Abstract
Songbirds, long of interest to basic neuroscientists, have great potential as a model system for translational neuroscience. Songbirds learn their complex vocal behavior in a manner that exemplifies general processes of perceptual and motor skill learning, and more specifically resembles human speech learning. Song is subserved by circuitry that is specialized for vocal learning and production, but that has strong similarities to mammalian brain pathways. The combination of a highly quantifiable behavior and discrete neural substrates facilitates understanding links between brain and behavior, both normally and in disease. Here we highlight 1) behavioral and mechanistic parallels between birdsong and aspects of speech and social communication, including insights into mirror neurons, the function of auditory feedback, and genes underlying social communication disorders, and 2) contributions of songbirds to understanding cortical-basal ganglia circuit function and dysfunction, including the possibility of harnessing adult neurogenesis for brain repair.
Keywords: Reinforcement learning, basal ganglia, speech, mirror neurons, hearing, neurogenesis (6)
Introduction
Humans are experts at vocal learning, an ability shared by few other vertebrates. No non-human primates or rodents have been shown to learn their vocal behavior, and there are only a few other known mammalian vocal learners - cetaceans, elephants, and some bats. Songbirds therefore represent an almost unique animal model: song learning has remarkable parallels to human speech learning (Fig. 1; Brenowitz et al. 2012, Doupe & Kuhl 1999, Marler 1970, Mooney 2009) and provides an opportunity for mechanistic investigation of vocal learning and its disorders.
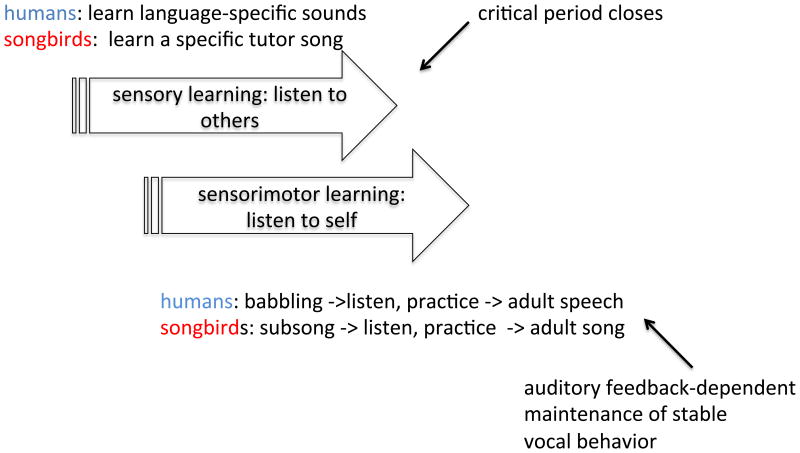
Figure 1.
Similarities between human speech and birdsong learning.
Because vocal learning involves both perceptual learning and motor skill learning, birdsong also provides a model for investigating these general learning processes and their disturbances. Additionally, song learning is shaped potently by social interactions and is limited to a ‘critical’ period during which experience plays an especially crucial role in nervous system development. Such factors similarly shape and constrain many aspects of behavior in other vertebrates including humans, making song learning an attractive system for understanding these constraints and how they might be overcome to enable nervous system repair in disease and injury.
Birdsong is a complex motor sequence that is readily quantified, making it a sensitive assay for investigating the effects of behavioral and neural manipulations. Moreover, song depends on dedicated and accessible brain nuclei (Fig. 2, ‘the song system’; Nottebohm et al. 1976) that have strong homologies to mammalian circuits (see sidebar ‘Avian and mammalian brains’). The specialization of this circuitry for a quantifiable, learned behavior facilitates investigation of links between brain and behavior, and offers the opportunity to control experience and manipulate brain function in a manner impossible in humans. Thus, animal models like songbirds can reveal basic mechanisms underlying normal and abnormal function. Accordingly, in this review, we take a ‘translational’ stance and discuss ways in which birdsong can contribute to biomedical research and ultimately human medicine.
Figure 2.
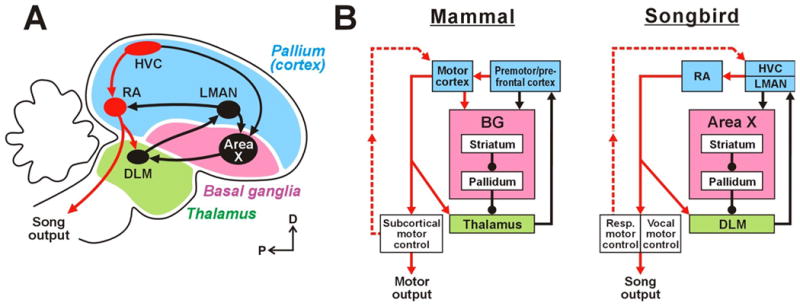
The song system.
We focus here on contributions of songbird research to studies of 1) speech and social communication, and 2) cortical-basal ganglia circuitry and motor and reward learning. We refer the reader to recent reviews of additional areas of basic and translational relevance of birdsong, including general auditory processing (Knudsen & Gentner 2010, Theunissen & Shaevitz 2006, Woolley 2012), central and peripheral vocal motor production (Fee & Scharff 2010, Schmidt et al. 2012, Riede & Goller 2010), and the effects of steroid hormones on brain and behavior (Ball & Balthazart 2010, Pinaud & Tremere 2012, Remage-Healey et al. 2010).
Background
Song, like speech, is learned in two, sometimes overlapping phases (Fig. 1). During a critical period for ‘sensory learning’, birds hear and form a memory of a specific adult vocal model (the ‘tutor song’) (see Doupe & Kuhl 1999, Mooney 2009 for reviews). Similarly, human infants progress from universal perception of speech sounds to perception that reflects exposure to a specific language. During subsequent ‘sensorimotor learning’, birds gradually shape their initial rambling, immature ‘subsong’ into mature song resembling that of the tutor, while humans turn their initial ‘babbling’ into the mature adult sounds of the language surrounding them. Both birds and humans must be able to hear themselves to refine their vocalizations. In songbirds, the tutor no longer needs to be present during this phase, indicating that a memory of the adult model, often called the ‘template’, can guide learning. For song, as for speech, there must be circuitry for producing vocalizations, circuitry for hearing and learning sounds of self and others, and linkage between the two.
The motor control of both song and speech involves high-level structures that ultimately coordinate the patterned breathing and vocal muscle activity necessary for vocalization. In songbirds, these areas are collectively known as the ‘song system’, and include a ‘motor pathway’ for song, and a cortical-basal ganglia circuit for song, the anterior forebrain pathway (AFP) (Fig. 2). The motor pathway includes the premotor cortical nucleus HVC (abbreviation used as proper name) and the robust nucleus of the arcopallium (RA), which is functionally equivalent to vocal motor cortex (we use the term ‘cortical’ to refer to song system nuclei that are equivalent to mammalian cortex; see sidebar ‘Avian and Mammalian Brains’). HVC is involved in generating the timing and sequencing of song (Fee & Scharff 2010, Hahnloser et al. 2002, Long & Fee 2008; see sidebar ‘Motor sequence generation’). RA receives inputs from HVC and projects directly to respiratory centers and to brainstem motor neurons controlling the vocal organ; the respiratory centers send recurrent information back to HVC via the thalamus, reflecting the importance of bidirectional coordination between telencephalic and brainstem structures in vocal control (Fig. 2; Schmidt et al. 2012). HVC and RA are required for normal song production throughout life; complete bilateral lesions of either of these nuclei cause song disruptions or even muteness, akin to human aphasias (Nottebohm et al. 1976). The AFP indirectly connects HVC and RA (Fig. 2), and like cortical-basal ganglia circuitry in mammals, functions importantly in motor learning (see Cortical Basal Ganglia Circuits and Reward and Motor Learning).
The songbird Field L, which is analogous to the primary auditory cortex of mammals, projects to a complex set of higher auditory areas known broadly as NCM (caudo-medial nidopallium) and CM (caudal mesopallium), and these high-level areas are the likely source of auditory inputs to the song system. Enhanced immediate early gene expression and neurophysiological activity in response to songs in CM/NCM suggest that they, in addition to the song system, are sites of operations critical to vocal learning, with analogies to speech-related regions of the human superior temporal gyrus (Knudsen & Gentner 2010, Moorman et al. 2012, Woolley 2012).
Speech and Social Communication
Influence of sensory exposure on vocal learning and the brain
Both humans and birds must hear adult vocal models in order to learn normal vocalizations. The requirement to imitate others results in different languages, dialects, and individual accents in humans, and in dialects within species of birds, as well as individual differences between birds tutored by different adults. The amount of early exposure to adult vocalizations contributes critically to vocal development. In humans, these data are largely correlative (Hurtado et al. 2008). In birds, controlled manipulations of experience demonstrate that insufficient exposure to vocal models can impair subsequent vocal learning (Catchpole & Slater 2008, Marler 1970). Interestingly, too much song model exposure can also impair learning (Tchernichovski et al. 1999). This suggests there may be an optimal balance between different kinds of experience (for example between listening to others and listening to self). These effects of early experience can be remarkably rapid. In songbirds, after the first exposure to a tutor, vocal performance can improve in less than a day (Derégnaucourt et al. 2005, Shank & Margoliash 2009). This is analogous to the almost immediate appearance of new speech sounds in infants exposed to novel sounds (Kuhl & Meltzoff 1996).
The quality of acoustic features heard during this early period additionally shapes learning. For humans, exaggerated features adopted by adults in speaking to infants, such as slowing of speech or increased pitch (‘infant-directed speech’), may preferentially shape perceptual learning for speech (Fernald 1985, Kuhl 2010). Similarly, for songbirds, some songs are more readily learned than others (Marler 1990), and key features within songs may enhance learning of associated sounds (Catchpole & Slater 2008; Soha & Marler 2000). Song learning also can be enhanced by factors such as hearing a greater variety of tutor songs (Tchernichovski et al. 1999), and impeded for instance by hearing tutor songs that are sung at too fast a tempo (Podos 1996). The controlled studies possible in songbirds enable investigation of such behavioral variables that may also be relevant to normal speech development and rehabilitation.
In songbirds it is possible to investigate directly how and where sensory exposure influences the brain. For example, brain activity can be locally and reversibly disrupted specifically when young birds are hearing auditory models (and not when they are actively rehearsing). Such experiments have identified several areas required for learning of the tutor song. These include not only high-level auditory areas (London & Clayton 2008), but also the song sensorimotor nuclei HVC and LMAN (Basham et al. 1996, Roberts et al. 2012). Consistent with the importance of vocal premotor regions to sensory learning, exposure to the tutor song can drive rapid changes in these regions, including dramatic changes to patterns of neural activity and to the structure and motility of dendritic spines (Roberts et al. 2010, Shank & Margoliash 2009). These findings suggest that perceptual learning for vocal production both shapes neural selectivity in auditory areas and organizes sensorimotor structures involved in production. These data reinforce that for song, and likely for speech, there is an intimate link between the mechanisms that contribute to sensory learning of vocal models and learning to produce those sounds.
