Abstract
Background:
HCV infection is characterized by a tendency towards chronicity. Acute HCV infection progresses to chronic infection in 70% of cases. Hepatitis C virus infection can cause progressive liver injury and lead to fibrosis and eventually cirrhosis. The degree of histologic fibrosis is an important marker of the stage of the disease. One of current standard treatment for CHC infection is the combination of PEG-IFN α and ribavirin.
Objectives:
The aim of the study was to investigate the effect of the therapy with Peginterferon alfa-2a or alfa-2b plus Ribavirin on evolution of liver fibrosis in patients with chronic hepatitis C. Also, our aim was to examine whether there was a difference between the genders in the efficacy of these antiviral therapy. Our goal also was to determine effect of the therapy with Peginterferon alfa-2a or alfa-2b plus Ribavirin on evolution of liver steatosis in patients with chronic hepatitis C.
Patients and Methods:
A retrospective study was made of chronic hepatitis C patients who had been treated from 2005 to April 2014 at the Clinic of Gastroenterohepatology, Clinical Center University of Sarajevo. We reviewed 40 patient medical records to collect demographic, epidemiological and clinical information, as information on liver biopsies that was performed prior to the antiviral therapy and FibroScan® test that was performed after the antiviral therapy. For the processing of data SPSS (Statistical Package for the Social Sciences Program) for Windows, ver. 21.0 statistical software was used. Comparisons between qualitative and quantitative variables were performed using the Student t-test. Mann Whitney U test was used to compare differences in variables such as fibrosis stage and steatosis grade. A value of p<0.05 was considered as statistically significant.
Results:
After treatment, there was a statistically significant increase in the number of patients with no fibrosis (p<0.05). There was no statistically significant reduction in the number of patients with cirrhosis (F4) (p>0.05). There was significantly higher decrease of fibrosis progression at the patients that were in an mild-to-moderate fibrosis (F1/F2/F3), patients that were in advanced stage of fibrosis (F4) at the time of the pre-treatment did not have a statistically significant fibrosis reduction. We found significant association in evolution of fibrosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2a (12,5) kD with ribavirin (p< 0.05). We also found significant association in evolution of steatosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2a (12,5) kD with ribavirin (p < 0.05). There was statistically significant differences (p<0.05) between genders within fibrosis qualitative evolution.
Conclusions:
There were significant regression of fibrosis especially at the patients that were in an mild-to-moderate fibrosis (F1/F2/F3), patients that were in advanced stage of fibrosis (F4) at the time of the pre-treatment did not have a statistically significant fibrosis reduction after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin. Our results showed significant improvement in steatosis in patients infected with HCV after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin. Those results provides further evidence for direct involvement of HCV and antiviral therapy in the pathogenesis of hepatic steatosis. Female gender showed a higher degree of fibrosis reduction.
Keywords: Antiviral Therapy, Regression
1. BACKGROUND
The hepatitis C virus (HCV) is a major factor in the development of chronic liver disease worldwide. HCV infection is characterized by a tendency towards chronicity. Acute HCV infection progresses to chronic infection in 70% of cases. Chronic hepatitis C is a major health problem with an estimated prevalence of 1.6%–2% worldwide. It is estimated that in Europe, more than 9 million chronic carriers are progressing to the late complications of HCV with approximately 86,000 deaths per year (1, 2).
Because of their high genetic variability, HCV has the ability to avoid immune response of the host. This virus is not directly cytopathic and liver damage are mostly caused by immune-mediated mechanisms, predominantly T helper cell response (3). Hepatitis C virus infection can cause progressive liver injury and lead to fibrosis and eventually cirrhosis. The degree of histologic fibrosis is an important marker of the stage of the disease (3). Most of the complications related to chronic infection occurs in patients who have established cirrhosis (4-6). Treatments that could stop or diminish the progression of fibrosis would theoretically be beneficial (7).
One of the current standard treatment for CHC infection especially in the low income countries, is the combination of PEG-IFN α and ribavirin. The efficacy endpoint of hepatitis C treatment is the ‘sustained virological response’ (SVR), defined by the absence of detectable HCV RNA in serum, as assessed by an HCV RNA assay with a lower limit of detection of 1000 copies/ml or less, 6 months after the end of treatment (8).
