giessen et al., 2011; Van Nortwick et al., 2013; Xu et al., 1998). These studies concluded that both aging and OA affect the shape of the CMC joint, but whether this effect is similar in men and women and whether male and female joints are innately different cannot be determined without baseline data from young healthy subjects.
Since current findings on the effect of sex on the articular morphology of the CMC joint remain controversial and since there are no reported data on young healthy joints, we revisited the question with a large dataset that contains healthy joints from two age groups and joints from older individuals with early stage OA. The purpose of this study was to determine if the articular shape of the CMC joint differs with sex, age, and early OA. We hypothesized that the shape of trapezial and metacarpal articular surfaces would not differ with sex, after normalizing for bone size, because sexual dimorphism in the human skeleton is typically paired with functional differences. We also hypothesized that the articular shape of the CMC joint would differ with age group, given existing evidence that articular joints remodel with ageing (Bullough, 1981) in response to compressive loading (Wolff et al., 1986). Finally, we hypothesized that there would be differences between patients with early stage OA and healthy controls because previous studies have proposed that changes in bony morphology may precede clinical symptoms of OA (Hutton et al., 1986).
2. METHODS
2.1 Subjects and Scanning
After receiving approval from our Institutional Review Board and obtaining informed consents, 68 asymptomatic subjects and 87 patients with signs of early CMC OA were enrolled in the study. The mean ± SD ages were 24.7 ± 4.5 yrs. for the 16 young asymptomatic men, 24.7 ± 3.2 yrs. for the 17 young asymptomatic women, 53.8 ± 8.7yrs. for the 16 older asymptomatic men, 55.8 ± 6.6 yrs. for the 19 older asymptomatic women, 60.2 ± 7.6 yrs. for the 39 arthritic men, and 51.5 ± 10.5 yrs. for the 48 arthritic women. The asymptomatic subjects were examined by a board-certified orthopaedic hand surgeon to ensure that they had no prior conditions that might have altered CMC joint morphology. The arthritic patients presented with basilar thumb pain, one or more positive clinical signs of OA, such as a positive grind test, and with or without radiographic evidence of OA (Eaton Stage I/II) (Eaton and Glickel, 1987). The wrists and thumbs of the dominant hands of all the asymptomatic subjects and the affected hands of the OA patients were imaged with a 16-slice clinical CT scanner (GE LightSpeed 16, General Electric, Milwaukee, WI) at tube settings of 80 kVp and 80 mA, slice thickness of 0.625 mm, and in-plane resolution of at least 0.4 mm × 0.4 mm (Fig. 1a).
Figure 1.
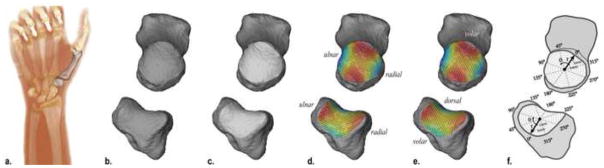
a) The wrists and thumbs of our participants were CT-scanned at a resolution of 0.4 mm × 0.4 mm × 0.625 mm or higher, and the CMC joints were segmented in Mimics; b) the trapezia and the first metacarpals were exported as meshed surfaces; c) the articular surfaces of the CMC joint were manually selected; d & e) fifth order polynomials were fit to the articular surfaces and principal curvatures were computed at 400pts/mm2—shown here at a lower resolution—and then averaged across the whole surface: kmin of the trapezial surface and kmax of the metacarpal surface correspond to curvature in the ulnar-radial direction, whereas kmax of the trapezial surface and kmin of the metacarpal surface correspond to curvature in the volar-dorsal direction; f) polar histograms of curvature, which preserve spatial information on local curvature were also used computed for the articular surfaces
2.2 Shape Analysis
The outer cortical bone surfaces of the trapezia and first metacarpals were semi-automatically segmented from the CT volume images using Mimics v12.11 (Materialise, Leuven, Belgium) and 3-D bone models were exported as meshed surfaces (Fig. 1b). The metacarpal and trapezial articular surfaces were manually extracted from the bone models using Geomagic® (Geomagic®, Research Triangle Park, NC) (Fig. 1c). Whole bone volumes (V), surface areas (SA), and articular surface areas (ASA) were computed from the meshed surfaces. The trapezial and metacarpal bone models, along with the selected articular surfaces, were isotropically scaled by , where Vavg is the average bone volume of the asymptomatic group and Vs is the subject-specific bone volume.