Songbirds provide a particularly clear example of learning that is limited to a critical or sensitive period early in life, with special relevance to the human critical period for language acquisition (Kuhl 2010). Many species of birds must hear a tutor song while young, and will not learn songs to which they are subsequently exposed (see Doupe & Kuhl 1999, Mooney 2009 for reviews). Many of the neuronal factors also implicated in mammalian development and critical period regulation change around the time that the song sensitive period normally closes, including spine density, axonal arborization, NMDA receptor current decay, and peri-neuronal nets (e.g. Balmer et al. 2009, Heinrich et al. 2005; Livingston et al. 2000, Roberts et al. 2010, Miller-Sims & Bottjer 2012). Because the critical period for tutor song memorization can be altered or extended by manipulations of experience, it should be possible to identify neural mechanisms that are causally linked to its regulation.
Bidirectional links between perception and production
Although perceptual learning guides the development of vocal production, it can also constrain our ability to perceive communication sounds. For example, in humans, exposure to a specific language can result in ‘categorical perception’, in which acoustically distinct versions of a single phoneme become grouped so that the capacity to distinguish between them is reduced. Songbirds also develop categorical perception of song elements, for instance for a particular range of interval times between elements of song (Fig. 3A,B; Nelson & Marler 1989, Prather et al. 2009). Such categorical perception is adaptive in creating an abstracted representation of sounds that enables recognition despite acoustic variation in production. However, in humans, such perceptual shaping also contributes to difficulty in perceiving or producing non-native phonemes. Indeed, the difficulty in changing speech, both normally and in vocal pathologies, may stem primarily from the stability of perceptual targets, rather than inflexibility of production mechanisms. Similarly, stability of perceptual targets contributes to the normal stability of adult song (Sober & Brainard 2009), suggesting that songbirds provide a useful model for investigating the neural basis of perceptual constraints on learning.
Figure 3.
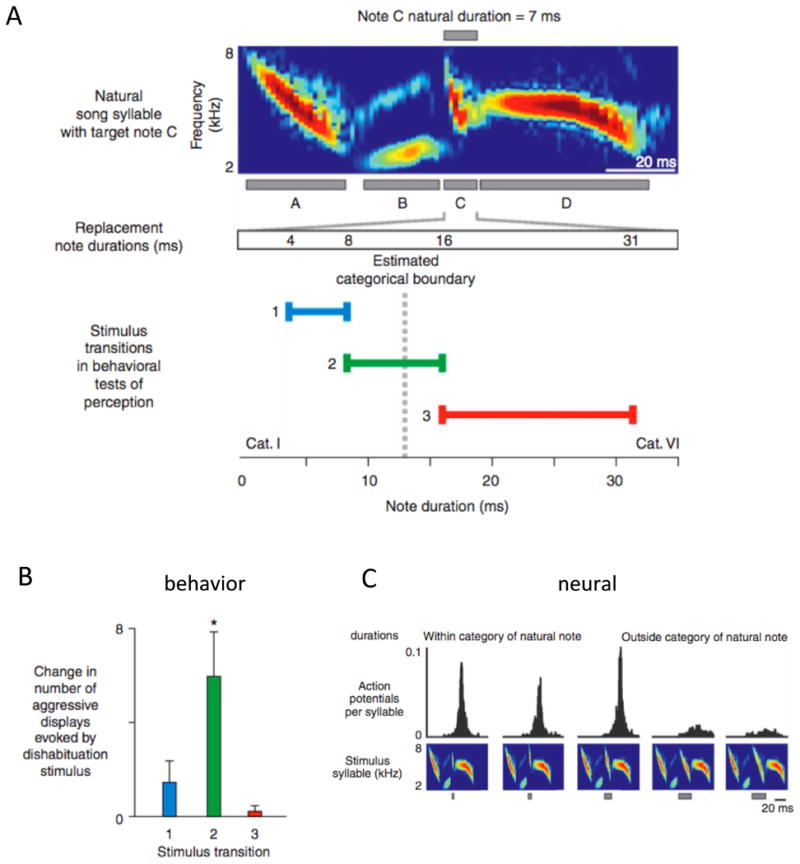
Behavioral and neural manifestations of categorical perception in songbirds.
The tight, bi-directional link between perception and production is evident in the nervous system. Human imaging studies indicate that sensorimotor structures for speech, such as Broca's area, are active both during production and perception. Damage or disruption of these areas, as well as of structures linked to comprehension, such as Wernicke's area, can cause both productive and receptive deficits (e.g. Hickok et al. 2011). Similarly, in adult birds, playback of the tutor song and the bird's own song can activate both high-level auditory areas and neurons throughout the song system (Dave & Margoliash 2000, Moorman et al. 2012, Prather et al. 2008, 2010; Solis & Doupe 1999). Moreover, damage to sensorimotor song nuclei (such as HVC, LMAN, and Area X) can cause difficulty in discriminating songs (Burt et al. 2000, Gentner et al. 2000, Scharff et al. 1998).
A particularly compelling example of the neural link between production and perception is provided by ‘mirror’ neurons, which are found in human and non-human primate cortical motor areas, including speech areas. These neurons are active during production of movements, but also exhibit similar firing patterns during observation or hearing of the same movements (Rizzolatti & Fabbri-Destro 2010). This sensorimotor correspondence suggests that mirror neurons have been shaped both by the production of gestures and by the sensory stimuli that these same gestures produce. Such a correspondence may contribute to a subject's understanding of sensory inputs from others, based on his or her own production of gestures. Thus, mirroring may be important not only in learning but also in subsequent mimicry and other social communication, and in disorders of the ability to understand and produce social gestures, such as autism (Rizzolatti & Fabbri-Destro 2010).
Songbirds provide striking examples of the kind of behavioral coordination and imitation that may require mirroring. Some species, such as lyrebirds and mockingbirds, can immediately produce imitations of newly heard sounds. Many species also engage in countersinging, in which birds sing songs in alternation, or duetting, in which two individuals cooperate to produce the alternating syllables of a single song (Catchpole & Slater 2008, Fortune et al. 2011). These behaviors indicate that songbirds can rapidly translate sensory inputs into the motor actions necessary to produce an imitation or the next gesture in a learned motor sequence.
At the neural level, the song system possesses song mirror neurons well-suited to subserve learning, mimicry, and social coordination (Dave & Margoliash 2000, Fortune et al. 2011, Prather et al. 2008). In adult birds, these mirror neurons exhibit motor-related activity during song production, and also fire strongly in response to playback of the sound of the bird's own song (BOS), much more than to other songs. The auditory activity of these ‘BOS-selective’ neurons exhibits precise and complex feature selectivity for the spectral structure, relative ordering and timing of song elements of the bird's own song (Dave & Margoliash 2000, Solis & Doupe 1999). For example, in swamp sparrows, HVC neurons fire most strongly for playback of song that matches the bird's behavioral boundaries for categorical perception of the same song (Fig. 3; Prather et al. 2009). Strikingly, in a duetting species, HVC neurons fire most when songs of both partners, in their normal alternation, are played back, and much less when either song is played alone (Fortune et al. 2011). This suggests that neurons have encoded not only BOS but also the duetting birds' combined, cooperative sensory output.
In birds, it is straightforward to study the development and learning-related properties of these mirror neurons. Many aspects of BOS selectivity clearly emerge only during vocal production learning (Solis & Doupe 1999, Volman 1993). Damage to the vocal periphery in both adult and juvenile birds results in altered auditory tuning of BOS-selective neurons (Roy & Mooney 2007, Solis & Doupe 2000), and birds prevented from developing normal song by peripheral muting, but otherwise exposed to a normal acoustic environment, exhibit degraded song perception (Pytte & Suthers 1999). Intriguingly, songbird mirror neurons can encode more than the sound of the BOS. In both LMAN and HVC, some neurons also respond to the songs of tutors that have been heard, and thus may be involved in encoding the tutor song memory or in matching of BOS to the tutor (Nick & Konishi 2005, Prather et al. 2010, Solis & Doupe 1999, 2000). The presence of such precise sensorimotor tuning in song-selective neurons provides an opportunity for investigating the mechanistic basis of interactions between perception and production, as well as what happens when this interaction is disrupted.
Behavioral and brain mechanisms of auditory feedback
Speech and song are both motor skills that rely crucially on auditory feedback. Congenitally deaf individuals have extreme difficulty developing normal patterns of articulation, and hearing loss even in adulthood can lead to gradual deterioration of speech, suggesting that auditory feedback continues to play an important role in calibrating vocal output. Moreover, perturbation of feedback, by altering loudness, timing, or pitch, can drive both online disruptions and compensatory adaptations of speech (see Houde & Nagarajan 2011 for review). Correspondingly, deficits in central feedback processing mechanisms may contribute to a variety of speech abnormalities, including deficits in speech acquisition and in control of phonation and sequencing of speech sounds, as in stuttering.
Auditory feedback is equally important in birds for learning, controlling, and maintaining production. Juvenile birds that are deafened after exposure to a tutor song fail to develop normal songs, and adult birds deafened or subjected to disruption of auditory feedback exhibit gradual song deterioration (Konishi 1965, Leonardo & Konishi 1999, Nordeen & Nordeen 1992). As in humans, delayed auditory feedback results in slowing and mis-sequencing of syllables, with the most disruptive delay corresponding approximately to the duration of a single syllable (Cynx & von Rad 2001, Sakata & Brainard 2006). Likewise, birds make gradual compensatory adjustments to the pitch at which they produce song to correct perceived errors in production (Sober & Brainard 2009).
Despite the importance of auditory feedback, we have relatively little understanding of the neural processes whereby information derived from auditory feedback is used to evaluate and adjust speech or song. Studies in humans suggest there are specialized substrates within vocal control and associated auditory regions that are specifically gated or modulated by the act of vocalizing. For example, responses in human superior temporal gyrus are decreased when a speaker hears himself during speaking versus when the same speech sounds are played back during quiescence (Ford & Mathalon 2012, Hickok et al. 2011, Price 2012). This could represent damping of the responsiveness of auditory neurons by motor preparatory or ‘efference copy’ signals, in advance of the predictable loud stimulus associated with vocalizing, a phenomenon widely observed across species (Ford & Mathalon 2012). However, in humans, alteration of the sound of the speaker's voice can increase neural activity during speaking to the level seen in response to playback (Price 2012). This suggests that efferent activity may specifically attenuate the expected sensory consequences of speech motor commands and thus enhance sensitivity to deviations from that expectation.
Several related phenomena are present in birds. First, in some species, the responses to playback of the bird's own song within premotor nuclei are strongest in sleeping or anesthetized birds and attenuated or absent in awake birds (Cardin & Schmidt 2004, Castelino & Schmidt 2010, Coleman et al. 2007). Such context-dependent modulation is one indication that auditory feedback from vocalizations is processed differently than other sounds, and is linked to mechanisms that control production. Second, transient perturbation of feedback during singing results in changes to the timing and sequencing of syllables at latencies of only a few tens of milliseconds. This indicates that sensory inputs must have rapid ‘online’ access to song premotor circuitry (Sakata & Brainard 2006). One locus where such neural responses to feedback perturbation have been observed is the premotor nucleus HVC of the Bengalese finch (Sakata & Brainard 2008; but see also Kozhevnikov & Fee 2007, Leonardo 2004, Prather et al. 2008), supporting the possibility that auditory signals within premotor circuitry may normally contribute to online control of song production and to song learning. Finally, in areas conventionally thought of as primarily auditory regions of the brain, some neurons exhibit strong responses only when feedback is perturbed (Keller & Hahnloser 2009). Such responses, like those in HVC and in human auditory cortex, could indicate that the actual sensory input differs from the expected consequences of vocalizing, and provide ideal signals for correcting vocal performance.