The IFNs are a family of proteins that are naturally produced by the cells of the immune system which presents antiviral, anti proliferative and immunomodulatory activity (9). Its mechanism of biological action occurs through the activation of specific genes, influencing cell growth and division, as well as modulating some immune system activities. Therefore, IFN-α has an indirect antiviral effect on HCV (10). Commercially, IFN-α is produced by means of recombinant DNA techniques and is available in preparations of two distinct subtypes (IFN-α 2a or IFN-α 2b)(11). The only difference between IFN-α 2a and IFN-α 2b is in the amino acid present at position 23 of the protein: IFN-α 2a has a lysine at that position, whereas IFN-α 2b has an arginine (12). Pegylated IFNs (PEG-IFNs) are produced through the binding of an inert molecule of polyethylene glycol to the recombinant IFN-α, thus reducing the renal clearance, altering the metabolism and increasing the half-life of the IFN molecule, although maintaining all of its immunostimulatory characteristics (13). After the binding with its specific receptor (interferon-α/β receptor, IFNAR) on the surface of the target cells, IFN-α activates an intracellular signaling cascade, which takes the induction of IFN-stimulated genes (ISGs), encoding antiviral proteins such as 2’5’-oligoadenylate synthetase (2’5’OAS), protein kinase RNA, and Mx protein establishing a non-virus-specific antiviral state inside the cell (12). Ribavirin is a synthetic nucleoside which is structurally similar to guanosine (14). Ribavirin enters into the eucaryotic cells rapidly and, after it undergoes intracellular phosphorylation, shows virustatic activity against a broad spectrum of DNA and RNA viruses (14). The exact mechanism of the antiviral action of ribavirin has not yet been totally elucidated (15). However, some studies suggest the following possible mechanisms: direct inhibition of HCV replication, inhibition of the enzyme inosine monophosphate dehydrogenase of the host, induction of mutagenesis in the viral RNA, immunomodulation by the induction of a T helper 1 (Th1)-type immune response (15).
2. OBJECTIVES
The aim of the study was to analyze the effect of the therapy with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin on evolution of liver fibrosis in patients with chronic hepatitis C.
Also, our aim was to examine whether there was a difference between the genders in the efficacy of these antiviral therapy.
Our aim also was to determine effect of the therapy with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin on evolution of liver steatosis in patients with chronic hepatitis C.
3. PATIENTS AND METHODS
A retrospective study was made in a group of chronic hepatitis C patients treated from 2005 to April 2014 at the Clinic of Gastroenterohepatology, Clinical Center University of Sarajevo. We reviewed 40 patient medical records to collect demographic, epidemiological and clinical information, as information on liver biopsies and FibroScan® test.
The diagnosis of chronic hepatitis C was based on elevated transaminase activity in serum testing positive for anti-HCV antibodies (third-generation ELISA) and the presence of HCV RNA (PCR) in the serum, as well as histological evidence of chronic hepatitis. To determine the HCV genotype, Versant kit (Lipa)® was used. The inclusion criteria were:
a) previous antiviral treatment for 24 or 48 weeks, depending on genotype, with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin using standardized doses and ways of administration;
b) a liver biopsy performed and reviewed by an pathologist, performed within six months prior to treatment;
c) examination with FibroScan® after treatment.
Patients associated with other diseases (acute, chronic or autoimmune either the liver or other organ systems) were excluded from the study.
Liver biopsies were analyzed always by the same pathologist from the Department of Pathology, Clinical Center University of Sarajevo applying the METAVIR fibrosis score. Steatosis was scored using the Brunt grading system in which steatosis is graded 0 to 3 based on percentage of hepatocytes involved.
3.1. Statistical analysis
For the processing of data SPSS (Statistical Package for the Social Sciences Program) for Windows, ver. 21.0 statistical software was used. Descriptive statistical analysis was used, and distributions of categorical variables were compared using Fisher tests. Continuous data were expressed as means, medians and standard deviation (SD). Comparisons between qualitative and quantitative variables were performed using the Student t-test. Mann Whitney U test was used to compare differences in variables such as fibrosis stage and steatosis grade. A value of p <0.05 was considered as statistically significant.
4. RESULTS
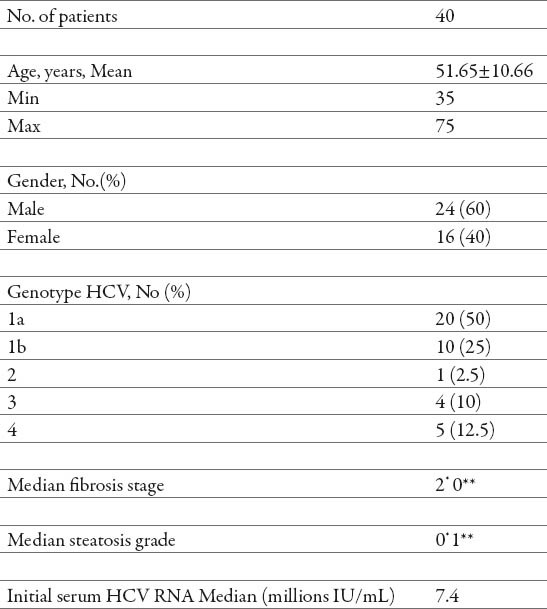
Forty patients met the inclusion criteria. Table 1 shows the main characteristics of the 40 patients included in this study.
