Abstract
Biomimetic nanofibrous scaffolds mimicking important features of the native extracellular matrix provide a promising strategy to restore functions or achieve favorable responses for tissue regeneration. This review provides a brief overview of current state-of-the-art research designing and using biomimetic electrospun nanofibers as scaffolds for tissue engineering. It begins with a brief introduction of electrospinning and nanofibers, with a focus on issues related to the biomimetic design aspects. The review next focuses on several typical biomimetic nanofibrous structures (e.g. aligned, aligned to random, spiral, tubular, and sheath membrane) that have great potential for tissue engineering scaffolds, and describes their fabrication, advantages, and applications in tissue engineering. The review concludes with perspectives on challenges and future directions for design, fabrication, and utilization of scaffolds based on electrospun nanofibers.
Tissue engineering is an emerging interdisciplinary field that applies biological and engineering principles to develop biological substitutes that restore, maintain, or improve tissue function1–4. It usually requires a scaffold to provide a transitional three-dimensional (3D) support for cell migration, attachment, and proliferation, as well as to provide a vector for delivery of biochemical factors5,6. The scaffold should also offer mechanical as well as biological influences to guide the maturation and integration of cells to form tissues7. Therefore, the major challenge in tissue engineering is to design and fabricate a suitable scaffold to fulfill the growing needs of the field.
With increasing understanding of the intricate interactions between cells and their microenvironment in tissues, more attention is now focused on the preparation of scaffolds that can imitate the componential and structural aspects of extracellular matrix (ECM) to facilitate cell recruiting/seeding, adhesion, proliferation, differentiation, and neo tissue genesis3,8,9. From a structural perspective, natural ECM consists of various interwoven protein fibers with diameters ranging from tens to hundreds of nanometers10. The nanoscale structure of ECM offers a natural network of nanofibers to support cells and to present an instructive background to guide cell behavior11,12. Developing scaffolds that imitate the architecture of tissues at the nanoscale is one of the major challenges in the field of tissue engineering13–15. Development of nanofibers has greatly improved the scope for preparing scaffolds that can imitate the architecture of natural human tissues in the nanoscale16.
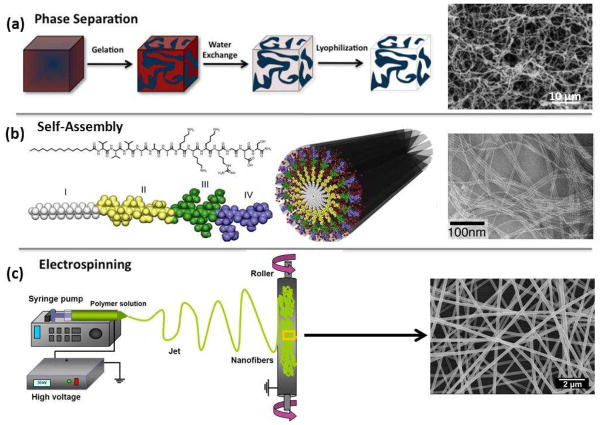
Various processing techniques (e.g. phase separation, self-assembly, and electrospinning) have been developed to fabricate nanofibrous scaffolds to be used as ECM substitutes (Fig. 1)9,17–22. Among them, the electrospinning process has attracted significant attention because of its ability to generate fibers similar to the fibrous structures of native ECM (Fig. 1c) and to process a wide range of materials, as well as the straightforward nature of the process and its cost-effectiveness23,24. The large surface area of electrospun nanofibers as well as their porous structure favors cell adhesion, proliferation, migration, and differentiation25–27. If necessary, the nanofibers can be further functionalized via incorporation with bioactive species (e.g. enzymes, DNAs, and growth factors) to better control the proliferation and differentiation of cells seeded on the scaffolds8. These attributes make electrospun nanofibers well-suited as scaffolds for tissue engineering.
Figure 1.
Schematic of current techniques (i.e., phase separation, self-assembly, and electrospinning) to create fibrillar structures in synthetic scaffolds. (a) Reprinted with permission from20. © 2012 Elsevier B.V.). (b) Reprinted with permission from21. © 2011 Elsevier B.V. Scanning electron microscopy (SEM) images adapted and reprinted with permission (a) from17. © 1999 WILEY-VCH Verlag GmbH & Co. and (b) from22. © 2002 The National Academy of Sciences of the USA.
This article gives a brief overview of recent work on designing and using biomimetic electrospun nanofibers as scaffolds for tissue engineering. First, we present a brief introduction of electrospinning and nanofibers, with a focus on issues related to the biomimetic design aspect. We then highlight a variety of biomimetic nanofibrous scaffolds fabricated via electrospinning, describing their fabrication, advantages, and applications in tissue engineering. Finally, we give conclusions along with perspectives on challenges and future directions of biomimetic scaffolds based on electrospun nanofibers.
Electrospinning of nanofibers
Electrospinning (also termed electrostatic spinning) has gained substantial attention in the last two decades triggered mainly by the potential applications of electrospun nanofibers in nanoscience and nanotechnology28–31. Particularly, remarkable features such as large specific surface area, high porosity, and spatial interconnectivity of electrospun nanofibers make them well suited for nutrient transport, cell communication, and efficient cellular responses32,33. The standard electrospinning system requires four major components: a spinneret with a metallic needle, a syringe pump, a high-voltage power supply, and a grounded collector (Fig. 1c)34. The electric field strength overcomes the surface tension of the droplet and generates a charged liquid jet35. The jet is then elongated and whipped continuously by electrostatic repulsion until it is deposited on the grounded collector36. The solvent evaporates on the way and the jet solidifies to form a nonwoven fibrous membrane. The processing flexibility of this technique enables fiber fabrication from a broad range of precursor materials such as synthetic polymers, natural polymers, semiconductors, ceramics, or their combinations37,38.
Currently, a variety of natural polymers such as collagen, gelatin, elastin, silk, and synthetic polymers such as poly(L-lactic acid) (PLLA), poly(glycolic acid) (PGA), poly(ε-caprolactone) (PCL) and poly(lactic-co-glycolic) acid (PLGA) have been electrospun as biomimetic and temporal substrates to modulate various cellular activities. However, synthetic or natural polymer alone cannot meet all the requirements for tissue engineering. Synthetic polymers have great flexibility in synthesis and modification, but these polymers lack cell affinity because of their low hydrophilicity and lack of surface cell recognition sites. Compared with synthetic polymers, natural polymers provide good biocompatibility but tend to display poor processing ability and mechanical properties32. Therefore, it is desirable to fabricate composite fibrous membranes comprising both synthetic polymers for the backbone and natural polymers for cellular attachment, which might possess not only suitable mechanical properties but also a bioactive surface39.