In songbirds, it is possible to test experimentally both how the brain encodes sensory feedback and how it uses feedback to shape vocal production. For example, the state-dependent neural mechanisms that engage or disengage auditory responsiveness in song control regions include neuromodulatory tone. Direct manipulation of neuromodulators can enhance or eliminate auditory responses in central vocal control structures (Cardin & Schmidt 2004; Shea & Margoliash 2010). Moreover, at a behavioral level, the presence of a female bird renders male song less sensitive to perturbations of auditory feedback (Sakata & Brainard 2009), perhaps also reflecting shifts in attentional and motivational systems arising from altered neuromodulatory tone.
For humans, some non-speech disorders also involve abnormal processing of auditory signals both of others and of self. For instance, schizophrenic patients may have impaired ‘efference copy’ mechanisms, evident as less damping of self-generated feedback. Similarly, these patients may mis-attribute delayed auditory feedback of their own voice to others (Ford & Mathalon 2012, Frith et al. 2000). These abnormalities are hypothesized to contribute to the generation of symptoms such as hallucinations and delusions. A mechanistic understanding of efference copy function during vocal behavior, and of how neuromodulatory systems act to alter neural and behavioral sensitivity to sensory feedback, may thus provide insights into self-monitoring systems relevant not only to speech but more generally to neuropsychiatric disorders.
Importance of social factors in vocal learning and brain organization
Both speech and birdsong are highly social behaviors, and social interactions can strongly influence multiple phases of vocal learning. For instance, social factors can determine what models juveniles choose to learn. For human infants, live exposure to foreign language phonemes preserves children's discriminatory ability for those phonemes, while exposure via video or audiotape does not (Kuhl 2010). Similarly, for highly social birds, like the zebra finch, song is better learned from live models than from passive playback of acoustic models (Catchpole & Slater 2008). However, if a young bird must press a key to interactively elicit song playback, the bird can learn well (Adret 1993; Tchernichovski et al. 2001). Moreover, birds will learn from interacting tutors beyond the age at which taped playback of song becomes ineffective, and will learn normally non-preferred (heterospecific) song if the heterospecific tutor is interactive tutor (Catchpole & Slater 2008).
Social cues are also important in vocal motor learning. Juvenile white-crowned sparrows singing a variety of almost mature songs stabilize and retain the variants that elicit counter-singing from other males (Nelson & Marler 1994), while young male cowbirds preferentially retain song variants that trigger visual courtship responses from females (West & King 1988).
Social factors in songbirds likely act in a variety of ways, including via changes in neuromodulators and steroid hormone levels (Riters 2011, Sasaki et al. 2006, White & Livingston 1999), and can exert influences regardless of acoustic experience. For example, starlings prevented from forming social bonds, even if they can hear normal songs, develop highly abnormal auditory cortices (Cousillas et al. 2008).
Mechanistic and neurophysiological studies in songbirds have identified many brain areas affected by social signals. The familiarity and social relevance of songs can affect the degree of expression of immediate early genes (IEG) in a number of high-level auditory areas (Knudsen & Gentner 2010, Moorman et al. 2012, Woolley & Doupe 2008), and in some cases, this induction can be prevented by blockade of norepinephrine receptors (Velho et al. 2012). The cortical basal ganglia circuit for song (the AFP; see Fig. 2) is also strongly affected by social cues. IEG induction, which is high in Area X and LMAN when birds sing alone, is dramatically decreased when birds sing to a female (Jarvis et al. 1998), and this is accompanied by marked changes in neuronal firing properties (Hessler & Doupe 1999a,b; Kao et al. 2008). Midbrain neuromodulatory areas that project strongly to the AFP, such as the noradrenergic locus coeruleus and the dopaminergic ventral tegmental area (VTA), likely mediate these social influences. Lesions of the locus coeruleus eliminate the social modulation of IEG induction in Area X (Castelino & Schmidt 2010). VTA neurons, which project strongly to the AFP, increase their firing in the presence of a female, and the amount of this increase correlates with the amount of female-directed singing (Hara et al. 2007, Huang & Hessler 2008, Riters 2011, Yanagihara & Hessler 2006). Intriguingly, VTA neurons in young birds also show enhanced IEG induction in response to tutor song versus that of unfamiliar males, which may reflect social salience of this signal or a possible function of the VTA in song memorization or evaluation during learning (Nordeen et al. 2009).
Interactions between social and vocal behavior are central to human disorders, such as autism spectrum disorders and fronto-temporal dementias, in which disruptions of social and linguistic capacities frequently co-occur. Songbirds provide an opportunity to directly manipulate the relevant behavioral and neural variables to understand their role in normal learning, as well as the nature of social and vocal deficits that arise when experience or activation of neural circuitry is abnormal. Moreover, many bird species have complex social structures and strong social bonds, which are subject to modification and disruption by experience (Elie et al. 2011). This makes songbirds potentially informative not just for social-vocal interaction but also for general brain mechanisms of social interaction, attention, and bonding.
Genetics of speech and social communication
Numerous genes that are implicated in human diseases are enriched in song system nuclei and exhibit differential expression both at specific points in development and during behaviors such as listening to song or singing (Fee & Scharff 2010, Wada et al. 2006, White 2010). This suggests that such genes play conserved roles in circuitry that underlies complex behaviors such as vocal learning, and that songbirds can provide insights into the functions of these genes both normally and in disease.
The gene FoxP2 provides a compelling example of parallels between songbirds and humans. FoxP2 codes for a transcription factor that when mutant in humans, leads to abnormalities in basal ganglia, cerebellum, and cortex, including Broca's area, and resultant language impairment with disordered sequencing of orofacial movements. In songbirds, as in humans, FoxP2 is expressed strongly in the striatum (Haesler et al. 2004, Teramitsu, et al. 2004). In addition, it is dynamically regulated during development and by adult behavioral state (Fee & Scharff 2010, White 2010). Knockdown of FoxP2 in song striatum impairs song learning, is accompanied by decreased spine density of striatal spiny neurons, and results in a highly variable song reminiscent of the abnormal speech of humans with mutant FoxP2 (Haesler et al. 2007, Schulz et al., 2010). Furthermore, the strong expression in several song nuclei of FoxP1, which dimerizes with FoxP2, led to the correct prediction that FoxP1 would also play a role in human speech (Teramitsu et al. 2004).
Many additional genes relevant to speech or other human disorders are differentially expressed in the song system. For example, one of FoxP2's interaction targets is contactin-associated protein2 (CNTNAP-2), a gene linked to autism and language impairment. In rodents, CNTNAP-2 expression is broad and relatively uniform, but in both human fetuses and songbirds, its expression is differentially enhanced in cortico-basal ganglia circuits (Panatoif et al. 2010), suggesting a specialized function in vocal learners. In addition, an analysis of genes based on patterns of coexpression during singing identified ∼2000 genes in Area X that are regulated by singing, including known regulators of synaptic plasticity as well as novel genes (Hilliard et al. 2012). The investigation of these genes will be further aided by the recent sequencing of the zebra finch genome (Warren et al. 2010) and by the generation of songbird brain gene expression libraries (http://www.zebrafinchatlas.org). Thus, songbirds can contribute to human research both by identifying novel genes of translational relevance and by enabling disruption of such genes via transgenesis or viral transfection (Agate et al. 2009, Haesler et al. 2007), in a system with sensitive behavioral and neural assays of vocal and social communication.
Cortico-Basal Ganglia Circuits and Reward and Motor Learning
Cortical-basal ganglia (CBG) circuits are critical for motor and cognitive processes, including control of movements and reward and reinforcement based learning. Accordingly, damage to CBG circuits can contribute to dysfunctions ranging from movement disorders to neuropsychiatric diseases. Yet a detailed understanding of how these circuits function normally and are disrupted in disease is lacking. This may in part be attributable to the very importance of CBG circuits to multiple functions, and the correspondingly complex and heterogeneous properties of neurons and subcircuits within the basal ganglia.
The anterior forebrain pathway (AFP; Fig. 2), is an avian CBG circuit that preserves core features of mammalian CBG circuits but is simplified and dedicated to song control and learning (see sidebar ‘Homology of avian and mammalian cortico-basal ganglia circuitry’). Cortico-striatal neurons in HVC project to Area X (Fig. 2), which has both striatal and pallidal components, and Area X pallidal neurons send inhibitory projections to the thalamic nucleus DLM. These thalamic neurons project onwards to the frontal cortical nucleus LMAN, thus completing a cortical-basal-ganglia thalamo-cortical loop. LMAN projects both to the song motor cortical nucleus RA and back to Area X. One simplified but advantageous feature of this architecture is that the output of the AFP flows primarily through LMAN en route to song premotor nucleus RA. Hence, the output of the song-related basal ganglia circuitry can be monitored and manipulated at a single bottleneck for behaviorally relevant signals.
The role of cortico-basal ganglia circuitry in motor and reinforcement learning
The specific contributions of basal ganglia circuits to learning are particularly evident in songbirds. In juvenile zebra finches, lesions of the cortical outflow nucleus of this circuit, LMAN, cause an abrupt reduction in song variability, and a failure of song to progress towards a good match with the tutor song (Bottjer et al. 1984, Scharff & Nottebohm 1991). Lesions of the striatopallidal Area X also disrupt song learning, but leave song structure highly variable (Scharff & Nottebohm 1991, Sohrabji et al. 1990). In adult finches, lesions of LMAN or Area X have little gross effect on song structure, but subtly reduce rendition-by-rendition variation in song and prevent a variety of forms of adult song plasticity (Brainard & Doupe 2000, Kao et al. 2005, Thompson & Johnson 2007, Williams & Mehta 1999). Similarly, for humans and mammalian models, disruptions of CBG circuits can cause more conspicuous deficits during learning than during execution of well-learned skills (Graybiel 2008).
In motor skill learning, which includes both song and speech, initially variable actions are refined over an extended period of rehearsal, during which sensory feedback is used to gradually improve performance relative to a goal. While multiple processes may contribute to such learning, these likely include reinforcement learning using ‘trial-and-error’ (Sutton & Barto 1998). Such reinforcement learning involves 1) the generation of behavioral variation, sometimes referred to as ‘motor exploration’, 2) the evaluation of which behavioral variants result in better versus worse outcomes, and 3) modifications to preferentially produce those variants that result in better outcomes. Studies in songbirds are helping to reveal the neural mechanisms that underlie these different processes.