Table 1.
Characteristics of the patients. pre-treatment **post-treatment
Tables 2 and 3 summarizes the information about the evolution in fibrosis, after treatment with PEG-IFN α and ribavirin.
Table 2.
Pre-treatment fibrosis stages
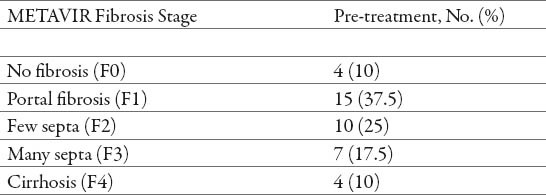
Table 3.
Post-treatment fibrosis stages
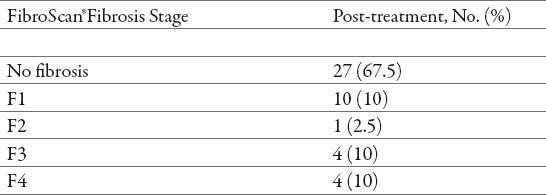
After treatment, there was a statistically significant increase in the number of patients with no fibrosis (p<0.05), and a significant reduction in the number of patients with fibrosis grade (F1), (F2) and (F3) (p<0.05). There was no statistically significant reduction in the number of patients with cirrhosis (F4) (p> 0.05) (Tables 2 and 3). After the treatment, the most frequent were patients with no fibrosis (67.5%), a minimum frequent were patients with grade (F2) (2.5%), while patients with fibrosis grade (F1), grade (F3) and cirrhosis (F4) had the same frequency (10%) (Tables 2 and 3).
There was significantly higher decrease of fibrosis progression at the patients that were in an mild-to-moderate fibrosis (F1/F2/F3), patients that were in advanced stage of fibrosis (F4) at the time of the pre-treatment did not have a statistically significant fibrosis reduction (Table 2 and 3).
Thirty-eight patients (95%) showed stabilization or regression of fibrosis (no progression) at the FibroScan® test performed after the treatment (Table 4). Before treatment, the vast majority of patients had evidence of fibrosis progression (fibrosis stage > 0) (Table 2). In contrast, after the treatment, most patients presented a tendency towards regression (Tables 3 and 4), therefore we found significant association in evolution of fibrosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin (p< 0.05) (Table 4).
Table 4.
Post-treatment fibrosis qualitative evolution.
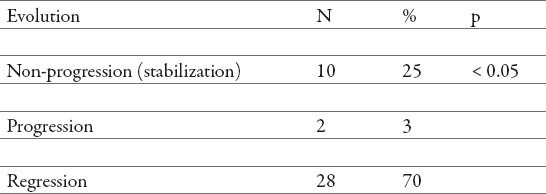
Table 5 summarize the information about the evolution in steatosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin.
Table 5.
Post-treatment steatosis qualitative evolution.
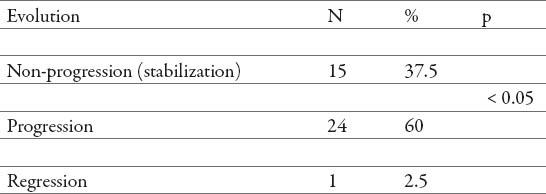
Twenty-four patient (60%) showed progression of steatosis (Table 5) after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin. Most patients showed a tendency towards progression, therefore we found significant association in evolution of steatosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin (p < 0.05) (Table 5), with limitation of the data, since presence of steatosis was measured by two different methods.
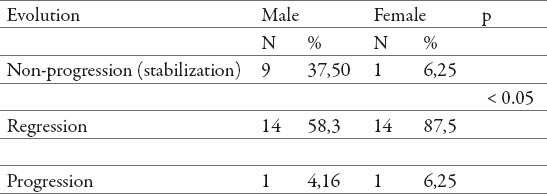
We also found the statistically significant differences (p<0.05) between genders in fibrosis qualitative evolution (Table 6), female gender showed a higher degree of fibrosis reduction.
Table 6.
