Abstract
Introduction:
Acrylamide is very toxic to various organs and associated with significant increase of oxidative stress and depletion of antioxidants. Alpha-lipoic acid enhances cellular antioxidant defense capacity, thereby protecting cells from oxidative stress.
Aim of the study:
This study aimed to evaluate the protective role of alpha-lipoic acid on the oxidative damage induced by acrylamide in testicular and epididymal tissues.
Material and methods:
Forty adult male rats were divided into four groups (10 rats each). Control group; acrylamide treated group administered acrylamide 0.05% (w/v) in drinking water for 21 days; alpha-lipoic acid group received basal diet supplemented with 1% alpha-lipoic acid and forth group was exposed to acrylamide and treated with alpha-lipoic acid at the same doses and treatment regimen mentioned before.
Results:
The administration of acrylamide resulted in significant elevation in testicular and epididymal malondialdehyde level (MDA) and significant reduction in the level of reduced glutathione (GSH) and the activities of glutathione-S-transferase (GST), glutathione peroxidase (GPX) and glutathione reductase (GR). Also, acrylamide significantly reduced serum total testosterone and progesterone but increased estradiol (E2) levels. Treatment with alpha-lipoic acid prior to acrylamide induced protective effects and attenuated these biochemical changes.
Conclusion:
Alpha-lipoic acid has been shown to possess antioxidant properties offering promising efficacy against oxidative stress induced by acrylamide administration.
Keywords: Acrylamide, lipoic acid, testis, testosterone, oxidative stress
1. INTRODUCTION
Acrylamide (ACR) is monomeric precursor for many industrially important polymeric products; however, the incidental formation of ACR during the cooking process of many common starchy foods is the primary source of human exposure in the range of 1 µg/kg/day (1). The likely major pathway in the formation of acrylamide in food cooked at high temperature involves the Maillard browning reaction between reducing sugars and asparagine, an amino acid present in potatoes and cereals (2).
In fact, the Maillard reaction is considered desirable in the production of starchy food because it imparts a palatable taste, even though it reduces the bioavailability of some amino acids, including taurine and lysine and increase ACR formation (3).
Acrylamide is metabolized by hepatic CYP 2E1 produces an epoxide, glycidamide (GA), which is reactive toward protein and nucleic acid nucleophiles (4). GA had been reported to be 100-1000 times more reactive with DNA than acrylamide, and considered as genotoxic in a variety of test systems (5).
Many studies were done to test the effects of oral acrylamide administration on the testicular functions and the effects include: severe testicular damage, significant reduction in mating, fertility, and pregnancy by formation of abnormal sperm and a decrease in sperm count which could be a result of alkylation of SH groups in the sperm nucleus and tail, depletion of GSH, or DNA damage in the testis (6).
Testicular toxicity had been reported in rodents exposed to ACR, changes in gonadal and pituitary hormones had been associated with histopathological and gene expression changes (7). One effective approach against environmental agents-induced toxicity is prevention, so identification and development of effective chemopreventive agents that block activation or enhance detoxification of environmental agents is an important aspect.
Alpha-Lipoic acid (LA) is a naturally occurring nutraceutical, whose therapeutic effect has been related to its antioxidant activity and its ability to repair oxidative damage (8). It is readily distributed and accumulates in several tissues where it is rapidly converted to its more potent antioxidant form dihydrolipoic acid (9). Because of its small size and high lipophilicity, it crosses biological membranes easily and quenches free radicals in both lipid and aqueous environments (10).
Pretreatment of spermatic cord torsion with LA significantly protects against ischemia/reperfusion injury by decreasing oxidative stress (11). LA is used as a protector against lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro (12).
The idea that free radical scavengers are protective against pollutant induced toxicity (13) has provoked the present study to investigate the potential protective effect of LA against acrylamide-induced oxidative toxicity in the reproductive system as represented by changes in sex hormone levels, and oxidative stress and antioxidant status in male rats. To the best of the author’s knowledge, the effect of LA on acrylamide treatment with respect to reproductive markers has not yet been established.