Average minimum and maximum curvatures across the whole articular surface were computed following the seminal work of Ateshian et al. (Ateshian et al., 1992). A fifth order polynomial surface was fit to the articular surface points and principal (minimum, kmin, and maximum, kmax) curvatures were computed at points uniformly sampled (400 points/mm2) across the fitted surface, f (x, y), as follows (Fig. 1d–e):
| (1) |
| (2) |
where
.
Average kmin, and kmax were then computed for each articular surface. Average curvature metrics are widely used (Ateshian et al., 1992; Marzke et al., 2012; Xu et al., 1998) indicators of overall curvedness,—kmin of the trapezial surface and kmax of the metacarpal surface correspond to curvature in the ulnar-radial direction (Fig. 1d), whereas kmax of the trapezial surface and kmin of the metacarpal surface correspond to curvature in the volar-dorsal direction (Fig. 1e)—but they do not preserve spatial information. The articular surfaces of two trapezia, for example, may have similar average curvatures, although one may be more curved on the volar half and the other more curved on the dorsal half.
In addition to average principal curvatures, we used polar histograms of curvature, which are structured descriptors of articular shapes that retain spatial information along with curvature measures (Halilaj et al., 2014). These histograms offer an anatomically meaningful comparison between articular surfaces, i.e. the radio-volar quadrant of a surface is compared with the respective quadrant on the other surface. To construct these histograms, the 3-D points on the articular surfaces were first collapsed into two dimensions (θ, r) or into polar coordinates. The saddle points of the articular surfaces were used as the poles and the volar-dorsal directions of principal curvature on the surfaces were used as the polar axes, with the coordinate systems oriented orthogonally to the surface normals of the inflection points of the saddles (Fig. 1f). The polar histograms were then three-dimensional histograms, with the physical coordinates (θ, r) being the first two dimensions and curvature being the third. A bin size of 7 was chosen for each dimension. Next, support vector machines (SVMs) with a linear separating function were used to find the optimal separating hyperplane between groups (sex, age, and health group) for the kmin and kmax histograms of both the trapezium and metacarpal. The shortest signed distances, ds, between each histogram and the optimal separating hyperplane were computed for each of the comparisons.
2.3 Statistical Analysis
Student’s t tests of the signed distances, ds, were used to test for group (age, sex, health) differences in the histogram representations, after testing for normality (Shapiro-Wilk) and equal variance. Since separate SVMs were used for sex, age, and health group comparisons of kmin and kmax histograms on each bone, separate t tests were used and Bonferroni adjustments were made accordingly (0.05/12). Generalized estimating equations were used to model size and average curvature metrics as functions of sex, age (categorical), and health group. A cell-means model approach was taken, with all the hypotheses set a priori, and carried out as orthogonal linear estimates, using the Holm test and maintaining a family-wise alpha of 0.05. The distribution used was selected based on the inspection of model residuals: average curvatures were normally distributed, while volume, surface areas, and the ratio of articular to total surface area were log-normally distributed.
3. RESULTS
In the younger asymptomatic subjects, sex had a significant effect on bone size, but not on the shape of the articular surfaces. Young men had significantly larger trapezial and metacarpal bone volumes, surface areas, and articular surface areas than young women, but the ratios of the articular surface areas to the whole bone surface areas were not significantly different between them (Fig. 2). The kmin and kmax histogram representations of the scaled trapezial and metacarpal articular surfaces were not statistically different between men and women (trapezium: pkmin=0.3112 and pkmax=0.1188; metacarpal: pkmin=0.1802, pkmax=0.0197). The average kmin and kmax were also not statistically different between male and female articular surfaces (Fig. 3a–d).
Figure 2.