Contributions of basal ganglia circuitry to the generation and regulation of behavioral variation
Motor exploration is a crucial substrate for learning, yet how such variability is generated has received relatively little attention from a biological perspective. Studies in songbirds indicate that such variability is actively generated by CBG circuitry. The observation that lesions of LMAN cause an abrupt reduction in song variability prompted speculation that one function of basal ganglia circuitry might be to introduce variability or ‘motor exploration’ into motor output for trial-and-error learning (Bottjer et al. 1984, Doya & Sejnowski 2000, Fiete et al. 2007, Kao & Brainard 2006, Kao et al. 2005, Olveczky et al. 2005, Scharff & Nottebohm 1991). For example, reversible inactivation of LMAN in singing birds, or interfering with LMAN synaptic inputs to RA, decreases variability of both RA activity and song structure within minutes (Olveczky et al. 2005, 2011; Stepanek & Doupe 2010, Warren et al. 2011). This suggests that LMAN introduces variability via synaptic influences on RA target neurons (Mooney 1992, Stark & Perkel 1999).
The ability to modulate behavioral variability is critical for trial-and-error learning, as too little variation can limit learning, and excess variability can interfere with performance. In songbirds, both behavioral and neural variability can be rapidly modulated by the social context in which a bird produces song. When a male sings courtship song (‘directed’ song) to a female, variability in syllable sequencing and in syllable structure is reduced relative to when males sing ‘undirected’ song alone (Kao et al. 2005, Kao & Brainard 2006, Sakata et al. 2008, Sossinka & Bohner 1980). This is especially striking in juveniles, where an immature and variable undirected song can be immediately transformed into nearly perfect adult song by the presence of a female (Kojima & Doupe 2011). These context-dependent changes in song variability are accompanied by dramatic changes in singing-related neural activity within LMAN of adult birds. During undirected song, neural activity is greater, more variable and more likely to occur in bursts than during directed song (Fig. 4; Hessler & Doupe 1999a,b; Kao et al. 2008). In young birds singing alone, LMAN activity is also dominated by bursts, and these are correlated with sound production (Aronov et al. 2008, Olveczky et al. 2005). Interestingly, immediate early gene expression within the AFP is also greater following periods of undirected song than periods of directed song (Jarvis et al. 1998). The elevated activity and variable bursting during undirected song are consistent with the idea that LMAN actively introduces variability into the motor pathway. Indeed, lesions or inactivation of LMAN reduce the variability normally present in undirected song to the level present in directed song (Hampton et al. 2009, Kao et al. 2005, Kao & Brainard 2006, Stepanek & Doupe 2010), suggesting that the ‘default’ state of the adult motor pathway (in the absence of extrinsic perturbations from LMAN) is to produce the low variability songs characteristic of directed singing.
Figure 4.
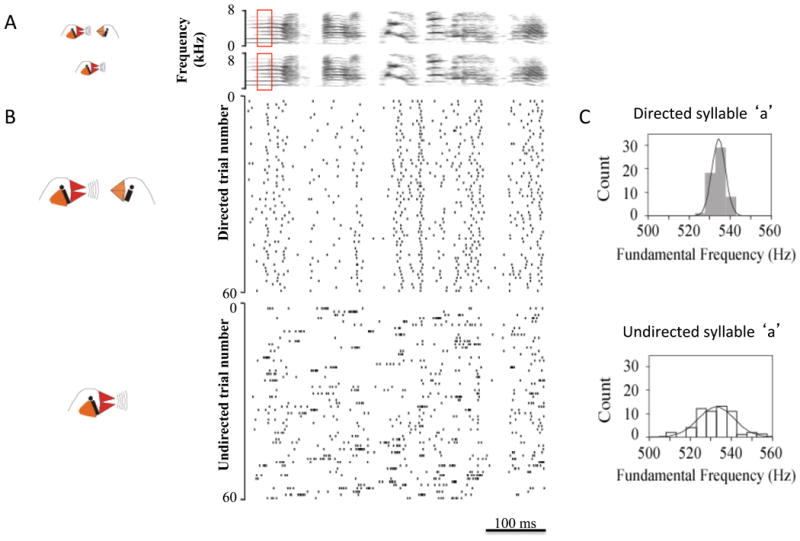
Rapid modulation of behavioral and neural variability by social context.
The active generation of behavioral variation during undirected singing could reflect ‘purposeful’ motor exploration. In principle, such motor exploration might enable trial-and-error refinement of vocalizations to match an acoustic target. While the importance of such variability to juvenile song learning remains to be tested, this variability can indeed subserve trial-and-error learning in adult birds. In particular, while the acoustic structure of ‘crystallized’ adult finch song normally remains very stereotyped, differential reinforcement of subtle variations in adult song can drive rapid changes in song (Andalman & Fee 2009, Charlesworth et al. 2011, Tumer & Brainard 2007). For example, if an aversive white noise burst is played each time a bird sings a particular syllable at a lower pitch (fundamental frequency) but not when the syllable is produced at a higher pitch, the bird gradually adjusts the pitch of the targeted syllable upwards so as to ‘escape’ the white noise (Fig. 5). These data reveal that even the very subtle rendition-to-rendition variations that are present in adult song can be utilized to enable adaptive changes to song structure, and support the idea that undirected song in adult birds may reflect a state of motor ‘practice’ in which motor exploration enables continuing optimization of song. In the case of explicit reinforcement with white noise, this optimization entails shifting song to avoid a disruptive stimulus. Under normal circumstances, this optimization might entail the adjustment of premotor commands to maintain a close match to the tutor song, despite central and peripheral changes to components of the song system that occur during aging or injury.
Figure 5. Trial-and-error learning in adult birdsong.
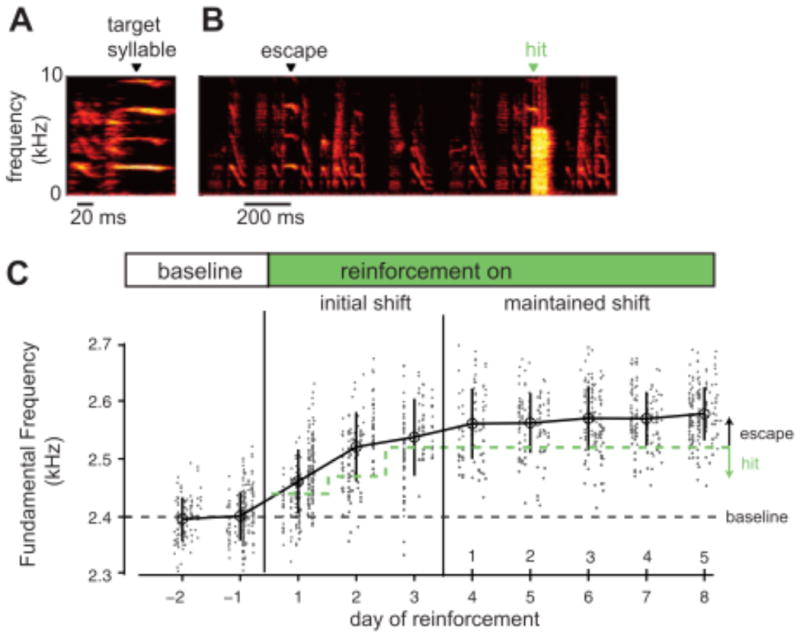
A corollary of the idea that undirected song reflects a practice state, during which AFP-dependent motor exploration enables fine-tuning of song, is the possibility that directed song reflects a performance state in which variability is reduced to produce the current ‘best version’ of song (in the context of courtship). Consistent with this possibility, males are less sensitive to altered auditory feedback when singing directed song than when singing alone (Sakata & Brainard 2009). Moreover, female birds tested with playback of recorded songs, without any additional cues, prefer directed songs over undirected songs (Woolley & Doupe 2008).
In mammalian systems, dopamine (DA) is hypothesized to play a role in the stability versus flexibility of behaviors. In songbirds, DA is one likely modulator of song variability via its actions on the AFP. Dopaminergic neurons send a strong projection to Area X (Gale et al. 2008, Person et al. 2008), and DA release in the AFP is enhanced by the presence of a female, as in rodent striatum (Sasaki et al. 2006). Moreover, both IEG induction and neural activity in the VTA are altered during directed singing, and VTA neurons show physiological changes following directed singing that are consistent with an elevated release of DA within VTA, (Hara et al. 2007, Huang & Hessler 2008, Riters 2011, Yanagihara & Hessler 2006). These data indicate that directed singing, which reduces song variability, engages neuromodulatory circuitry that encodes salience and reward. Finally, blockade of D1 receptors in the AFP decreases the excitability of Area X neurons and the strength of the synaptic inputs onto them (Ding & Perkel 2002, Ding et al. 2003), and correspondingly interferes with modulation of variability by social context, pointing to a causal role of DA in modulating song variability (Leblois et al. 2010).
Together, these data suggest that the AFP is a useful model both for understanding how cortical basal ganglia circuits contribute to behavioral variation important to learning, and for how such variability is dynamically regulated, for example between practice and performance states. More broadly, the context-dependent changes that are readily apparent in songbirds may provide insights into human behavioral disruptions such as tremor, stuttering and other speech disorders, “choking” during performance, and difficulties with movement initiation, each of which can exhibit strong context-dependence in the severity of their manifestations.
Contributions of basal ganglia circuits to implementing adaptive changes to behavior
In mammalian systems, CBG circuits are broadly linked to reward based action selection and learning (Graybiel 2008). In songbirds, studies indicate a central role of the AFP not only in generating variability but also in the subsequent selection and implementation of more successful behavioral variants. These studies generally support an ‘actor-critic’ model of basal ganglia function (Sutton & Barto 1998), schematized in Figure 6 in the context of reinforcement-driven changes to fundamental frequency. According to this model, at baseline (prior to learning), varying patterns of activity from LMAN ‘act’ to drive rendition-to-rendition variation in RA activity and song structure (Andalman & Fee 2009, Doya & Sejnowski 2000, Kao et al. 2005, Olveczky et al. 2005, 2011; Sober et al. 2008, Warren et al. 2011). During initial stages of learning, some patterns of LMAN activity cause syllable renditions that elicit more favorable feedback (i.e. escape from white noise bursts). These adaptive patterns of LMAN activity then are reinforced by favorable feedback from a ‘critic’ so that over time they become more prevalent. Hence, LMAN activity is altered over the course of learning to bias vocal output in an adaptive direction.
Figure 6. Actor-critic model of AFP contributions to reinforcement learning.