Fibrosis qualitative evolution according to gender
5. DISCUSSION
Regression of liver fibrosis has been a main topic of research and discussion in the community of liver experts for decades. Regression or reversibility of fibrosis has been perceived to be not much more than a myth. However, recent data have convincingly demonstrated the occurrence of fibrosis regression in a wide spectrum of chronic liver diseases, including viral chronic hepatitis. In our study, the vast majority of patients had evidence of fibrosis before treatment (fibrosis stage > 0). In contrast, after treatment, most patients displayed a tendency towards regression, i.e. 38 patients showed stabilization or regression of fibrosis (no progression) at the FibroScan® test (Table 3). Therefore, we found significant association in evolution of fibrosis after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin (p=0.000, p<0.05) (Table 4). Similar results have been found in other studies (16, 17, 18,19) i.e. there is growing clinical evidence that fibrosis can regress and possibly even resolve in a number of liver diseases. It is difficult to believe from a clinical perspective that established cirrhosis may resolve to a pre-cirrhotic state. There are however, a number of studies reporting evidence of such reversal (20, 21, 22, 23). This evidence is predominantly based on changes in histological stage.
The observation of hepatic fibrosis regression in 28 patients is a very important piece of information, since spontaneous regression of hepatic fibrosis is not usually seen in patients without treatment. We speculate that the regression in fibrosis is due to an anti fibrogenic effect of interferon, regardless of its antiviral action. Poynard et al. demonstrated that hepatitis C is indeed a progressive fibrotic disease, which is the reason for its morbidity and mortality (24). Therefore, a therapeutic intervention to decrease or revert hepatic fibrosis progression will probably have a relevant clinical impact. On the other hand, the relevant fibrosis stabilization rates in patients with mild-to-moderate, as well as advanced fibrosis (10%), are probably due to the anti-inflammatory action of ribavirin, rebetol and interferon, with the possibility that the ribavirin and rebetol indirectly facilitated the interferon anti fibrogenic effect through its anti-inflammatory action.
In our study, steatosis was significantly increased after antiviral therapy (p<0.05). Our results are in agreement with the results of previous studies (25, 26) that have determined significant progression in steatosis in patients infected with HCV. This provides further evidence for direct involvement of HCV and antiviral therapy in the pathogenesis of hepatic steatosis. We believe that the low grade of steatosis before therapy is responsible for the efficacy of antiviral therapy because steatosis negatively influences the rate of response to antiviral treatment, as confirmed by large clinical trials. Management of steatosis in chronic hepatitis C requires knowledge of its pathogenesis and may involve both life-style changes and pharmacological interventions, although the latter remain largely experimental and it remains to be explored in future studies.
The reasons for statistically significant differences (p<0.05) between genders within fibrosis qualitative evolution are not known, but the reduction may be the cause of the estrogen-mediated inhibition of IL-6 production by the Kupfer cells in women, which reduces damage to the liver. Also, males usually shows a greater exposure to harmful life-style factors such as smoking and alcohol and higher rate of hepatitis virus infections.
6. CONCLUSIONS
In patients with chronic hepatitis C, there were significant regression of fibrosis especially in a group of the patients with mild-to-moderate fibrosis (F1/F2/F3), patients that were in advanced stage of fibrosis (F4) at the time of the pre-treatment did not have a statistically significant fibrosis reduction after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin.
Our results showed significant increase in steatosis in patients infected with HCV after treatment with PEG-IFN α2a (40) kD and PEG-IFNα2b (12,5) kD with ribavirin. This provides further evidence for direct involvement of HCV and antiviral therapy in the pathogenesis of hepatic steatosis. Those results should be taken in to the consideration, since the presence of liver steatosis was analyzed by two different methods.