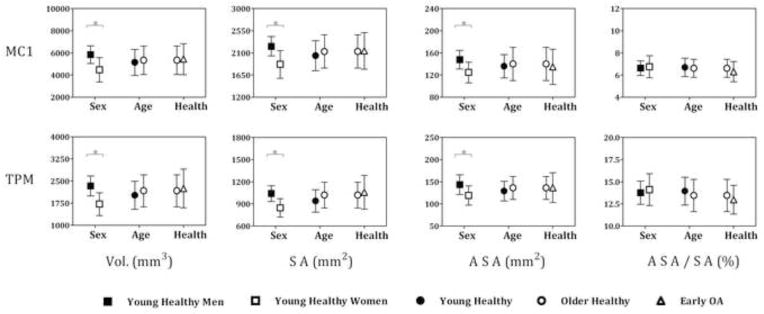
For both the trapezium (TPM) and metacarpal (MC1), bone volumes (Vol.), whole bone surface areas (SA), and articular surface areas (ASA) were significantly different between sex groups, but not between age and health groups, whereas the ratios of the articular surface areas to whole bone surface areas (ASA/SA) were not different between sex, age, or health groups
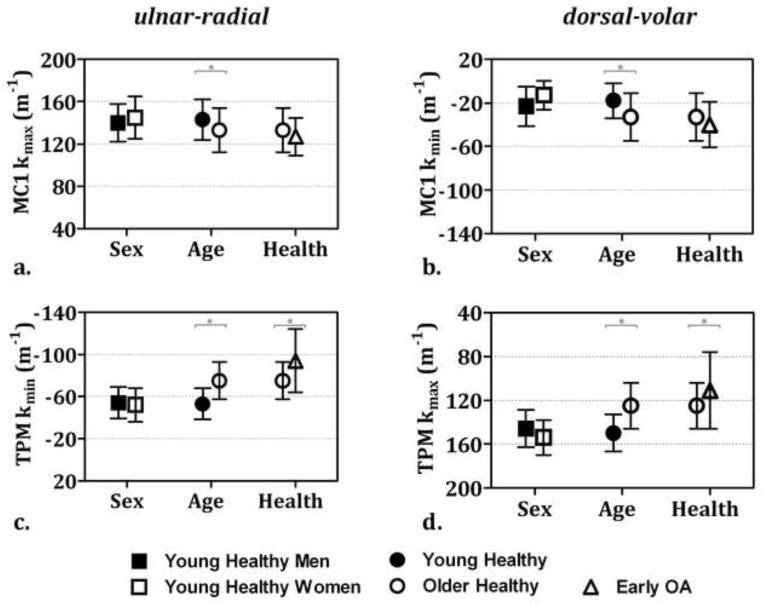
Figure 3.
Mean (±SD) average curvatures: a & b) differed between age groups in the metacarpal (MC1) and c & d) differed between age and health groups in the trapezium (TPM) (* p < 0.05 considered statistically significant)
Age, however, had a significant effect on the articular surface shapes of healthy trapezia and metacarpals. Since the effect of age did not differ significantly between men and women, the data were pooled for the analysis of age groups. The kmin and kmax histograms of the trapezium and the kmax histograms of the metacarpal were significantly different between age groups (trapezium: pkmin=0.0034 and pkmax<0.0001; metacarpal: pkmin=0.1806, pkmax=0.0008). The trapezia of older subjects had significantly higher average kmin, which corresponds to curvature in the ulnar-radial direction (Fig. 3c), and significantly lower average kmax, which corresponds to curvature in the volar-dorsal direction (Fig. 3d), than the trapezia of younger subjects. The metacarpals of older subjects had significantly higher average kmin, which corresponds to curvature in the volar-dorsal direction (Fig. 3b), and significantly lower average kmax, which corresponds to curvature in the ulnar-radial direction (Fig. 3a), than the metacarpals of younger subjects. Bone volumes, surface areas, articular surface areas, and the ratios of the articular surface areas to whole bone surface areas were not statistically different between the young and older subjects (Fig. 2).