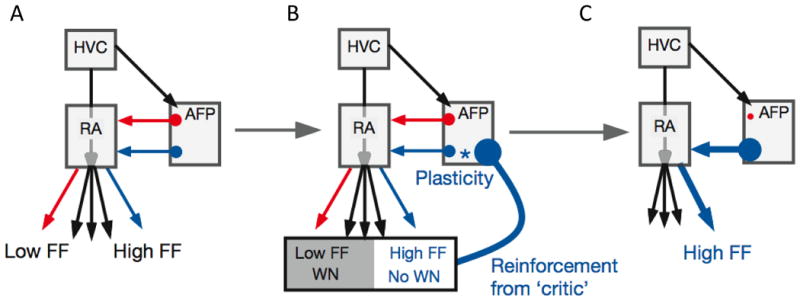
Multiple lines of evidence support this model. First, lesion experiments indicate that signals from LMAN are necessary for learning and can influence song structure (Bottjer et al. 1984, Brainard & Doupe 2000, Kao & Brainard 2006, Thompson & Johnson 2007, Williams & Mehta 1999). Second, recording experiments indicate that LMAN activity during singing is temporally patterned, consistent with the possibility that LMAN provides input to RA that implements changes to song structure at specific times in song (Hessler & Doupe 1999a,b; Kao et al. 2008, Leonardo 2004). Third, lesions of adult Area X strip LMAN of its patterned bursting during singing without decreasing its overall firing rate, and simultaneously eliminate song plasticity in response to altered auditory feedback (Kojima et al. 2013). Finally, alterations in LMAN activity elicited by local microstimulation can acutely alter features of song, including the fundamental frequency and amplitude of individual syllables, indicating that signals from LMAN can acutely bias vocal output in a directed fashion (Kao et al. 2005).
Experiments in adult birds in which explicit reinforcement is used to direct changes to fundamental frequency provide further evidence that such biasing contributes to the initial expression of learning (Andalman & Fee 2009, Warren et al. 2011). During baseline singing (prior to learning), inactivation of LMAN causes a reduction in rendition-to-rendition variability of fundamental frequency for individual syllables, with little shift in the mean fundamental frequency. However, after the fundamental frequency of a targeted syllable is driven upwards by differential reinforcement (Fig. 7), inactivation of LMAN causes not only a reduction in variability but also a reversion of the mean fundamental frequency towards the original baseline (Andalman & Fee 2009, Warren et al. 2011). Infusions of NMDA receptor antagonists into RA, which preferentially block LMAN synapses onto RA neurons, cause a quantitatively matched reversion of fundamental frequency towards baseline (Warren et al. 2011). Hence, a parsimonious account for these findings is that during initial stages of learning, specific patterns of LMAN activity direct adaptive changes to song structure via their action on targets in RA, and reversion following LMAN inactivation reflects the removal of this biasing signal.
Figure 7. Contributions of LMAN to the expression of learning in adult song.
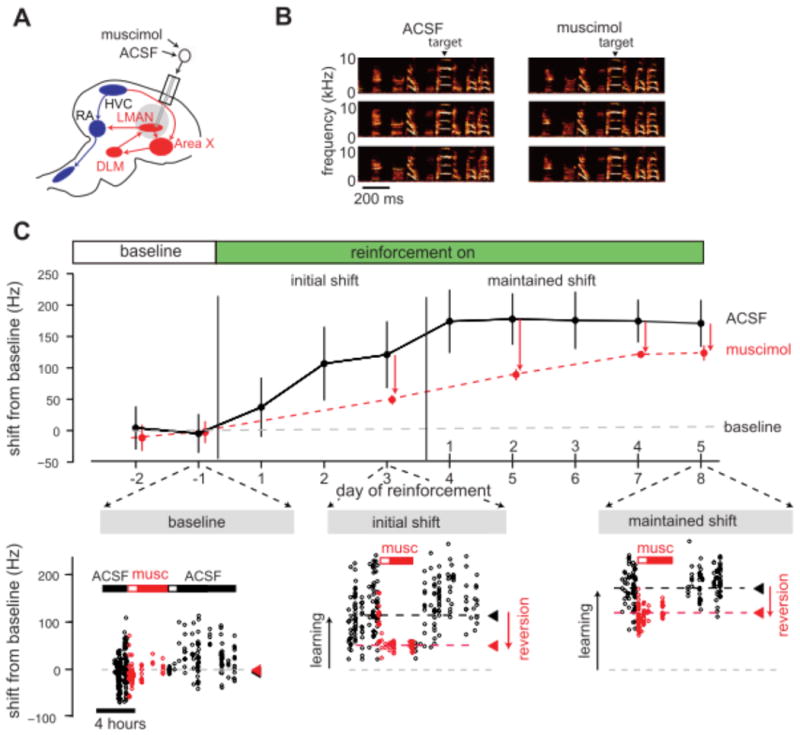
While important aspects of this model remain to be tested, including whether and how LMAN activity changes during adult reinforcement learning, these findings indicate crucial contributions of LMAN to the initial expression of such learning. It remains unclear whether similar contributions obtain for juvenile sensorimotor learning, in which auditory feedback guides developing vocalizations towards an acoustic target. However, in adults, vocal error correction in the absence of external instruction similarly depends on contributions of LMAN to the initial expression of learning (Warren et al. 2011). This suggests that the AFP plays a general role in directing adaptive changes to behavior during initial stages of learning.
This model of AFP contributions to the initial expression of learning implies that there is adaptive plasticity within the AFP or its upstream inputs. To achieve such adaptive plasticity, the nervous system must integrate information about neural activity patterns during a particular syllable rendition with feedback about resultant consequences (i.e. the presence or absence of reinforcement). In particular, because learned changes to song structure are precisely localized to reinforced features of song, there must be a correspondingly detailed record of the timing and structure of associated neural activity (Charlesworth et al. 2011, Tumer & Brainard 2007). The AFP is well-positioned to receive information both about motor actions (an efference copy) from HVC and RA (Goldberg & Fee 2012, Kozhevnikov & Fee 2007, Prather et al. 2008), and feedback about the outcomes resulting from those actions, either as auditory inputs via HVC, or from neuromodulatory centers, such as the VTA, that may provide signals reflecting an evaluation of outcomes relative to expectation. Indeed, synaptic mechanisms of plasticity in Area X are modulated by dopaminergic tone, rendering Area X one likely locus for plasticity underlying initial learning (Ding & Perkel 2004, Fee & Goldberg 2011).
Basal ganglia dependent learning without basal ganglia dependent variation
The actor-critic model outlined above posits that basal ganglia circuitry monitors the consequences of behavioral variations that it generates, and then implements those variations that result in better outcomes. However, experiments in adult songbirds indicate that basal ganglia circuitry can contribute to learning even when it is prevented from generating behavioral variation (Charlesworth et al. 2012). The AFP can be prevented from contributing to song variation by infusing NMDA receptor blockers into RA, which interrupts LMAN to RA synapses (Mooney 1992, Olveczky et al. 2005, Stark & Perkel 1999). During this interruption, variation in the pitch of individual syllables is reduced, but there is still some residual variation that arises from elsewhere, most likely the motor pathway itself. If this residual variation is differentially reinforced, by playing white noise in response to lower versus higher pitched syllables, there is no expression of learning as long as the input from LMAN to RA remains interrupted. Surprisingly, however, as soon as input from LMAN to RA is restored, there is an immediate appearance of appropriate learned changes to song - an upward shift in pitch that is as large as would have occurred if AFP input to RA had remained intact. In contrast, if activity within the AFP itself is disrupted during differential reinforcement, no learning develops. These data indicate that the AFP is required for the acquisition and expression of learning, but that this learning does not require that the AFP act as the source of motor exploration for learning. While normally the AFP may generate variation in song, it can also integrate information about variation arising from elsewhere.
The immediate appearance of learning that is specific to reinforced features of song when the output of the AFP is unblocked indicates the importance of precise and bi-directional coordination between the AFP and the motor pathway in learning (Charlesworth et al. 2012). First, the specificity of this learning implies that the AFP receives detailed information about the song features produced by the motor pathway during reinforcement, likely as a copy of premotor commands in HVC and RA (Fee & Goldberg 2011, Goldberg & Fee 2012; Troyer & Doupe 2000a). Hence, these findings support the suggestion that efference copy signals from motor cortex to basal ganglia circuitry may play a fundamental role in mammalian skill learning (Crapse & Sommer 2008). Second, these results imply that the AFP is able to change its output to specifically implement the song features that were reinforced. Anatomically defined topographic loops that could mediate interactions between cortex and basal ganglia are well-described in both mammals and songbirds (Johnson et al. 1995, Luo et al. 2001, Vates et al. 1997). However, the capacity of the AFP to precisely direct activity within the motor pathway indicates surprisingly fine-scale functional coordination both in the projections from the motor pathway to the AFP and in the projections from the AFP back to the motor pathway. These might be mediated by segregated functional loops between the AFP and the motor pathway, each encoding a particular feature of song, such as high fundamental frequency in a particular syllable. Under normal conditions, with AFP output intact, such functional loops could enable the AFP to amplify and bias specific behavioral features, functions that have been attributed to mammalian basal ganglia circuits (Turner & Desmurget 2010). More generally, these results suggest that CBG circuits such as the AFP can act as a specialized hub that can both regulate and monitor variability in other brain regions and then direct those regions to produce patterns of variation that result in better outcomes.
Consolidation of motor skill learning
Numerous forms of learning, including motor skill learning, are stabilized or ‘consolidated’ over time, often involving a shift in behavioral control from one region to another (Censor et al. 2012). Adult reinforcement learning in the songbird provides a clear example of such consolidation. Although the cortical outflow of the AFP, LMAN, must be active for the initial expression of adult learning, the degree to which learned changes revert towards baseline during LMAN inactivation gradually decreases over time (Andalman & Fee 2009, Warren et al. 2011). Thus, learning that initially requires the AFP is transferred to downstream structures in the motor pathway. One likely locus for this more slowly developing component of learning is at HVC-RA synapses within the motor pathway. These synapses influence the activity of RA neurons (Hahnloser et al. 2002, Long & Fee 2008; see sidebar ‘Motor sequence generation’) and thereby shape the spectral structure of ongoing song (Leonardo & Fee 2005, Sober et al. 2008), and they are a site where LMAN inputs to RA are well-poised to drive synaptic plasticity (Mooney 1992, Sizemore & Perkel 2011). LMAN could instruct adaptive changes in motor circuitry by persistently imposing patterns of RA activity that eventually become engrained by spike timing dependent plasticity mechanisms acting on HVC to RA synapses (Troyer & Doupe 2000b, Swinehart & Abbott, 2005). In this case, LMAN would be a source of signals that drive the formation of longer lasting ‘habits’ but need not be the repository of those habits. Such song consolidation is consistent with other cases in which mammalian striatal activity initially implements adaptive changes in behavior but ultimately drives downstream consolidation of those changes (e.g. Pasupathy & Miller 2005, Censor et al. 2012).
Disorders of basal ganglia circuitry
Given the importance of CBG circuits in motor and reinforcement learning, these pathways are also a locus of many neurological and psychiatric diseases. These include movement disorders, such as Parkinson's and Huntington's diseases, as well as psychiatric disorders, including obsessive-compulsive disorder, psychoses, and addictions. As outlined above, the simplified CBG circuit for song has facilitated identification of ways in which such circuits contribute to normal learning. By extension, the avian CBG circuit may also be useful for investigating disorders of such circuits.