Biomimicry via electrospinning
Through orchestrating parameters of electrospinning, controllable fibrous structures can be successfully fabricated40,41 and therefore provide excellent prospects for construction of biomimetic structures. Benefitting from the easily tunable compositions and structures of electrospun fibers, a variety of fascinating structures resembling natural objects (e.g. lotus leaf, silver ragwort leaf, rice leaf, honeycomb, polar bear fur, spider webs, soap-bubble, ECM, etc.) have been successfully biomimicked via electrospinning (Fig. 2)41,42. Particularly, biomimetic processing for tissue engineering has focused on emulating or duplicating ECM structures and functions using mostly natural or synthetic components43. A detailed review of biomimicry based on the electrospinning technique was recently published by Lin and co-workers44.
Figure 2.
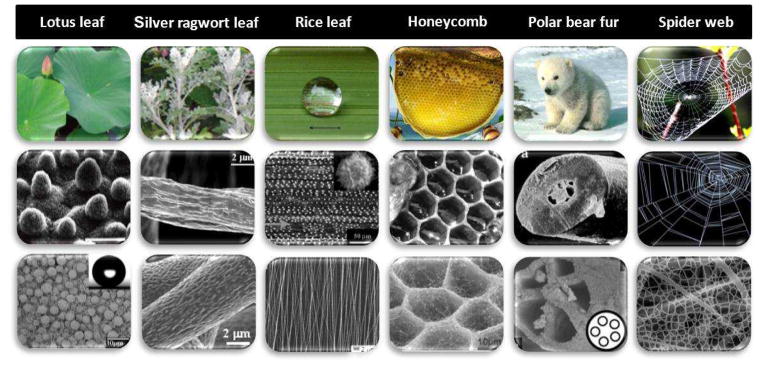
Biomimetic electrospun nanofibrous structures inspired from nature. In each case the first row shows a photograph of the biological feature, the second row shows the optical or SEM images of corresponding micro- and nanometer-scale structures, and the third row shows the SEM images of inspired electrospun nanofibrous structures.
Biomimetic nanofibrous scaffolds for tissue engineering
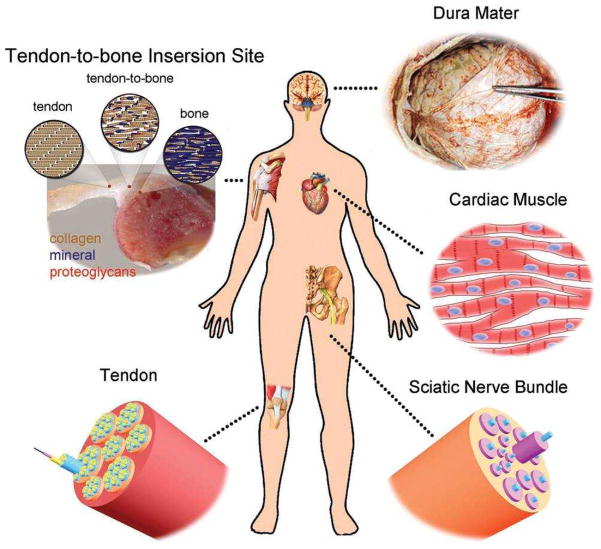
Nanofibers can be electrospun in various patterns depending on the biomedical application. For example, core-shell fibers can facilitate drug delivery in which drugs will be encapsulated in a nonreactive coating and safely delivered to the target sites45. Biomimetic substitutes have been developed to replicate natural tissues for use in the repair of destroyed tissues such as skin, bone, dura mater, sciatic nerve, articular cartilage, and tendon in order to improve functional outcomes (Fig. 3)8,46,47. In skin tissue regeneration, for example, nanofibers have shown great potential to mimic skin ECM in both morphology and composition, and thus may be promising tissue engineering scaffolds for skin substitutes48. The high surface area of nanofibrous scaffolds allows oxygen permeability and prevents fluid accumulation at the wound site, making them ideal substrates for wound dressings45. Therefore, it is important to understand the structural and biological properties of tissues in order to gain insight into choosing the materials that best suit reconstruction of these tissues33. Bone tissue, as an example, has a hierarchical organization over length scales ranging from macro- to nano-structured (ECM) components2. When designing biomimetic scaffolds for bone tissue engineering, the following aspects should be considered: They must (1) mimic the nanofibrous collagen ECM; (2) be highly porous to allow for cell ingrowth and efficient mass transport of nutrients, oxygen, growth factors, and waste products; and (3) be able to withstand mechanical stresses during tissue neogenesis9,49,50. In this section, we discuss several biomimetic nanofibrous scaffolds (from simple to complex) fabricated via electrospinning and describe their fabrication, advantages, and applications in tissue engineering.
Figure 3.
Illustration of some typical examples of tissues in the human body whose regeneration would benefit from the use of nanofiber-based scaffolds that could be readily fabricated by electrospinning. (Reprinted with permission from8. © 2012 WILEY-VCH Verlag GmbH & Co.)
Aligned nanofibrous scaffolds
Electrospun nanofibers are typically collected into a nonwoven membrane, which generally gives random fiber orientation and poor mechanical properties. Most native ECMs found in tissues or organs (e.g. sciatic nerve, heart, tendon, and blood vessel), however, have anisotropic architecture, which is important for tissue function8. Therefore, a well-defined architecture is believed to be necessary in order to precisely imitate native ECM for guiding cell growth or tissue regeneration51,52. To this end, electrospun nanofibrous scaffolds with various alignments (e.g. axially aligned, yarn, and radially aligned) have shown superior capacity in shaping cell morphology, guiding cell migration, and affecting cell differentiation when compared to other types of scaffolds both in vitro and in vivo48,53,54. More significantly, specific cellular behaviors (e.g. cell adhesion, migration, and differentiation) of nanofibers may lead to favorable adaptation of cells in this nanoscale microenvironment. A number of strategies have been developed for controlling electrospun nanofiber alignment. Overall, these methods can be classified into three major categories (i.e. mechanical, electrostatic, and magnetic) depending on the type of forces involved55–58.
Why alignment?