Early OA had a significant effect on the shape of both the trapezial and the metacarpal articular surfaces. The kmin and kmax histograms of the trapezial, as well as the kmax histograms of the metacarpal, articular surfaces were significantly different between older asymptomatic subjects and OA patients (trapezium: pkmin<0.0001 and pkmax<0.0001; metacarpal: pkmin=0.0071, pkmax<0.0001). The average kmin of the trapezium, or curvature in the ulnar-radial direction, was significantly higher, whereas the average kmax, or curvature in the volar-dorsal direction, was significantly lower in OA patients than in normal controls. Average curvatures of the metacarpal were not significantly different between the controls and the OA patients. There were also no statistically significant differences in bone sizes, articular surface areas, or the ratios of the articular surface areas to whole bone surface areas between healthy older subjects and OA patients (Fig. 2).
Overall, the average principal curvatures of the trapezium were higher in the dorsal-volar direction (e.g., mean ± SD in healthy young subjects was 150 ± 17 mm−1) than in the ulnar-radial direction (−53 ± 15 mm−1); whereas those of the metacarpal were higher in the ulnar-radial direction (143 ± 19 mm−1) than in the dorsal-volar direction (−18 ± 16 mm−1) (Fig. 3a–d). The average principal curvatures of the metacarpal and trapezium were more similar in the ulnar-radial direction (143 ± 19 mm−1 and −53 ± 15 mm−1, respectively) than in the dorsal-volar direction (−18 ± 16 mm−1 and 150 ± 17 mm−1, respectively). The observed age- or health-group differences in these average curvatures were associated with increased similarity between the trapezial and metacarpal articular surfaces, e.g., from −53 ± 15 m−1 and 143 ± 19 m−1 in the younger subjects to −75 ± 18 m−1 and 133 ± 21 m−1 in the older subjects.
4. DISCUSSION
The aim of this study was to determine the effects of sex, age, and early OA on the articular shapes of the trapezium and first metacarpal. We analyzed the articular shapes of healthy joints from cohorts of young (18 – 25 yrs.) and older (45 – 75 yrs.) asymptomatic volunteers, as well as joints from patients with early-stage OA (Eaton Stage I/II)(Eaton and Glickel, 1987). As expected, we found that men had larger bones than women; the ratios of the articular surface areas to the whole bone surface areas, however, were not different between them. Interestingly, we found a significant effect of age and early OA on the shapes of both the trapezial and first metacarpal articular surfaces, but we did not find a statistically significant effect of sex. The trapezia of older subjects were more curved in the ulnar-radial direction and less curved in the volar-dorsal direction than the trapezia of younger subjects, whereas the metacarpals of older subjects were less curved in the ulnar-radial direction and more curved in the volar-dorsal direction than the metacarpals of younger subjects. OA had a similar effect, which was more emphasized in the trapezium than in the metacarpal.
When interpreting these results it is important to consider the following facets of the study. First, the analysis presented here was carried out on subchondral bone surfaces, and while the findings may not be representative of differences in the curvature of cartilage surfaces, previous studies have reported that group differences in cartilage curvature are also detectable in subchondral bone curvature (Xu et al., 1998). Second, this is not a longitudinal study, therefore the direct effects of aging and OA cannot be determined with certainty. Third, differences in articular morphology may be indicative, but are not representative of differences in joint congruence, which was not examined in this study. Joint congruence is a complex measure that varies with thumb position. Nevertheless, the study of morphology alone is an intuitive first step that provides valuable insight into joint mechanics and addresses previous conjectures about the relationship between CMC joint shape and CMC OA. It must be noted that here we used two methods to assess group differences because they provide complementary information—one is more sensitive to local shape differences, while the other provides a scalar measure of average differences across the whole surface. In the case of the metacarpal, the histogram method detected differences due to pathology, which the average curvature metrics did not detect, whereas the average curvature method detected changes due to age in the dorsal-volar direction, which the histogram method did not detect. These findings reinforce that ageing causes structured patterns of change (Bullough, 1981), whereas pathology can introduce more irregular and localized changes (Van Nortwick et al., 2013). Lastly, while it may be possible that there are sex differences that were not captured by our sample size, some previous studies that have reported differences were based on smaller samples.