In Parkinson's disease and other human diseases of basal ganglia, disruptions of movement control (and perhaps also of cognitive function) do not reflect merely the absence of adaptive contributions of CBG activity to behavior, but also the addition of abnormal signals that actively corrupt behavior. In Parkinson's disease, neural activity in CBG circuits is excessively synchronized and is dominated by highly abnormal burst-firing (e.g. Hammond et al. 2007). Correspondingly, surgical elimination or alteration (via electrical stimulation) of components of human CBG circuitry can dramatically improve symptoms. Despite the clinical importance of these observations, how abnormal patterns of activity within CBG circuits interfere with normal function and how interventions such as deep brain stimulation exert their beneficial effects (and deleterious side effects) are difficult to investigate in the human brain.
In songbirds, the consequences of abnormal activity within CBG circuits may similarly be much more disruptive than the complete absence of activity. Lesions of LMAN or Area X have relatively modest effects on the structure of adult song (though acute effects can be more disruptive in juvenile birds or in cases where song structure is more variable) (Aronov et al. 2008, Bottjer et al., Kobayashi et al. 2001, Nordeen & Nordeen 1993, Scharff & Nottebohm 1991, Sohrabji et al. 1990). However, if abnormal patterns of activity are artificially introduced into LMAN by electrical or pharmacological perturbations, song is more dramatically disrupted (Hamaguchi & Mooney 2012, Kao et al. 2005). Most strikingly, abnormalities of adult song that develop in response to a variety of insults to the nervous system (including peripheral damage to hearing or production mechanisms, as well as central damage to HVC) can be prevented or even reversed by lesion or inactivation of LMAN (Brainard & Doupe 2000, Nordeen & Nordeen 2010, Thompson et al. 2007, Williams & Mehta 1999).
The importance of CBG circuitry in songbirds for regulating behavioral variability supports a view that aberrant regulation of variability may be an important component of many cortical-basal ganglia diseases. For example, Huntington's disease is characterized by excessive and uncontrolled movement variability. Similarly, Parkinson's disease, which is characterized by decreased movement amplitude and speed, also includes abnormalities in movement variability. Abnormalities of speech in Parkinsons' disease can include low volume and reduced amplitude of articulation, but also include increased variability of articulation, which can contribute significantly to loss of intelligibility (Skodda 2011). Similarly, freezing of gait in Parkinson's disease may follow steps that have increased variability in their size and timing (e.g. Hausdorff et al. 2003). At the neuropsychiatric level, cognitive disturbances that may reflect abnormal basal ganglia and frontal function similarly can be construed as expressing too little variability (as in obsessive compulsive disorder and depression) or too much variability (psychosis or attention deficit disorders).
Parkinson's disease and other basal ganglia disorders are also characerized by difficulty in initiating movements, which can depend strongly on the context of movement generation. Parkinsonian patients who are impaired in spontaneous movement generation are often much better at movements elicited by a sensory cue or an arousing stimulus (Berardelli et al. 2001, Carlson et al. 2012). Parkinson's disease reflects in part a loss of dopaminergic inputs to striatum. In this regard, the bird's normal switch in performance between social contexts resembles the patients' switch between spontaneously generated and cued movements. While birds can sing in isolation (uncued), presentation of a female often elicits immediate initiation of song (cued), consistent with the idea that neuromodulatory changes, such as elevation of dopamine release in Area X, may act to promote movement initiation.
These similarities to findings in human diseases suggest that studies in the songbird can help reveal how pathological activity arising from CBG circuits contributes to movement and neuropsychiatric symptoms, and provide insights into mechanisms whereby these signals can be controlled or corrected. Moreover, as for speech and communication, songbirds can provide a sensitive assay for the cellular, circuit, and behavioral effects of the expression of genes identified in human neurodegenerative and neuropsychiatric diseases.
Adult neurogenesis
One impediment to brain repair in the case of disease or injury, both in CBG circuits and more broadly, is our inability to replace neurons that are lost or damaged. Correspondingly, an increasingly promising approach to enabling adult brain repair is leveraging and extending the capacity for adult neurogenesis and the functional incorporation of new neurons into the adult brain. In songbirds the phenomenon of adult neurogenesis is especially robust, with large-scale neurogenesis in HVC, as well as in high-level auditory structures, striatum, and hippocampus (Nottebohm 2004), and played a key role in overturning the dogma that the adult nervous system does not generate or incorporate new neurons.
There are numerous similarities between avian and mammalian adult neurogenesis, including lifespan and fate restrictions, progenitor cell types, and sensitivity to hormones and other experiential factors, but songbird neurogenesis also exhibits several differences that might be usefully harnessed for human health (Gould 2007, Nottebohm 2004). These include much greater levels of neurogenesis, distributed over more brain regions, than observed in mammals. Comparative molecular and genetic studies in mammals and birds could reveal how to upregulate cell division, migration and functional incorporation rates in mammals in key additional areas. For instance, striatal spiny neurons that are the targets of neurodegenerative diseases such as Huntington's disease are specifically replaced in adult songbirds, raising the possibility that they could be renewed therapeutically (Rochefort et al. 2007). In addition, HVC neurons replaced in adult songbirds are premotor neurons that grow long projections into RA, through a heavily myelinated tract, and insert without disrupting already learned song (Kirn et al. 1991). Understanding the molecular and cellular processes that enable this incorporation in adult songbirds has clear relevance to brain and spinal cord repair in humans.
Finally, songbirds are well-suited to test how new neurons link to learning and behavior. The amount of neurogenesis in songbirds is correlated with periods of learning and repair (Nottebohm 2004), but there is also marked turnover and insertion of new neurons in birds that do not change their song (Tramontin & Brenowitz 1999, Pytte et al. 2012). Manipulating neurogenesis in songbirds could provide sensitive behavioral and neural tests of the role of new neurons. An understanding of how new neurons contribute to sensory and sensorimotor learning might be particularly clear in birds, and will be relevant to understanding and therapeutically manipulating neurogenesis in mammals.
Conclusions
No single animal model is likely to recapitulate all the features of complex neurological and psychiatric diseases, but we argue here that songbirds, with their easily quantifiable learned behavior and dedicated circuit, are a uniquely useful addition to more traditional model systems. Songbirds show remarkable parallels to humans in their links between sensory processing and motor output, their use and regulation of auditory feedback, their neural sensitivity to social signals, and the genes and circuits underlying vocal and motor skill learning. They have provided new insights into cortical-basal ganglia circuitry, a major locus of human neural disease, including into the function of such circuitry in reinforcement learning and the social modulation of behavior. Finally, they provide striking examples of time-limited neural plasticity and of functional insertion of newborn adult neurons into brain circuits, with great potential for mechanistic understanding of these phenomena. It is our hope that enhanced dialogue between songbird and human researchers, with attention to both the similarities and differences between systems, can provide new therapeutic insights.
Contributor Information
Michael S. Brainard, Email: msb@phy.ucsf.edu, UCSF Center for Integrative Neuroscience, Depts of Physiology and Psychiatry, University of California, San Francisco, Box 0444, San Francisco 94143-0444.
Allison J. Doupe, Email: ajd@phy.ucsf.edu, UCSF Center for Integrative Neuroscience, Depts of Psychiatry and Physiology, University of California, San Francisco, Box 0444, San Francisco 94143-0444.
Literature Cited
- Adret P. Operant conditioning, song learning and imprinting to taped song in the zebra finch. Anim Beh. 1993;46:149–59. [Google Scholar]
- Agate RJ, Scott BB, Haripal B, Lois C, Nottebohm F. Transgenic songbirds offer an opportunity to develop a genetic model for vocal learning. Proc Natl Acad Sci USA. 2009;106:17963–7. doi: 10.1073/pnas.0909139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci USA. 2009;106:12518–23. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–4. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Seasonal and hormonal modulation of neurotransmitter systems in the song control circuit. J Chem Neuroanat. 2010;39:82–95. doi: 10.1016/j.jchemneu.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;14(29):12878–85. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham M, Nordeen E, Nordeen K. Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata) Neurobiol Learn Mem. 1996;66:295–304. doi: 10.1006/nlme.1996.0071. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M. Pathophysiology of bradykinesia in Parkinson's disease. Brain. 2001;124:2131–46. doi: 10.1093/brain/124.11.2131. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Altenau B. Parallel pathways for vocal learning in basal ganglia of songbirds. Nat Neurosci. 2009;13:153–5. doi: 10.1038/nn.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–18. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–3. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–6. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Perkel DJ, Osterhout L. Language and birdsong: Introduction to the special issue. Brain Lang. 2010;115:1–2. doi: 10.1016/j.bandl.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt J, Lent K, Beecher M, Brenowitz E. Lesions of the anterior forebrain song control pathway in female canaries affect song perception in an operant task. J Neurobiol. 2000;42:487. doi: 10.1002/(sici)1097-4695(200003)42:4<487::aid-neu9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004;24:7745–53. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Almeida QJ, Franks IM. Using a startling acoustic stimulus to investigate underlying mechanisms of bradykinesia in Parkinson's disease. Neuropsychologia. 2012;51:392–399. doi: 10.1016/j.neuropsychologia.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur J Neurosci. 2005;21:1962–72. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Schmidt MF. What birdsong can teach us about the central noradrenergic system. J Chem Neuroanat. 2010;39:96–111. doi: 10.1016/j.jchemneu.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song: Biological Themes and Variations. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Censor N, Sagi D, Cohen LG. Common mechanisms of human perceptual and motor learning. Nat Rev Neurosci. 2012;13:658–64. doi: 10.1038/nrn3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JD, Tumer EC, Warren TL, Brainard MS. Learning the microstructure of successful behavior. Nat Neurosci. 2011;14:373–80. doi: 10.1038/nn.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth JD, Warren TL, Brainard MS. Covert skill learning in a cortical-basal ganglia circuit. Nature. 2012;486:251–5. doi: 10.1038/nature11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Roy A, Wild JM, Mooney R. Thalamic gating of auditory responses in telencephalic song control nuclei. J Neurosci. 2007;27:10024–36. doi: 10.1523/JNEUROSCI.2215-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousillas H, George I, Henry L, Richard JP, Hausberger M. Linking social and vocal brains: could social segregation prevent a proper development of a central auditory area in a female songbird? PLoS One. 2008;3:e2194. doi: 10.1371/journal.pone.0002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crapse TB, Sommer MA. Corollary discharge across the animal kingdom. Nat Rev Neurosci. 2008;9:587–600. doi: 10.1038/nrn2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cynx J, von Rad U. Immediate and transitory effects of delayed auditory feedback on bird song production. Animal Behaviour. 2001;62:305–12. [Google Scholar]