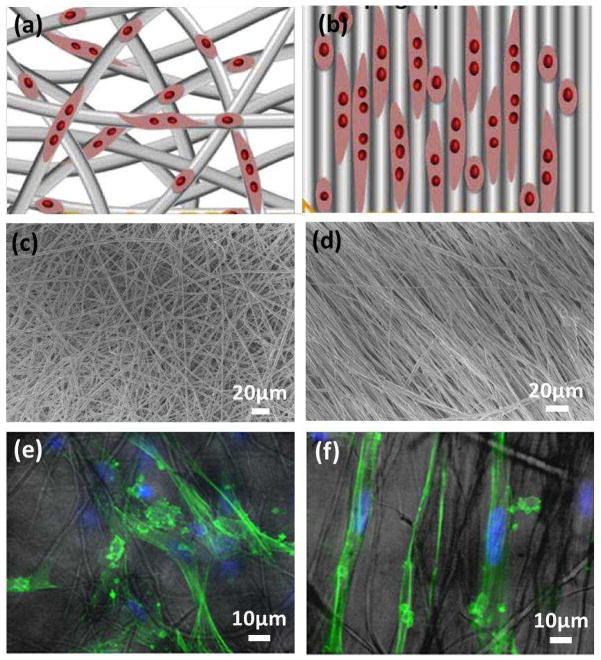
Aligned fibrous scaffolds are advantageous in replicating the ECM for a specific tissue such as cardiac tissue, where the ventricular myocardium is composed of perpendicularly interwoven collagen strips59.An aligned electrospun nanofibrous scaffold can guide the migration and extension of cells8,60. For instance, Chew and co-workers showed that aligned electrospun PCL scaffolds were able to provide contact guidance to cultured human Schwann cells (hSCs), resulting in an elongation and alignment of cells along the axes of the fibers61. As shown in Fig. 4, the effect of contact guidance provided by the aligned fibers appeared to be more dramatic than the randomly oriented fibers. When cultured on aligned fibers, the cytoskeleton and nuclei aligne and elongate on the fiber axes. The effect of contact guidance of aligned electrospun fibers on cell morphological changes was also evident in other cell types (e.g. neural stem cell) 62.
Figure 4.
Schematic of nanofibers with (a) random orientation and (b) alignment for the guidance of cell migration and extension. (Reprinted with permission from60. © 2012 Elsevier B.V.) (c–f) SEM micrographs of PCL scaffolds for hSC culture: (c) randomly oriented and (d) aligned PCL electrospun fibers; and (e, f) their corresponding fluorescent-light images overlay of hSCs cultured on PCL scaffolds for 3 days. (Reprinted with permission from61. © 2008 Elsevier B.V.)
Applications of aligned nanofibrous scaffolds in tissue engineering
Significant efforts have been made in the development of aligned nanofibrous structures as tissue-engineered scaffolds for bone63,64, cartilage65, dural53, and other tissues. Natural bone has significant anisotropic mechanical properties with highly oriented ECM and bone cells. Therefore, aligned electrospun nanofibers show great potential as a bone tissue engineering scaffold. Jose et al.63 demonstrated that uniaxially aligned PLGA/hydroxyapatite nanofibrous composite membranes could serve as ideal scaffolds for bone tissue engineering and found that the concentration of hydroxyapatite in the composites played an important role in the structure and mechanical behavior of the scaffolds.
Apart from uniaxially aligned nanofibers, some other unique biomimetic aligned fibrous structures have also been developed for tissue engineering applications. Electrospun 3D nanofibrous matrices with high spatial interconnectivity, high porosity, and controlled alignment have been well studied to direct cell orientation and migration (Fig. 5a)66. For instance, Cai et al.64 demonstrated a practical 3D macroporous nanofibrous (MNF) scaffold from aligned electrospun nanofibrous yarns for bone tissue engineering (Fig. 5b). Human embryonic stem cell-derived mesenchymal stem cells (hESC-MSCs) were well attached on the 3D MNF scaffolds and the cells changed their original rounded shape to elongated and spindle-like shapes (Fig. 5c). In vivo, radiography and histology results showed that the MNF scaffold treated bone defect had fine 3D bony tissue formation around the scaffold as well as inside the scaffold at 6 weeks. This study demonstrated that the 3D MNF scaffold could provide a structural support for hESC-MSC growth and guide bone formation, which may help promote the clinical translation of electrospun nanofibers for regenerative medicine in the future64.
Figure 5.
(a) Schematic illustration of a biomimetic nanofibrous scaffold with integrated synthetic osteogenic microenvironment. The porous scaffold can not only provide a physical structure that accommodates cells and tissue formation, but also serve as an ECM-mimicking matrix to enhance cell-scaffold interactions and delivery of bioactive agents and/or stem cells in a 3D controlled manner. (Reprinted with permission from66. © 2012 Elsevier B.V.) (b, c) SEM micrographs of 3D MNF scaffolds (b) without and (c) with in vitro growth of hESC-MSCs cells (white line indicates the direction of aligned nanofiber, black line indicates the direction of cell elongation). (Reprinted with permission from64. © 2012 WILEY-VCH Verlag GmbH & Co.)
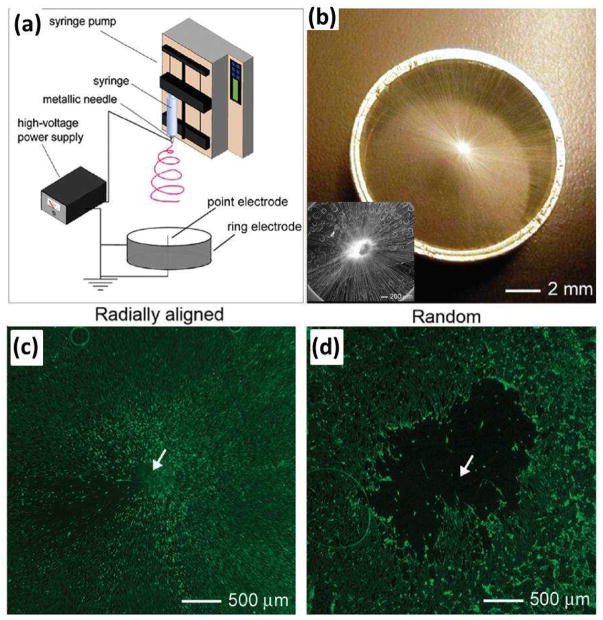
To imitate the dura mater and to develop artificial dural substitutes to promote cell migration from the surrounding tissue to the center of a dural defect, Xie and co-workers demonstrated the fabrication of an electrospun nanofibrous scaffold consisting of radially aligned PCL nanofibers (Fig. 6) and evaluated its potential application as a dural substitute53. This nanofibrous scaffold was prepared by utilizing a collector composed of a central point electrode and a peripheral ring electrode (Fig. 6a and b). They demonstrated that this novel class of scaffolds was able to present nanoscale topographic cues to cultured cells, directing and enhancing their migration from the periphery to the center. As shown in Fig. 6c, dural fibroblasts stained with fluorescein diacetate (FDA) migrated from the surrounding tissue along the radially aligned nanofibers toward the center of the circular scaffold after incubation for 4 days. In contrast, a void was observed after the same period of incubation time for a scaffold made of random fibers (Fig. 6d), indicating a faster migration rate for the cells on radially aligned nanofibers. Scaffolds based on radially aligned, electrospun nanofibers show great potential as artificial dural substitutes and may be particularly useful as biomedical patches or grafts to induce wound closure and tissue regeneration.
Figure 6.