Our finding of significant differences between the articular surfaces of young healthy subjects and older healthy subjects is suggestive of bone surface remodeling with aging and is in agreement with previous findings on the CMC joint and other joints (Bullough et al., 1973; Goodfellow and Bullough, 1967; Ogston, 1878; Xu et al., 1998). Average curvature values indicate that the trapezial and metacarpal articular surfaces become more conforming with aging. These patterns are consistent with similar findings from a cadaver study of the CMC joint that compared middle-aged (40–59 years) specimens with elderly (60–79 years) specimens (Xu et al., 1998) and with earlier observations that older joints are flatter (Ogston, 1878) and more conforming than younger joints (Bullough et al., 1973; Goodfellow and Bullough, 1967). Whether these shape alterations lead to improved joint congruence, however, cannot be determined at this point. Xu et al. arrived at that conclusion by utilizing a congruence measure that is based solely on the average principal curvatures of the joint (Xu et al., 1998), but ongruence is heavily dependent on joint position. The trapezial and metacarpal saddles are innately very different, and their flattening may result in better geometrical similarity, which may lead to altered, but not greater, contact during physiological joint positions. Bullough hypothesized that as the joint surfaces remodel with aging, joint contact may shift to areas that were previously low load bearing, where cartilage is weak and unready to withstand loading. His reasoning is founded on existing evidence that cartilage wear starts the radial side of the of the CMC joint, which is postulated to be a low bearing region (Ateshian et al., 1995; Koff et al., 2003; Momose, 1994; Shi et al., 1995), rather than the volar-ulnar quadrant, which exhibits greater trabecular bone volume, thickness, and connectivity (Lee et al., 2013).
Similarly, the effect of early OA on the articular surfaces of the CMC joint reported here, which has not been reported before, may contribute to a shift, but not an increase, in joint contact area. Xu et al., who reported that joint morphology does not change in the early stages of CMC OA, but that it does in the late stages of the disease, also found that although the joint remodeling that occurs with both aging and late-stage OA improves the similarity between the trapezial and metacarpal saddles, it does not result in increased contact areas (Xu et al., 1998). The decrease of maximum curvature and the increase of minimum curvature in the trapezium is in agreement with the two previously observed patterns of arthritic explanted trapezia: the “dish,” where the whole saddle turns into a concave surface and the “cirque,” where only the volar half of the saddle becomes concave (Van Nortwick et al., 2013). How these shape changes affect the pathomechaics of the joint will be better elucidated in an in vivo study of joint contact and congruence at different thumb positions.
Our finding of no significant sex differences in the articular shape of the trapezium contrasts previous findings that identified sex differences in explanted trapezia (Eaton and Littler, 1973; North and Rutledge, 1983; Ateshian et al., 1992; Xu et al., 1998; Kovler et al., 2004), but is in agreement with a more recent study that found no differences (Marzke et al., 2012). Our finding, based on in vivo morphological measurements of both young and older subjects, suggests that inherent sex differences in articular shape are not responsible for the higher incidence of OA in women. Previous studies that reported sex-related differences were limited to older cadaveric specimens (Ateshian et al., 1992; Kovler et al., 2004; Xu et al., 1998), most of which exhibited visible signs of OA. Therefore, it is possible that the previously reported sex differences may reflect surface alterations due to aging and pathology.
Comparative studies of human and nonhuman primate CMC joints indicate that the human joint has evolved to balance the capability for a wide range of motion with the ability to distribute loads across the joint during manipulative tasks (Marzke et al., 2010; Tocheri et al., 2005), which are carried out by both men and women. Currently, there is little support for diverging patterns of joint-shape evolution between the sexes. Size may play a role on the mechanics of the joint and studying its effect may merit additional attention. Activities of daily living, which represent a substantial portion of overall thumb use, are similar between men and women. As a result, smaller joints likely experience higher stresses during the execution of the same task. Examining subsets of low-load versus high-load thumb activities, with men and women in each group, may provide valuable information on how trapezial size relates to pathology.
In conclusion, the current study does not support the previously upheld theory that inherent sex differences in articular bone morphology lead to the higher prevalence of CMC OA in women. Age and the onset of OA, not sex, were found to affect the shape of the CMC articulation. Since, clinically, CMC OA is more prevalent in women than in men, sex-related differences in other intrinsic factors may play a leading role on the ethiopathogenesis of this disease. The findings presented here advance the current understanding of CMC joint mechanics, and, along with concurrent studies of CMC joint contact and kinematics, they help narrow the scope of future investigation motivated to answer why CMC OA is mainly a disease of aging women.