Female gender showed a higher degree of fibrosis reduction.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Méndez-Sánchez N, Villa AR, Zamora-Valdés D, et al. Worldwide mortality from cirrhosis. Annals of Hepatology. 2007;6(3):194–195. [PubMed] [Google Scholar]
- 2.Esteban JI, Sauleda S, Quer J. The changing epidemiology of hepatitis C virus infection in Europe. J Hepatol. 2008 Jan;48(1):148–162. doi: 10.1016/j.jhep.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg W. Mechanisms of immune escape in viral hepatitis. Gut. 1999;44:759–764. doi: 10.1136/gut.44.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Opolon P For the OBSVIRC, METAVIR, CLINIVIR and DOSVIRC groups. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet. 1997;349:825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Mason A. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;34:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 6.Deuffic S, Buffat L, Poynard T, Valleron AJ. Modeling the hepatitis C virus epidemic in France. Hepatology. 1999;29:1596–1601. doi: 10.1002/hep.510290528. [DOI] [PubMed] [Google Scholar]
- 7.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis Cvirus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. doi: 10.1056/NEJM199908193410802. [DOI] [PubMed] [Google Scholar]
- 8.Sobesky R, Mathurin P, Charlotte F, et al. Modeling the impact of interferon alfa treatment on liver fibrosis progression in chronic hepatitis C: a dynamic view. Gastroenterology. 1999;116:378–386. doi: 10.1016/s0016-5085(99)70135-6. [DOI] [PubMed] [Google Scholar]
- 9.Manns MP, McHutchison JG, Gordon SC, et al. PEG-Interferon alfa-2b in combination with ribavirin compared to interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C. Lancet. 2001;358:958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 10.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436(7053):967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 11.Souvignet C, Lejeune O, Trepo C. Interferon-based treatment of chronic hepatitis C. Biochimie. 2007;89(6-7):894–898. doi: 10.1016/j.biochi.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Foster GR. Review article: pegylatedinterferons: chemical and clinical differences. Aliment Pharmacol Ther. 2004;20(8):825–830. doi: 10.1111/j.1365-2036.2004.02170.x. [DOI] [PubMed] [Google Scholar]
- 13.Pestka S. The human interferon alpha species and receptors. Biopolymers. 2000;55(4):254–287. doi: 10.1002/1097-0282(2000)55:4<254::AID-BIP1001>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Reddy KR, Wright TL, Pockros PJ, et al. Efficacy and safety of pegylated (40-kd) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33(2):433–438. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 15.Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker WB. Metabolism and antiviral activity of ribavirin. Virus Res. 2005;107(2):165–171. doi: 10.1016/j.virusres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Abe S, Tabaru A, Ono M, et al. High-dose interferon-a therapy lowers the levels of serum fibrogenesis markers over 5 years in chronic hepatitis C. Hepatology Research. 2003;25:22–31. doi: 10.1016/s1386-6346(02)00203-6. [DOI] [PubMed] [Google Scholar]
- 18.Pol S, Carnot F, Nalpas B, et al. Reversibility of hepatitis C virus-related cirrhosis. Human Pathol. 2004;35(1):107–112. doi: 10.1016/j.humpath.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Prado K, Patzina R, Bergamaschi D, Focaccia R. Histological Response Study of Chronic Viral Hepatitis C Patients Treated With Interferon Alone or Combined With Ribavirin. BJID. 2008;12(5):362–367. doi: 10.1590/s1413-86702008000500004. [DOI] [PubMed] [Google Scholar]
- 20.Mallet V, Gilgenkrantz H, Serpaggi J, et al. Cirrhosis Regression in Chronic Hepatitis C: An Old Tale With a New Ending. Gastroenterology. 2009;136(4):1447–1449. doi: 10.1053/j.gastro.2009.02.033. [DOI] [PubMed] [Google Scholar]
- 21.Germani G, Hytiroglou P, Fotiadu A, et al. Assessment of Fibrosis and Cirrhosis in Liver Biopsies. Semin Liver Dis. 2011;31(1):82–90. doi: 10.1055/s-0031-1272836. [DOI] [PubMed] [Google Scholar]
- 22.Palumbo E. Pegylated Interferon and Ribavirin Treatment for Hepatitis C Virus Infection. Ther Adv Chronic Dis. 2011;2(1):39–45. doi: 10.1177/2040622310384308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellis EL, Mann DA. Clinical evidence for the regression of liver fibrosis. J Hepatol. 2012;56(5):1171–1180. doi: 10.1016/j.jhep.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 24.Poynard T, Moussalli J, Munteanu M, et al. Slow regression of liver fibrosis presumed by repeated biomarkers after virological cure in patients with chronic hepatitis C. Journal of Hepatology. 2013;59(4):675–683. doi: 10.1016/j.jhep.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Vukobrat-Bijedic Z, Mehmedovic A, Redzepovic A, Gogov B. Use of Serum Levels of Proinflammatory Cytokine IL-1a in Chronic Hepatitis B. Med Arh. 2014 Feb;68(2):94–97. doi: 10.5455/medarh.2014.68.94-97. doi:10.5455/medarh.2014.68.94-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castéra L, Hézode C, Roudot-Thoraval F, et al. Effect of antiviral treatment on evolution of liver steatosis in patients with chronic hepatitis C: indirect evidence of a role of hepatitis C virus genotype 3 in steatosis. Gut. 2004;53(3):420–424. doi: 10.1136/gut.2002.009936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung CH, Kuo FY, Wang JH, et al. Impact of steatosis on long-term histological outcome in chronic hepatitis C after antiviral therapy. Antivir Ther. 2006;11(4):483–489. [PubMed] [Google Scholar]