- Dave AS, Margoliash D. Song replay during sleep and computational rules for sensorimotor vocal learning. Science. 2000;290:812–6. doi: 10.1126/science.290.5492.812. [DOI] [PubMed] [Google Scholar]
- Derégnaucourt S, Mitra PP, Fehér O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433:710–6. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ, Farries MA. Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia. J Neurosci. 2003;23:6086–95. doi: 10.1523/JNEUROSCI.23-14-06086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Dopamine modulates excitability of spiny neurons in the avian basal ganglia. J Neurosci. 2002;22:5210–8. doi: 10.1523/JNEUROSCI.22-12-05210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Perkel DJ. Long-term potentiation in an avian basal ganglia nucleus essential for vocal learning. J Neurosci. 2004;24:488–94. doi: 10.1523/JNEUROSCI.4358-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Ann Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Perkel DJ, Reiner A, Stern EA. Birdbrains could teach basal ganglia research a new song. Trends Neurosci. 2005;28:353–63. doi: 10.1016/j.tins.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Doya K, Sejnowski T. A computational model of avian song learning. In: Gazzaniga M, editor. The New Cognitive Neurosciences. Cambridge, MA: MIT Press; 2000. pp. 469–82. [Google Scholar]
- Dugas-Ford J, Rowell JJ, Ragsdale CW. Cell-type homologies and the origins of the neocortex. Proc Natl Acad Sci. 2012;109:16974–9. doi: 10.1073/pnas.1204773109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elie JE, Soula HA, Mathevon N, Vignal C. Same-sex pair-bonds are equivalent to male-female bonds in a life-long socially monogamous songbird. Behavioral Ecology and Sociobiology. 2011;65:2197–220. [Google Scholar]
- Farries MA, Perkel DJ. A telencephalic nucleus essential for song learning contains neurons with physiological characteristics of both striatum and globus pallidus. J Neurosci. 2002;22:3776–87. doi: 10.1523/JNEUROSCI.22-09-03776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Goldberg JH. A hypothesis for basal ganglia-dependent reinforcement learning in the songbird. Neuroscience. 2011;198:152–70. doi: 10.1016/j.neuroscience.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Scharff C. The songbird as a model for the generation and learning of complex sequential behaviors. ILAR J. 2010;51:362–77. doi: 10.1093/ilar.51.4.362. [DOI] [PubMed] [Google Scholar]
- Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, et al. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS One. 2008;3:e1768. doi: 10.1371/journal.pone.0001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A. Four-month-old infants prefer to listen to motherese. Infant Behav Dev. 1985;8:181–95. [Google Scholar]
- Fiete IR, Fee MS, Seung HS. Model of birdsong learning based on gradient estimation by dynamic perturbation of neural conductances. J Neurophysiol. 2007;98:2038–57. doi: 10.1152/jn.01311.2006. [DOI] [PubMed] [Google Scholar]
- Ford JM, Mathalon DH. Anticipating the future: automatic prediction failures in schizophrenia. Int J Psychophysiol. 2012;83:232–9. doi: 10.1016/j.ijpsycho.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Rodríguez C, Li D, Ball GF, Coleman MJ. Neural mechanisms for the coordination of duet singing in wrens. Science. 2011;334:666–70. doi: 10.1126/science.1209867. [DOI] [PubMed] [Google Scholar]
- Frith CD, Blakemore S, Wolpert DM. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Rev. 2000;31:357–63. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Anatomy of a songbird basal ganglia circuit essential for vocal learning and plasticity. J Chem Neuroanat. 2010;39:124–31. doi: 10.1016/j.jchemneu.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Bentley GE, Ball GF. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J Neurobiol. 2000;42:117–33. doi: 10.1002/(sici)1097-4695(200001)42:1<117::aid-neu11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. Vocal babbling in songbirds requires the basal ganglia-recipient motor thalamus but not the basal ganglia. J Neurophysiol. 2011;105:2729–39. doi: 10.1152/jn.00823.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JH, Fee MS. A cortical motor nucleus drives the basal ganglia-recipient thalamus in singing birds. Nat Neurosci. 2012;15:620–7. doi: 10.1038/nn.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Haesler S, Rochefort C, Georgi B, Licznerski P, Osten P, Scharff C. Incomplete and inaccurate vocal imitation after knockdown of FoxP2 in Songbird basal ganglia nucleus Area X. PLoS Biol. 2007;5:e321. doi: 10.1371/journal.pbio.0050321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, et al. FoxP2 expression in avian vocal learners and non-learners. J Neurosci. 2004;24:3164–75. doi: 10.1523/JNEUROSCI.4369-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahnloser RH, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- Hamaguchi K, Mooney R. Recurrent interactions between the input and output of a songbird cortico-basal ganglia pathway are implicated in vocal sequence variability. J Neurosci. 2012;32:11671–8. doi: 10.1523/JNEUROSCI.1666-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Bergman H, Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends in Neuroscience. 2007;30:357–64. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–16. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey CD, Coen P, Tank DW. Choice-specific sequences in parietal cortex during a virtual-navigation decision task. Nature. 2012;484:62–8. doi: 10.1038/nature10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Schaafsma JD, Balash Y, Bartels AL, Gurevich T, Giladi N. Impaired regulation of stride variability in Parkinson's disease subjects with freezing of gait. Exp Brain Res. 2003;2:187–94. doi: 10.1007/s00221-002-1354-8. [DOI] [PubMed] [Google Scholar]
- Heinrich JE, Nordeen KW, Nordeen EJ. Dissociation between extension of the sensitive period for avian vocal learning and dendritic spine loss in the song nucleus lMAN. Neurobiol Learn Mem. 2005;83:143–50. doi: 10.1016/j.nlm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Singing-related neural activity in a dorsal forebrain-basal ganglia circuit of adult zebra finches. J Neurosci. 1999a;19:10461–81. doi: 10.1523/JNEUROSCI.19-23-10461.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999b;2:209–11. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Hickok G, Houde J, Rong F. Sensorimotor integration in speech processing: computational basis and neural organization. Neuron. 2011;69:407–22. doi: 10.1016/j.neuron.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard AT, Miller JE, Fraley ER, Horvath S, White SA. Molecular microcircuitry underlies functional specification in a basal ganglia circuit dedicated to vocal learning. Neuron. 2012;73:537–52. doi: 10.1016/j.neuron.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde JF, Nagarajan SS. Speech production as state feedback control. Front Hum Neurosci. 2011;5:82. doi: 10.3389/fnhum.2011.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YC, Hessler NA. Social modulation during songbird courtship potentiates midbrain dopaminergic neurons. PLoS ONE. 2008;3:e3281. doi: 10.1371/journal.pone.0003281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado N, Marchman VA, Fernald A. Does input influence uptake? Links between maternal talk, processing speed and vocabulary size in Spanish-learning children. Dev Sci. 2008;11:F31–9. doi: 10.1111/j.1467-7687.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Z, Bergman H. Pathophysiology of the basal ganglia and movement disorders: from animal models to human clinical applications. Neurosci Biobehav Rev. 2008;32:367–77. doi: 10.1016/j.neubiorev.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–88. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Johnson F, Sablan M, Bottjer SW. Topographic organization of a pathway involved with vocal learning in zebra finches. J Comp Neurol. 1995;358:268–75. doi: 10.1002/cne.903580208. [DOI] [PubMed] [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. Journal of Neurophysiol. 2006;96:1441–55. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–43. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–47. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller GB, Hahnloser RH. Neural processing of auditory feedback during vocal practice in a songbird. Nature. 2009;457:187–90. doi: 10.1038/nature07467. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J Neurosci. 1991;11:1756–62. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen DP, Gentner TQ. Mechanisms of song perception in oscine birds. Brain Lang. 2010;115:59–68. doi: 10.1016/j.bandl.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Uno H, Okanoya K. Partial lesions in the anterior forebrain pathway affect song production in adult Bengalese finches. Neuroreport. 2001;12:353–8. doi: 10.1097/00001756-200102120-00034. [DOI] [PubMed] [Google Scholar]
- Kojima S, Doupe AJ. Social performance reveals unexpected vocal competency in young songbirds. Proc Natl Acad Sci USA. 2011;108:1687–92. doi: 10.1073/pnas.1010502108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima S, Kao M, Doupe A. Tasking-related “cortical” bursting depends on basal ganglia input and is linked to vocal plasticity. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1216308110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift fur Tierpsychologie. 1965;22:770–83. [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–83. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–27. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Meltzoff AN. Infant vocalizations in response to speech: vocal imitation and development change. J Acoust Soc Am. 1996;100:2425–38. doi: 10.1121/1.417951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A, Wendel BJ, Perkel DJ. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J Neurosci. 2010;30:5730–43. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Fee MS. Ensemble coding of vocal control in birdsong. J Neurosci. 2005;25:652–61. doi: 10.1523/JNEUROSCI.3036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardo A, Konishi M. Decrystallization of adult birdsong by perturbation of auditory feedback. Nature. 1999;399:466–70. doi: 10.1038/20933. [DOI] [PubMed] [Google Scholar]
- Leonardo A. Experimental test of the birdsong error-correction model. Proc Natl Acad Sci U S A. 2004;101:16935–40. doi: 10.1073/pnas.0407870101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston FS, White SA, Mooney R. Slow NMDA-EPSCs at synapses critical for song development are not required for song learning in zebra finches. Nat Neurosci. 2000;3:482–8. doi: 10.1038/74857. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–86. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Fee MS. Using temperature to analyze temporal dynamics in the songbird motor pathway. Nature. 2008;456:189–94. doi: 10.1038/nature07448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long MA, Jin DZ, Fee MS. Support for a synaptic chain model of neuronal sequence generation. Nature. 2010;468:394–9. doi: 10.1038/nature09514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Ding L, Perkel DJ. An avian basal ganglia pathway essential for vocal learning forms a closed topographic loop. J Neurosci. 2001;21:6836–45. doi: 10.1523/JNEUROSCI.21-17-06836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Birdsong and speech development: could there be parallels? American Scientist. 1970;58:669–73. [PubMed] [Google Scholar]
- Marler P. Innate learning preferences: signals for communication. Dev Psychobiol. 1990;23:557–68. doi: 10.1002/dev.420230703. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–22. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Sims VC, Bottjer SW. Auditory experience refines cortico-basal ganglia inputs to motor cortex via remapping of single axons during vocal learning in zebra finches. J Neurophysiol. 2012;107:1142–56. doi: 10.1152/jn.00614.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Synaptic basis for developmental plasticity in a birdsong nucleus. J Neurosci. 1992;12:2464–77. doi: 10.1523/JNEUROSCI.12-07-02464.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–69. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Moorman S, Mello CV, Bolhuis JJ. From songs to synapses: molecular mechanisms of birdsong memory. Molecular mechanisms of auditory learning in songbirds involve immediate early genes, including zenk and arc, the ERK/MAPK pathway and synapsins. Bioessays. 2011;33:377–85. doi: 10.1002/bies.201000150. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Categorical perception of a natural stimulus continuum: birdsong. Science. 1989;244:976–8. doi: 10.1126/science.2727689. [DOI] [PubMed] [Google Scholar]