Acknowledgments
The project described was supported by Grant Number AR059185 from NIAMS/NIH and the American Foundation for Surgery of the Hand (AFSH). Its contents are the responsibility of the authors and do not necessarily represent the official views of the NIAMS or NIH. The authors would like to thank Jason T Machan for his assistance with the statistical analysis, Michael J. Rainbow for useful conversations on CMC joint curvature, Joel B Schwartz, James C Tarrant, Robert Chang, Tarpit K Patel for their assistance with subject scanning and data processing, as well as Arlene Garcia and Debbie Kenny for coordinating subject recruitment.
Footnotes
Conflicts of Interest Statement: The authors have no financial or personal relationships that could bias this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Engin. 2004;33:447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Ark JW, Rosenwasser MP, Pawluk RJ, Soslowsky LJ, Mow VC. Contact areas in the thumb carpometacarpal joint. J Orthop Res Off Publ Orthop Res Soc. 1995;13:450–458. doi: 10.1002/jor.1100130320. [DOI] [PubMed] [Google Scholar]
- Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25:591–607. doi: 10.1016/0021-9290(92)90102-7. [DOI] [PubMed] [Google Scholar]
- Aune S. Osteo-arthritis in the first carpo-metacarpal joint; an investigation of 22 cases. Acta Chir Scand. 1955;109:449–56. [PubMed] [Google Scholar]
- Bullough P, Goodfellow J, O’Conner J. The relationship between degenerative changes and load-bearing in the human hip. J Bone Joint Surg Br. 1973;55:746–758. [PubMed] [Google Scholar]
- Bullough PG. The geometry of diarthrodial joints, its physiologic maintenance, and the possible significance of age-related changes in geometry-to-load distribution and the development of osteoarthritis. Clin Orthop. 1981:61–66. [PubMed] [Google Scholar]
- Carter DR, Wong M. The role of mechanical loading histories in the development of diarthrodial joints. J Orthop Res Off Publ Orthop Res Soc. 1988;6:804–816. doi: 10.1002/jor.1100060604. [DOI] [PubMed] [Google Scholar]
- Dahaghin S, Bierma-Zeinstra SMA, Ginai AZ, Pols HAP, Hazes JMW, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987;3:455–471. [PubMed] [Google Scholar]
- Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg Am. 1973;55:1655–1666. [PubMed] [Google Scholar]
- Giessen M, Raedt S, Stilling M, Hansen TB, Maas M, Streekstra GJ, Vliet LJ, Vos FM. Localized Component Analysis for Arthritis Detection in the Trapeziometacarpal Joint. In: Fichtinger G, Martel A, Peters T, editors. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2011. Springer Berlin Heidelberg; Berlin, Heidelberg: 2011. pp. 360–367. [DOI] [PubMed] [Google Scholar]
- Goodfellow JW, Bullough PG. The pattern of ageing of the articular cartilage of the elbow joint. J Bone Joint Surg Br. 1967;49:175–181. [PubMed] [Google Scholar]
- Haara MM, Heliövaara M, Kröger H, Arokoski JPA, Manninen P, Kärkkäinen A, Knekt P, Impivaara O, Aromaa A. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86-A:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- Halilaj E, Laidlaw DH, Moore DC, Crisco JJ. Polar Histograms of Curvature for Quantifying Skeletal Joint Shape and Congruence. 2014 doi: 10.1115/1.4027938. Submitt. Publ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter DJ, Wilson DR. Imaging the role of biomechanics in osteoarthritis. Rheum Clin North Am. 2009a;35:465–83. doi: 10.1016/j.rdc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Hunter DJ, Wilson DR. Role of alignment and biomechanics in osteoarthritis and implications for imaging. Radiol Clin North Am. 2009b;47:553–66. doi: 10.1016/j.rcl.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Hutton CW, Higgs ER, Jackson PC, Watt I, Dieppe PA. 99mTc HMDP bone scanning in generalised nodal osteoarthritis. II. The four hour bone scan image predicts radiographic change. Ann Rheum Dis. 1986;45:622–626. doi: 10.1136/ard.45.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff MF, Ugwonali OF, Strauch RJ, Rosenwasser MP, Ateshian GA, Mow VC. Sequential wear patterns of the articular cartilage of the thumb carpometacarpal joint in osteoarthritis. J Hand Surg. 2003;28:597–604. doi: 10.1016/s0363-5023(03)00145-x. [DOI] [PubMed] [Google Scholar]