(a) Electrospinning setup for generating scaffolds consisting of radially aligned nanofibers. (b) Photograph of a scaffold of radially aligned nanofibers directly deposited on the ring collector. Inset of (b) shows the SEM image of the radial alignment nanofibers. (c, d) Fluorescence micrographs comparing the migration of cells when dura tissues were cultured on scaffolds of random and radially aligned nanofibers, respectively, for 4 days. The dashed circle line indicates the border of dura cells after seeding at day 0. (Reprinted with permission from53. © 2010 American Chemical Society.)
Aligned to random nanofibrous scaffolds
Tendons are the connective tissues that bridge muscle to bone and allow transmission of forces to produce joint movement67. They are attached to bones across a specialized transitional tissue with varying structures and compositions. Tendon-related injuries are among the most common injuries to the body. The clinical repair of a tendon-to-bone insertion site often fails due to the lack of regeneration of the complex transitional tissue that normally exits at the uninjured attachment8,68. To imitate the gradients in composition and structure that exist at the uninjured tendon-to-bone insertion, Li et al.69 demonstrated the fabrication of a gradient of mineral on the surface of a nanofiber-based scaffold, which could imitate the composition and mechanical function of the tendon-to-bone insertion site. They also demonstrated the fabrication of a nanofibrous scaffold with an “aligned-to-random” transition in the same scaffold by utilization of a specially designed collector, which could imitate the structural organization of collagen fibers at the tendon-to-bone insertion site67. Specifically, the aligned portion could imitate the high level of alignment for collagen fibers in a normal tendon and the random portion could recapitulate the less ordered organization of collagen fibers in a bone. Tendon fibroblasts cultured on such an “aligned-to-random” electrospun nanofiber scaffold exhibited highly organized and haphazardly oriented morphologies on the aligned and random portions, respectively. Electrospun nanofiber scaffolds have been demonstrated as a useful platform for repairing injury at a tendon-to-bone insertion site. Future work should focus on investigating the potential of these scaffolds for healing tendon-to-bone insertion in an in vivo rotator cuff model.
Spiral-structured nanofibrous scaffolds
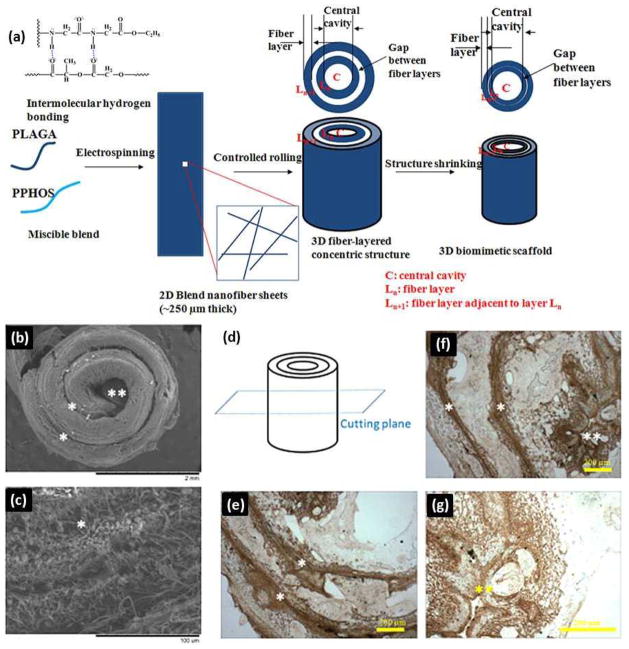
Given the pressing clinical need, the market for bone defect reconstruction-based treatments in orthopaedics is growing at a rapid rate70. Current challenges include the engineering of materials that can match both the mechanical and biological context of real bone tissue matrix and support the vascularization of large tissue constructs71. Successful bone regeneration benefits from 3D bioresorbable scaffolds that imitate the hierarchical architecture and mechanical characteristics of native tissue ECM72,73. Inspired by the complex hierarchical structures that enable bone functions, Deng and coworkers designed and constructed a 3D biomimetic scaffold by rolling electrospun nanofiber matrices in a concentric manner with an open central cavity to imitate native bone structurally and mechanically (Fig. 7a)72. The fabricated biomimetic scaffolds provide highly desirable properties for bone regeneration. Fig. 7b–g shows ECM deposition throughout 3D scaffold architecture during cell culture. Osteoblast cell layers were formed on the surfaces of biomimetic scaffolds after 28 days of culture (Fig. 7b). Robust osteoblast matrix deposition was found to bridge the gap space between the scaffold’s concentric walls as well as on the nanofiber layers (Fig. 7c), suggesting that the cells were functioning actively. Moreover, the scaffolds exhibited similar lamellar ECM organization to that of native bone. The immunohistochemical staining images shown in Fig. 7e–g demonstrated that robust secretion of osteopontin formed inside the central cavity and the gap space between laminate layers throughout the scaffold.
Figure 7.
(a) Schematics of 3D biomimetic scaffold design and fabrication. ECM deposition throughout 3D scaffold architecture during cell culture. (b, c) SEM images showing the morphologies of cell-seeded 3D biomimetic scaffolds after 28 days of culture: (b) Cell layers covering the scaffold; and (c) ECM deposited by the cells bridging the gaps in the concentric pattern by 28 days. Cells could migrate through 250 μm thick concentric fiber laminates from both the surfaces leading to a homogeneous ECM deposition and cellular activity throughout the biomimetic scaffold. (d–g) Immunohistochemical staining for osteopontin, a prominent component of the mineralized ECM, illustrating a homogenous ECM distribution throughout the scaffold at day 28: (d) Schematics of the select plane for immunohistochemical staining. (e, f) A robust stain for osteopontin bridging the gap between the concentric layers for the lower portion as well as upper and center portion of the 3D biomimetic scaffold. (g) Higher magnification image of the central cavity showing the robust stain for osteopontin. (*) indicates inter-lamellar space whereas (**) indicates central cavity. PLAGA: poly(lactide-co-glycolide), PPHOS: poly[(glycine ethyl glycinato)1(phenylphenoxy)1phosphazene] (Reprinted with permission from72. © 2011 WILEY-VCH Verlag GmbH & Co.)
Another approach for fabricating biomimetic spiral shaped scaffolds involved coating electrospun nanofibers on spiral scaffolds, to provide a substrate that imitates the native ECM and the essential contact guidance cues10,74. The spiral structure with open geometries, large surface areas, and porosity is helpful for improving nutrient transport and cell penetration into the scaffolds, which is otherwise limited in conventional tissue-engineered scaffolds for large bone defect repair10. Compared with other geometries (e.g. cylindrical and tubular scaffolds), the spiral scaffolds exhibited improved functional performance when dynamic conditions were imitated. Moreover, the spiral walls are thinner than the walls of the tubular and cylindrical scaffolds and are subject to cellular invasion from both sides of the wall, and hence, have a greater ratio of interior to exterior cells than the other scaffolds74.