- Nelson DA, Marler P. Selection-based learning in bird song development. Proc Natl Acad Sci U S A. 1994;91:10498–501. doi: 10.1073/pnas.91.22.10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Neural song preference during vocal learning in the zebra finch depends on age and state. J Neurobiol. 2005;62:231–42. doi: 10.1002/neu.20087. [DOI] [PubMed] [Google Scholar]
- Nordeen EJ, Holtzman DA, Nordeen KW. Increased fos expression among midbrain dopaminergic cell groups during birdsong tutoring. Eur J Neurosci. 2009;30:662–70. doi: 10.1111/j.1460-9568.2009.06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Deafening-induced vocal deterioration in adult songbirds is reversed by disrupting a basal ganglia-forebrain circuit. J Neurosci. 2010;30:7392–400. doi: 10.1523/JNEUROSCI.6181-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–86. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–58. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ölveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106:386–97. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaitof SC, Abrahams BS, Dong H, Geschwind DH, White SA. Language–related Cntnap2 gene is differentially expressed in sexually dimorphic song nuclei essential for vocal learning in songbirds. J Comp Neurol. 2010;18:1995–2018. doi: 10.1002/cne.22318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller EK. Different time courses of learning-related activity in the prefrontal cortex and striatum. Nature. 2005;433:873–6. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. Unitary IPSPs drive precise thalamic spiking in a circuit required for learning. Neuron. 2005;46:129–40. doi: 10.1016/j.neuron.2004.12.057. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Tremere LA. Control of central auditory processing by a brain-generated oestrogen. Nat Rev Neurosci. 2012;13:521–7. doi: 10.1038/nrn3291. [DOI] [PubMed] [Google Scholar]
- Podos J. Motor constraints on vocal development in a songbird. Anim Behav. 1996;51:1061–70. [Google Scholar]
- Prather JF, Nowicki S, Anderson RC, Peters S, Mooney R. Neural correlates of categorical perception in learned vocal communication. Nat Neurosci. 2009;12:221–8. doi: 10.1038/nn.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–10. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- Prather JF, Peters S, Nowicki S, Mooney R. Persistent representation of juvenile experience in the adult songbird brain. J Neurosci. 2010;30:10586–98. doi: 10.1523/JNEUROSCI.6042-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech spoken language and reading. Neuroimage. 2012;62:816–47. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytte CL, Suthers RA. A bird's own song contributes to conspecific song perception. Neuroreport. 1999;10:1773–8. doi: 10.1097/00001756-199906030-00027. [DOI] [PubMed] [Google Scholar]
- Riede T, Goller F. Peripheral mechanisms for vocal production in birds - differences and similarities to human speech and singing. Brain Lang. 2010;115:69–80. doi: 10.1016/j.bandl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Mello CV, Jarvis ED. Songbirds and the revised avian brain nomenclature. Ann N Y Acad Sci. 2004;1016:77–108. doi: 10.1196/annals.1298.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, London SE, Schlinger BA. Birdsong and the neural production of steroids. J Chem Neuroanat. 2010;39:72–81. doi: 10.1016/j.jchemneu.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV. Pleasure seeking and birdsong. Neurosci Biobehav Rev. 2011;35:1837–45. doi: 10.1016/j.neubiorev.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp Brain Res. 2010;200:223–37. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- Roberts TF, Gobes SMH, Murugan M, Olveczky BP, Mooney R. Motor circuits are required to encode a sensory model for imitative learning. Nat Neurosci. 2012;15:1454–9. doi: 10.1038/nn.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts TF, Tschida KA, Klein ME, Mooney R. Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning. Nature. 2010;463:948–52. doi: 10.1038/nature08759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochefort C, He X, Scotto-Lomassese S, Scharff C. Recruitment of FoxP2-expressing neurons to area X varies during song development. Dev Neurobiol. 2007;67:809–17. doi: 10.1002/dneu.20393. [DOI] [PubMed] [Google Scholar]
- Roy A, Mooney R. Auditory plasticity in a basal ganglia-forebrain pathway during decrystallization of adult birdsong. J Neurosci. 2007;27:6374–87. doi: 10.1523/JNEUROSCI.0894-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Real-time contributions of auditory feedback to avian vocal motor control. J Neurosci. 2006;26:9619–28. doi: 10.1523/JNEUROSCI.2027-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Online contributions of auditory feedback to neural activity in avian song control circuitry. J Neurosci. 2008;28:11378–90. doi: 10.1523/JNEUROSCI.3254-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Brainard MS. Social context rapidly modulates the influence of auditory feedback on avian vocal motor control. J Neurophysiol. 2009;102:2485–97. doi: 10.1152/jn.00340.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata JT, Hampton CM, Brainard MS. Social modulation of sequence and syllable variability in adult birdsong. J Neurophysiol. 2008;99:1700–11. doi: 10.1152/jn.01296.2007. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–4. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F, Cynx J. Conspecific and heterospecific song discrimination in male zebra finches with lesions in the anterior forebrain pathway. J Neurobiol. 1998;36:81–90. [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, McLean J, Goller F. Breathing and vocal control: the respiratory system as both a driver and a target of telencephalic vocal motor circuits in songbirds. Exp Physiol. 2012;97:455–61. doi: 10.1113/expphysiol.2011.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SB, Haesler S, Scharff C, Rochefort C. Knockdown of FoxP2 alters spine density in Area X of the zebra finch. Genes Brain Behav. 2010;9:732–40. doi: 10.1111/j.1601-183X.2010.00607.x. [DOI] [PubMed] [Google Scholar]
- Shank SS, Margoliash D. Sleep and sensorimotor integration during early vocal learning in a songbird. Nature. 2009;458:73–7. doi: 10.1038/nature07615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea SD, Margoliash D. Behavioral state-dependent reconfiguration of song-related network activity and cholinergic systems. Journal of Chemical Neuroanat. 2010;39:132–40. doi: 10.1016/j.jchemneu.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizemore M, Perkel DJ. Premotor synaptic plasticity limited to the critical period for song learning. Proc Natl Acad Sci USA. 2011;108:17492–7. doi: 10.1073/pnas.1104255108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skodda S. Aspects of speech rate and regularity in Parkinson's disease. J Neurol Sci. 2011;310:231–6. doi: 10.1016/j.jns.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Slater PJB, Eales LA, Clayton NS. Advances in the study of behavior. Vol. 18. Academic Press, Inc.; 1988. Song learning in zebra finches (“Taeniopygia guttata”): Progress and prospects; pp. 1–34. [Google Scholar]
- Sober SJ, Brainard MS. Adult birdsong is actively maintained by error correction. Nat Neurosci. 2009;12:927–31. doi: 10.1038/nn.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sober SJ, Wohlgemuth MJ, Brainard MS. Central contributions to acoustic variation in birdsong. J Neurosci. 2008;28:10370–9. doi: 10.1523/JNEUROSCI.2448-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soha JA, Marler P. A species-specific acoustic cue for selective song learning in the white-crowned sparrow. Anim Behav. 2000;60:297–306. doi: 10.1006/anbe.2000.1499. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Solis MM, Doupe AJ. Contributions of tutor and bird's own song experience to neural selectivity in the songbird anterior forebrain. J Neurosci. 1999;19:4559–84. doi: 10.1523/JNEUROSCI.19-11-04559.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis MM, Doupe AJ. Compromised neural selectivity for song in birds with impaired sensorimotor learning. Neuron. 2000;25:109–21. doi: 10.1016/s0896-6273(00)80875-2. [DOI] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra finch poephila guttata castanotis. Zeitschrift fur Tierpsychologie. 1980;53:123–32. [Google Scholar]
- Stark LL, Perkel DJ. Two-stage, input-specific synaptic maturation in a nucleus essential for vocal production in the zebra finch. J Neurosci. 1999;19:9107–16. doi: 10.1523/JNEUROSCI.19-20-09107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanek L, Doupe AJ. Activity in a cortical-basal ganglia circuit for song is required for social context-dependent vocal variability. J Neurophysiol. 2010;104:2474–86. doi: 10.1152/jn.00977.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: an Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Swinehart CD, Abbott LF. Supervised learning through neuronal response modulation. Neural Comput. 2005;17:609–31. doi: 10.1162/0899766053019980. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Lints T, Mitra PP, Nottebohm F. Vocal imitation in zebra finches is inversely related to model abundance. Proc Natl Acad Sci USA. 1999;96:12901–4. doi: 10.1073/pnas.96.22.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernichovski O, Mitra PP, Lints T, Nottebohm F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science. 2001;291:2564–9. doi: 10.1126/science.1058522. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–63. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen FE, Shaevitz SS. Auditory processing of vocal sounds in birds. Curr Opin Neurobiol. 2006;16:400–7. doi: 10.1016/j.conb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J Neurosci. 2007;27:12308–20. doi: 10.1523/JNEUROSCI.2853-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–26. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning I. Efference copy and the learning of song syllables. J Neurophysiol. 2000a;84:1204–23. doi: 10.1152/jn.2000.84.3.1204. [DOI] [PubMed] [Google Scholar]
- Troyer TW, Doupe AJ. An associational model of birdsong sensorimotor learning II. Temporal hierarchies and the learning of song sequence. J Neurophysiol. 2000b;84:1224–39. doi: 10.1152/jn.2000.84.3.1224. [DOI] [PubMed] [Google Scholar]
- Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallized’ adult birdsong. Nature. 2007;450:1240–4. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M. Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol. 2010;20:704–716. doi: 10.1016/j.conb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo-“cortical” loops in the song system of oscine songbirds. Journal of Comparative Neurology. 1997;380:275–90. [PubMed] [Google Scholar]
- Volman SF. Development of neural selectivity for birdsong during vocal learning. J Neurosci. 1993;13:4737–47. doi: 10.1523/JNEUROSCI.13-11-04737.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho TA, Lu K, Ribeiro S, Pinaud R, Vicario D, Mello CV. Noradrenergic control of gene expression and long-term neuronal adaptation evoked by learned vocalizations in songbirds. PLoS One. 2012;7:e36276. doi: 10.1371/journal.pone.0036276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Howard JT, McConnell P, Whitney O, et al. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc Natl Acad Sci. 2006;103:15212–7. doi: 10.1073/pnas.0607098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Brzozowska-Prechtl A, Karten HJ. Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci. 2010;107:12676–81. doi: 10.1073/pnas.1006645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren TL, Tumer EC, Charlesworth JD, Brainard MS. Mechanisms and time course of vocal learning and consolidation in the adult songbird. J Neurophysiol. 2011;106:1806–21. doi: 10.1152/jn.00311.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Clayton DF, Ellegren H, Arnold AP, Hillier LW, et al. The genome of a songbird. Nature. 2010;464:757–62. doi: 10.1038/nature08819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, King AP. Female visual displays affect the development of male song in the cowbird. Nature. 1988;334:244–6. doi: 10.1038/334244a0. [DOI] [PubMed] [Google Scholar]
- White S, Livingston F. Androgens modulate NMDA receptor-mediated EPSCs in the zebra finch song system. J Neurophysiol. 1999;82:2221–34. doi: 10.1152/jn.1999.82.5.2221. [DOI] [PubMed] [Google Scholar]
- White SA. Genes and vocal learning. Brain Lang. 2010;115:21–8. doi: 10.1016/j.bandl.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams H, Mehta N. Changes in adult zebra finch song require a forebrain nucleus that is not necessary for song production. J Neurobiol. 1999;39:14–28. [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. Plos Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM. Early experience shapes vocal neural coding and perception in songbirds. Dev Psychobiol. 2012;54:612–31. doi: 10.1002/dev.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–27. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]