- Kovler M, Lundon K, McKee N, Agur A. The human first carpometacarpal joint: osteoarthritic degeneration and 3-dimensional modeling. J Hand Ther Off J Am Soc Hand Ther. 2004;17:393–400. [PubMed] [Google Scholar]
- Lee AT, Williams AA, Lee J, Cheng R, Lindsey DP, Ladd AL. Trapezium trabecular morphology in carpometacarpal arthritis. J Hand Surg. 2013;38:309–315. doi: 10.1016/j.jhsa.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzke MW, Tocheri MW, Marzke RF, Femiani JD. Three-dimensional quantitative comparative analysis of trapezial-metacarpal joint surface curvatures in human populations. J Hand Surg. 2012;37:72–76. doi: 10.1016/j.jhsa.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Marzke MW, Tocheri MW, Steinberg B, Femiani JD, Reece SP, Linscheid RL, Orr CM, Marzke RF. Comparative 3D quantitative analyses of trapeziometacarpal joint surface curvatures among living catarrhines and fossil hominins. Am J Phys Anthropol. 2010;141:38–51. doi: 10.1002/ajpa.21112. [DOI] [PubMed] [Google Scholar]
- Momose T. Cartilage degeneration and measurement of the contact area of the trapeziometacarpal joint: morphological observation. Nihon Seikeigeka Gakkai Zasshi. 1994;68:426–434. [PubMed] [Google Scholar]
- North ER, Rutledge WM. The trapezium-thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15:201–6. doi: 10.1016/s0072-968x(83)80014-x. [DOI] [PubMed] [Google Scholar]
- Ogston A. The Growth and Maintenance of the Articular Ends of Adult Bones. J Anat Physiol. 1878;12:503–517. [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Hashizume H, Inoue H, Miyake T, Nagayama N. Finite element analysis of pathogenesis of osteoarthritis in the first carpometacarpal joint. Acta Med Okayama. 1995;49:43–51. doi: 10.18926/AMO/30414. [DOI] [PubMed] [Google Scholar]
- Smith SA, Kuczynski K. Observations on the joints of the hand. The Hand. 1978;10:226–231. doi: 10.1016/s0072-968x(78)80042-4. [DOI] [PubMed] [Google Scholar]
- Tocheri MW, Razdan A, Williams RC, Marzke MW. A 3D quantitative comparison of trapezium and trapezoid relative articular and nonarticular surface areas in modern humans and great apes. J Hum Evol. 2005;49:570–586. doi: 10.1016/j.jhevol.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Van Nortwick S, Berger A, Cheng R, Lee J, Ladd A. Trapezial Topography in Thumb Carpometacarpal Arthritis. J Wrist Surg. 2013;02:263–270. doi: 10.1055/s-0033-1350088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthr Cartil OARS Osteoarthr Res Soc. 2006;14:953–957. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Wilson DR, McWalter EJ, Johnston JD. The measurement of joint mechanics and their role in osteoarthritis genesis and progression. Rheum Clin North Am. 2008;34:605–22. doi: 10.1016/j.rdc.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Wolff J, Maquet P, Furlong R. The Law of Bone Remodelling. Springer-Verlag; 1986. [Google Scholar]
- Xu L, Strauch RJ, Ateshian GA, Pawluk RJ, Mow VC, Rosenwasser MP. Topography of the osteoarthritic thumb carpometacarpal joint and its variations with regard to gender, age, site, and osteoarthritic stage. J Hand Surg. 1998;23:454–464. doi: 10.1016/S0363-5023(05)80463-0. [DOI] [PubMed] [Google Scholar]