Tubular conduit scaffolds
Electrospun nanofibers can be further used to generate scaffolds with complex architectures such as tubular conduits. Tubular conduits comprised of electrospun fibers can be easily fabricated by collecting nanofibers over a rotating rod of desired diameter (<5 mm) and length (e.g. ~15 cm length for coronary bypass). For example, a trilayer tubular conduit of 20 cm length and 4 mm inner diameter was fabricated by sequential electrospinning of blends of polydioxanone (PDO) and proteins onto a small diameter rod (4 mm), which imitates the complex matrix structure of native arteries (Fig. 8)75. Apart from this one-step molding process, a tubular conduit can also be prepared by rolling up electrospun fibrous membranes and securing the edges with solvent, glue, or heating76. This strategy allows fabrication of multilayered conduits with aligned fibers in the inner layer and random fibers in the outer layer, which could support better nutrient transport and cell outgrowth without compromising contact guidance8.
Figure 8.
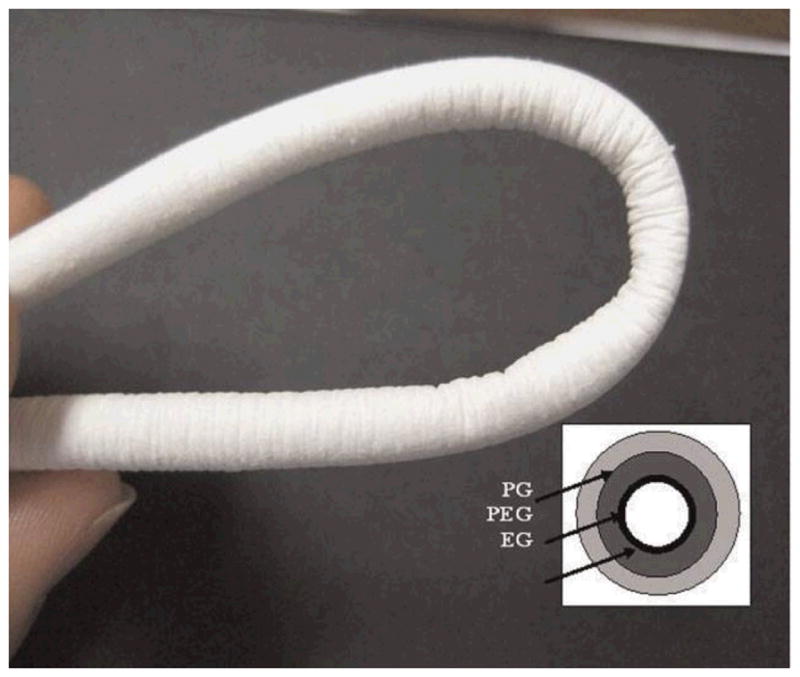
Photograph of tubular conduit of 20 cm length and 4 mm inner diameter. Inset is a schematic showing the trilayer tubular conduit (EG/PEG/PG) with spatially designed layers of elastin/gelatin (EG), PDO/elastin/gelatine (PEG), and PDO/gelatine (PG). The lumen layer is rich in protein and outer layers are rich in PDO. (Reprinted with permission from75. © 2009 WILEY-VCH Verlag GmbH & Co.)
One of the major applications of electrospun nanofibrous tubular conduits is in vascular tissue regeneration. This is because electrospun nanofibrous tubular conduits resemble the hollow structures of vascular or neural tissues. Seamless, nonwoven, bioresorbable vascular prosthetics composed of submicron fibers were fabricated using electrospinning77, and electrospun collagen and elastin fibers showed promise in vascular tissue engineering77. While natural polymer grafts offer excellent cell-matrix interactions, they often lack the mechanical properties of synthetic polymers like PLLA and PGA. When used as a scaffold, these natural polymers rapidly lose strength and dimensional stability, due to gelation and rapid hydrolysis in culture media75. Therefore, collagen and elastin-based substances alone are unlikely candidates for vascular tissue engineering, especially under the periodically loaded stress field typical of vascular structures78. Similarly, while synthetic polymer grafts offer excellent mechanical properties and easily tailored degradation, they often lack the bioactivity of natural polymers77. As such, there has been a great need to create bioresorbable vascular prosthetics that incorporate both the strength of synthetics and the bioactivity of natural polymers. The use of bio-artificial blend nanofibers could be ideal since they not only imitate the dimensions and compositions of ECM, but also have good mechanical properties. Stitzel et al.79 fabricated tubular scaffolds of electrospun PLGA-collagen-elastin ternary blend fibers, and achieved adequate mechanical strength and elasticity and appropriate bioactivities. The electrospinning process allows control of the grafts’ mechanical and bioactive properties, which allows for the creation of more dynamic grafts that can closely imitate the behavior and signaling of a native artery80.
Another application of electrospun nanofibrous tubular conduits is in nerve repair76. When direct suturing of two opposing nerve stumps during surgery is not feasible, scaffolds are often used to bridge the damaged nerve gap and to guide nerve regeneration81. The scaffold used in nerve tissue engineering applications requires optimal guidance effect, mechanical strength, and cellular compatibility82. Electrospun fibrous tubular conduits have shown potential as scaffolds in nerve regeneration applications, as their aligned fibers can provide guidance for axonal growth and the fibrous structure imitates the nerve microenvironment83. It has been reported that electrospun fibers could support oriented neurite outgrowth and glial cell migration from dorsal root ganglia explants84. In vivo studies indicated that nerve conduits with an inner layer of aligned fibers led to improved peripheral nerve regeneration85. In vitro investigation showed that, when the filament size was in the subcellular size range, growth cones could easily sense the energy differences of different outgrowth directions. Yao et al.82 developed a fibrous PCL conduit with aligned fibers on the interior surface via electrospinning and determined the optimal fiber diameter in the subcellular size range for neurite extension and directional growth. Neurite length on aligned fibers, with fiber diameters of 3.7 ± 0.5 μm and 5.9 ± 0.9 μm, was significantly longer than neurite length on randomly oriented fibers. However, further research should include efforts to design conduits with a suitable degradation profile and optimized fiber organization for better guidance of regenerative axon growth76.
Biomimetic sheath membrane
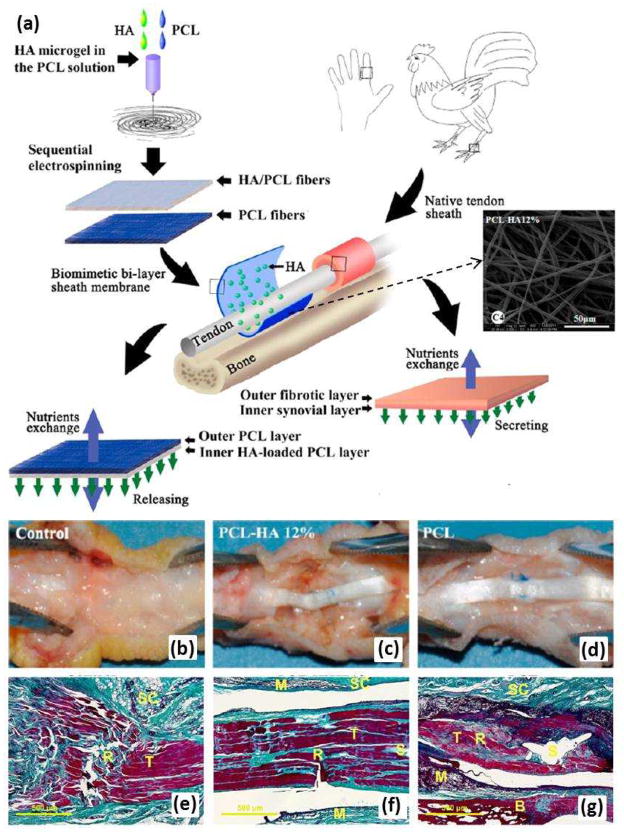
The membrane-shaped tendon sheath consists of an outer fibrotic layer and an inner synovial layer. The fibrotic layer prevents exogenous healing of the tendon as an effective biological barrier while the synovial layer secretes synovial fluid [e.g. hyaluronic acid (HA)] to enable tendon gliding86. In order to repair damage, biological replication of the tendon sheath is necessary because it allows the tendon to glide smoothly within the sheath. To replicate the hierarchical architecture and complex biologic functions of a native sheath, a biomimetic sheath should consist structurally of an outer antiadhesion layer and an inner lubricant layer. Liu et al.87 fabricated a sheath membrane by sequential electrospinning, producing a biomimetic bilayer sheath membrane consisting of an HA-loaded PCL fibrous membrane as the inner layer and a PCL fibrous membrane as the outer layer, which imitates a native sheath (Fig. 9a). Large and severe peritendinous adhesion areas were found at the repair site in the untreated control group (Fig. 9b and e). In the tendons treated with biomimetic bilayer sheath membrane, no formation of adhesions was observed between the repaired tendons and the peritendinous tissues (Fig. 9c and f). Although the adhesion area could be separated by blunt dissection, loose bundles of fibrous tissue were observed in PCL fibrous membrane treatments (Fig. 9d and g). In vitro and in vivo results showed that the outer PCL layer could reproduce the antiadhesive role of the outer fibrotic layer while the inner HA loaded PCL layer could imitate the biological function of HA secretion to promote tendon healing and gliding, showing preliminary promise in promoting tendon gliding and preventing adhesion.
Figure 9.
(a) After formation of HA microgel in the PCL solution, a biomimetic bilayer sheath membrane was fabricated by sequential electrospinning, producing a sheath membrane consisting of a PCL-HA fibrous membrane as the inner layer and a PCL fibrous membrane as the outer layer to imitate the synovial layer and the fibrotic layer, respectively, of native sheath. (b-d) Gross evaluation of a chicken model of flexor digitorum profundus tendon repair after 21 days. (b) Untreated control group; (c) group treated with sheath membrane with inner PCL-HA PCL layer; (d) group treated with PCL membrane. (eg) Histological assessments of the tendons repaired using each of the treatments. Masson’s trichrome staining of untreated repair site (e); repair site wrapped with sheath membrane with inner PCL-HA PCL layer (f); repair site wrapped with PCL membrane (g). Subcutaneous tissue (SC), tendon (T), sutured site (S), bone (B), and materials (M) could be detected. (Reprinted with permission from87. © 2012 American Chemical Society.)
Conclusions and outlook
Electrospinning has emerged as an extremely promising method for the preparation of tissue engineering scaffolds. This technique offers advantages for the preparation of scaffolds in terms of resembling the fibrillar structures of ECM, large surface areas, ease of functionalization, and controllable mechanical properties, all of which may lead to improvements in the ability to provide a true biomimetic microenvironment to the developing tissue. Yet important issues regarding its application in tissue engineering, such as achievement of in-depth penetration of cells into scaffolds and control of pore sizes, biomechanical properties, and solvent toxicity still need further investigation32. Here, we reviewed the utilization of the electrospinning approach to design and fabricate nanofibrous materials that can be used as biomimetic scaffolds for tissue engineering.
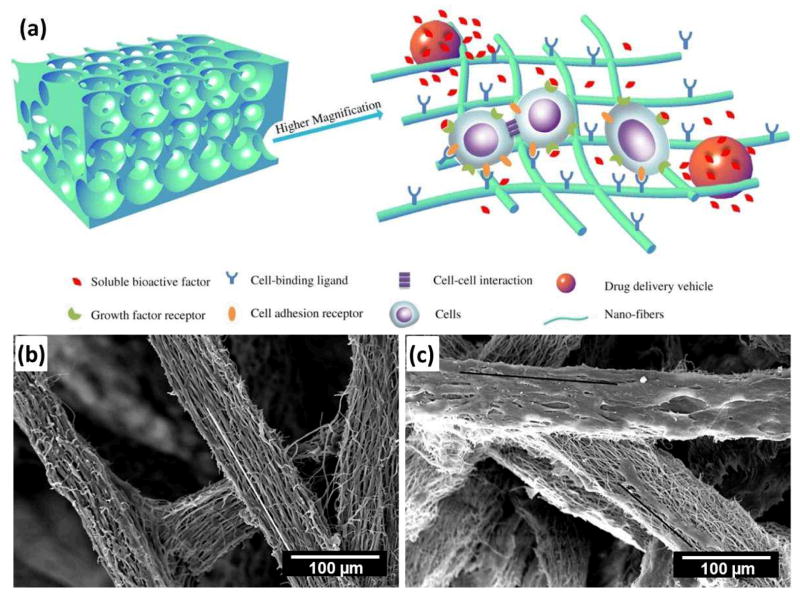
Despite recent advances toward the development of biomimetic nanofibrous scaffolds for tissue engineering applications, several challenges still remain. Generally, electrospun nanofibers were collected as two-dimensional nonwoven, which limits their applications in 3D tissues. Therefore, one challenge that must be addressed is the complexity of creating 3D porous scaffolds of a clinically relevant 3D shape88,89. Fortunately, several attempts have emerged that promise to bridge the gap, for instance, tubular and spiral-structured nanofibrous scaffolds have been fabricated by combining electrospinning with some additive manufacturing technologies, which provide a potential solution to solve this problem. It was recently reported that tissue-mimicking 3D porous scaffolds could be developed by the alternate stacking of cells and thin nanofiber substrate through a layer-by-layer approach90. It is expected that the approach can be applied to 3D skin and bone structures33. Additionally, it is crucial to develop a strategy capable of producing fibers with a diameter identical to that of native ECM fibers (a diameter less than 100 nm, preferably in the range of 10–50 nm) while maintaining high porosity for cell infiltration and migration8. The novel electro-spinning/netting (ESN) technique91–94 overcomes the bottleneck problem of electrospinning and offers a versatile method for generating spider-web-like nano-nets with ultrafine fiber diameter (less than 50 nm) while maintaining high porosity (Fig. 10), which make nano-nets optimal candidates for the fabrication of tissue-engineering scaffolds.
Figure 10.
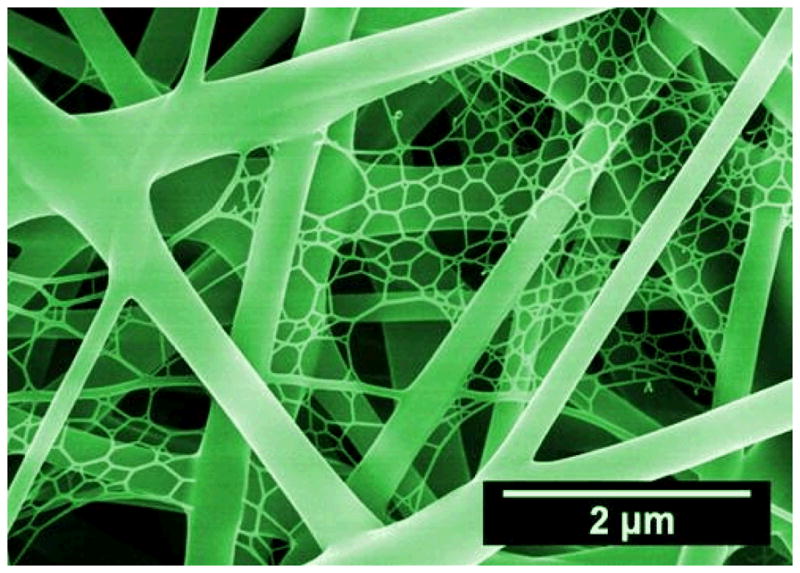
SEM image of nano-fiber/net fabricated by ESN technique comprising common electrospun nanofibers and spider-web-like nano-nets. (Reprinted with permission from93. © 2010 IOP Publishing Ltd.)
Although many challenges remain, electrospinning exhibited great potential in the fabrication of fibrous scaffolds with controllable compositions and structures, enabling scientists from various disciplines to design and generate novel scaffolds incorporating various biomimetic characteristics at genetic, molecular, and nanometer scales3. We envision a continuous expansion of the electrospinning approach in biomimetic scaffold design in the coming decades, which will stimulate further research and advances in the exciting field of tissue engineering.
Acknowledgments
We acknowledge financial support from the West Virginia Higher Education Policy Commission/Division of Science Research, NSF, and AO Foundation (Project S-13-15L was supported by the AO Foundation). We thank Suzanne Danley for proofreading.
References
- 1.Dvir T, et al. Nat Nanotechnol. 2011;6:13. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens MM, George JH. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 3.Ma PX. Adv Drug Deliv Rev. 2008;60:184. doi: 10.1016/j.addr.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YP, et al. Angew Chem Int Edit. 2012;51:2864. [Google Scholar]
- 5.Zhang XH, et al. Adv Drug Deliv Rev. 2009;61:988. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du J, et al. Biomaterials. 2011;32:5427. doi: 10.1016/j.biomaterials.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du JA, Yarema KJ. Adv Drug Deliv Rev. 2010;62:671. doi: 10.1016/j.addr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu W, et al. Adv Health Mater. 2012;1:10. [Google Scholar]
- 9.Holzwarth JM, Ma PX. Biomaterials. 2011;32:9622. doi: 10.1016/j.biomaterials.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JP, et al. J Biomed Mater Res Part A. 2010;93A:753. doi: 10.1002/jbm.a.32591. [DOI] [PubMed] [Google Scholar]
- 11.Liao S, et al. Biomed Mater. 2006;1:R45. doi: 10.1088/1748-6041/1/3/R01. [DOI] [PubMed] [Google Scholar]
- 12.Jiang BB, et al. Biomacromolecules. 2010;11:3630. doi: 10.1021/bm1010855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith LG, Naughton G. Science. 2002;295:1009. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg M, et al. J Biomater Sci-Polym Ed. 2007;18:241. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li YP, et al. ACS Nano. 2012;6:9485. doi: 10.1021/nn302317j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasita R, Katti DS. Int J Nanomed. 2006;1:15. [Google Scholar]
- 17.Ma PX, Zhang RY. J Biomed Mater Res. 1999;46:60. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 18.Holmes TC, et al. Proc Natl Acad Sci U S A. 2000;97:6728. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu XH, et al. Nat Mater. 2011;10:398. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wade RJ, Burdick JA. Mater Today. 2012;15:454. [Google Scholar]
- 21.Matson JB, et al. Curr Opin Solid State Mat Sci. 2011;15:225. doi: 10.1016/j.cossms.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartgerink JD, et al. Proc Natl Acad Sci U S A. 2002;99:5133. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li WJ, et al. J Biomed Mater Res. 2002;60:613. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 24.Bhardwaj N, Kundu SC. Biotechnol Adv. 2010;28:325. doi: 10.1016/j.biotechadv.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 25.Bhattarai SR, et al. Biomaterials. 2004;25:2595. doi: 10.1016/j.biomaterials.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Shi J, et al. ACS Appl Mater Interfaces. 2010;2:1025. doi: 10.1021/am9007962. [DOI] [PubMed] [Google Scholar]
- 27.Pant HR, et al. Colloid Surf B-Biointerfaces. 2011;88:587. doi: 10.1016/j.colsurfb.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 28.Ramakrishna S, et al. Mater Today. 2006;9:40. [Google Scholar]
- 29.Liu J, et al. Small. 2009;5:536. doi: 10.1002/smll.200801440. [DOI] [PubMed] [Google Scholar]
- 30.Fong H, et al. Polymer. 1999;40:4585. [Google Scholar]
- 31.Li D, Xia YN. Adv Mater. 2004;16:1151. [Google Scholar]
- 32.Agarwal S, et al. Adv Mater. 2009;21:3343. doi: 10.1002/adma.200803092. [DOI] [PubMed] [Google Scholar]
- 33.Jang JH, et al. Adv Drug Deliv Rev. 2009;61:1065. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 34.Huang ZM, et al. Compos Sci Technol. 2003;63:2223. [Google Scholar]
- 35.Hohman MM, et al. Phys Fluids. 2001;13:2201. [Google Scholar]
- 36.Yarin AL, et al. J Appl Phys. 2001;89:3018. [Google Scholar]
- 37.Bognitzki M, et al. Adv Mater. 2001;13:70. [Google Scholar]
- 38.Greiner A, Wendorff JH. Angew Chem Int Edit. 2007;46:5670. doi: 10.1002/anie.200604646. [DOI] [PubMed] [Google Scholar]
- 39.Ulery BD, et al. J Polym Sci Pt B-Polym Phys. 2011;49:832. doi: 10.1002/polb.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ding B, et al. Mater Today. 2010;13:16. doi: 10.1016/S1369-7021(10)70200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang XF, et al. Nano Today. 2011;6:510. [Google Scholar]
- 42.Roach P, et al. Soft Matter. 2008;4:224. doi: 10.1039/b712575p. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Hunt JA. J Mater Chem. 2007;17:3974. [Google Scholar]
- 44.Lin JY, et al. Crit Rev Solid State Mat Sci. 2012;37:94. [Google Scholar]
- 45.Reddy VJ, et al. Wound Repair Regen. 2013;21:1. [Google Scholar]
- 46.Wang J, Yu X. Acta Biomater. 2010;6:3004. doi: 10.1016/j.actbio.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 47.Nguyen TBL, Lee BT. J Biomater Appl. 2012;27:255. doi: 10.1177/0885328211401932. [DOI] [PubMed] [Google Scholar]
- 48.Huang CY, et al. Biomaterials. 2012;33:1791. doi: 10.1016/j.biomaterials.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stevens MM. Mater Today. 2008;11:18. [Google Scholar]
- 50.Deng M, et al. IEEE Trans Nanobiosci. 2012;11:3. doi: 10.1109/TNB.2011.2179554. [DOI] [PubMed] [Google Scholar]
- 51.Kim TG, et al. Adv Funct Mater. 2012;22:2446. [Google Scholar]
- 52.Yang F, et al. Biomaterials. 2005;26:2603. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 53.Xie J, et al. ACS Nano. 2010;4:5027. doi: 10.1021/nn101554u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan LS, Xu ZK. J Biomed Mater Res A. 2009;89:168. doi: 10.1002/jbm.a.31907. [DOI] [PubMed] [Google Scholar]
- 55.Li D, et al. Adv Mater. 2004;16:361. [Google Scholar]
- 56.Yang D, et al. Adv Mater. 2007;19:3702. [Google Scholar]
- 57.Matthews JA, et al. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 58.Teo WE, Ramakrishna S. Nanotechnology. 2006;17:R89. doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 59.Engelmayr GC, et al. Nat Mater. 2008;7:1003. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen M, et al. Acta Biomater. 2013;9:5562. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Chew SY, et al. Biomaterials. 2008;29:653. doi: 10.1016/j.biomaterials.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.He L, et al. Acta Biomater. 2010;6:2960. doi: 10.1016/j.actbio.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 63.Jose MV, et al. Acta Biomater. 2009;5:305. doi: 10.1016/j.actbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 64.Cai YZ, et al. J Biomed Mater Res A. 2012;100:1187. doi: 10.1002/jbm.a.34063. [DOI] [PubMed] [Google Scholar]
- 65.Chen JP, Su CH. Acta Biomater. 2011;7:234. doi: 10.1016/j.actbio.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Zhang ZP, et al. Adv Drug Deliv Rev. 2012;64:1129. doi: 10.1016/j.addr.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie JW, et al. Nanoscale. 2010;2:923. [Google Scholar]
- 68.Richardson LE, et al. Trends Biotechnol. 2007;25:409. doi: 10.1016/j.tibtech.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Li XR, et al. Nano Lett. 2009;9:2763. doi: 10.1021/nl901582f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang BB, Li BY. J Biomed Mater Res Part B. 2009;88B:332. doi: 10.1002/jbm.b.31021. [DOI] [PubMed] [Google Scholar]
- 71.Li BY, et al. Biomaterials. 2009;30:2552. doi: 10.1016/j.biomaterials.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deng M, et al. Adv Funct Mater. 2011;21:2641. [Google Scholar]
- 73.Lee JH, et al. Biomaterials. 2012;33:999. doi: 10.1016/j.biomaterials.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 74.Valmikinathan CM, et al. Mater Sci Eng C-Mater Biol Appl. 2011;31:22. doi: 10.1016/j.msec.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas V, et al. Biotechnol Bioeng. 2009;104:1025. doi: 10.1002/bit.22467. [DOI] [PubMed] [Google Scholar]
- 76.Xie JW, et al. Nanoscale. 2010;2:35. [Google Scholar]
- 77.Sell SA, Bowlin GL. J Mater Chem. 2008;18:260. [Google Scholar]
- 78.Lee SJ, et al. J Biomed Mater Res Part A. 2007;83A:999. doi: 10.1002/jbm.a.31287. [DOI] [PubMed] [Google Scholar]
- 79.Stitzel J, et al. Biomaterials. 2006;27:1088. doi: 10.1016/j.biomaterials.2005.07.048. [DOI] [PubMed] [Google Scholar]
- 80.Agarwal S, et al. Polymer. 2008;49:5603. [Google Scholar]
- 81.Bashur CA, et al. Biomaterials. 2006;27:5681. doi: 10.1016/j.biomaterials.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 82.Yao L, et al. J Biomed Mater Res Part B. 2009;90B:483. doi: 10.1002/jbm.b.31308. [DOI] [PubMed] [Google Scholar]
- 83.Yu LMY, et al. Mater Today. 2008;11:36. [Google Scholar]
- 84.Schnell E, et al. Biomaterials. 2007;28:3012. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 85.Chew SY, et al. Adv Funct Mater. 2007;17:1288. doi: 10.1002/adfm.200600441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharma P, Maffulli N. J Bone Joint Surg-Am Vol. 2005;87A:187. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 87.Liu S, et al. Biomacromolecules. 2012;13:3611. doi: 10.1021/bm301022p. [DOI] [PubMed] [Google Scholar]
- 88.Holzwarth JM, Ma PX. J Mater Chem. 2011;21:10243. [Google Scholar]
- 89.Wang HJ, van Blitterswijk CA. Biomaterials. 2010;31:4322. doi: 10.1016/j.biomaterials.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 90.Yang XC, et al. Tissue Eng Pt A. 2009;15:945. doi: 10.1089/ten.tea.2007.0280. [DOI] [PubMed] [Google Scholar]
- 91.Wang XF, et al. Nanoscale. 2011;3:911. doi: 10.1039/c0nr00783h. [DOI] [PubMed] [Google Scholar]
- 92.Ding B, et al. J Mater Chem. 2011;21:12784. [Google Scholar]
- 93.Wang XF, et al. Nanotechnology. 2010;21:055502. doi: 10.1088/0957-4484/21/5/055502. [DOI] [PubMed] [Google Scholar]
- 94.Barakat NAM, et al. Polymer. 2009;50:4389. [Google Scholar]