2. MATERIAL AND METHODS
2.1. Chemicals and Reagents
Acrylamide (CAS number 79-06-1) and alpha-lipoic acid were obtained from Sigma Chemicals Co. (St. Quentin-Fallavier, France), 5,5- dithiobis-2-nitrobenzoic acid, and 1- chloro-2,4-dinitrobenzene, reduced glutathione, thiobarbituric acid, and butylated hydroxytoluene were purchased from Fluka (Buchs, Switzerland), and testosterone, progesterone and estradiol hormones ELISA kit from Cayman Chemical company. All the other reagents were of analytical, high-performance liquid chromatography, or the best available pharmaceutical grade.
2.2. Animals and experimental design
Forty Male rats weighing 210 ± 7 g were housed 10 per cage with wood shavings as bedding, and were maintained under a controlled environment at 25 ± 2°C, 55 ± 5% relative humidity, and a 12 h/12 h light/dark cycle throughout the experimental period. The animal experiments were carried out in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, and the study protocol was approved by the local authorities. After 2 weeks of acclimatization, the rats were randomly divided into four groups of 10 rats each. Group I was the control group received normal basal diet, Group II and Group IV were administered acrylamide 0.05% (w/v) in drinking water for 21 days, group IV received beside acrylamide alpha-lipoic acid 1% mixed with diet 7 day before and along with acrylamide treatment, and group III were received alpha-lipoic acid 1% mixed with diet for 28 days.
Twenty-four hours after the last administration of alpha-lipoic acid or ACR, the rats were anesthetized with diethyl ether. Then, blood samples were collected from the inner canthus of the eye by heparinized capillary tube into clean test tubes. Following standing in room temperature for at least 30 min, the blood was centrifuged at 3400 xg for 10 min and the serum was separated, transferred to eppendorf tubes, and stored at -20°C prior till biochemical analysis. Immediately after the collection of blood samples, the animals were euthanized, and their testis and epididymis were quickly excised, rinsed in ice-cold saline and used immediately or stored frozen at -80°C until analysis.
2.3. Hormonal assays
ELISA procedure was used for quantitative determination of serum total testosterone, progesterone and estradiol levels (Dima Gesellschaft Fur Diagnostika [GmbH], Germany) according to manufacturer’s instructions.
2.4. Indices of antioxidant and oxidative stress
Tissue homogenates were separately prepared from frozen testis and epididymis samples in 10 volumes of 0.1 M Tris–EDTA buffer (pH 7.4) and centrifuged at 8000 xg for 30 min at 4°C. Aliquots of the supernatant were utilized for the spectrophotometrical assessment of the levels of the following: lipid peroxidation (LPO), assessed as the production of the thiobarbituric acid reactive substances (TBARS) in the presence of BHT (14); reduced glutathione, by using Ellman’s reagent (15); glutathione S-transferase (GST, EC 2.5.1.18) activity, as the rate of GSH conjugation of CDNB (16); glutathione peroxidase (GPx, EC 1.11.1.9) activity was determined using reduced glutathione and cummene hydroperxide as substrate by the modified method of (17) and glutathione reductase (GR) activity was measured according to the method of (18).
2.5. Histopathology
The excised testes and epididymis were fixed in Bouin’s solution, for 24 h and processed using standard laboratory procedures for histology. The tissue was embedded in paraffin blocks, sectioned perpendicular to the longest axis of the testis with 5-cm thickness, and stained routinely with hematoxylin and eosin. Stained sections were examined using light microscopy at power 200 and 400 for detection of histopathological changes (19).
2.6. Statistical analysis
Data were analyzed using the SPSS package. Results are expressed as mean ± SEM with the experiment repeated at least three times. Statistical evaluations were done using the analysis of variance (ANOVA). A p value of < 0.05 was considered significant.
3. RESULTS
3.1. Serum sex hormone levels
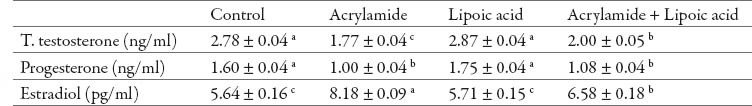
Compared to control rats, acrylamide treated rats exhibited a significant decrease in serum total testosterone and progesterone levels while serum estradiol was significantly increased (P<0.05; Table 1). Alpha-lipoic acid non-significantly increased serum total testosterone, progesterone and estradiol levels. Administration of alpha-lipoic acid together with acrylamide significantly increased serum total testosterone level, non-significantly increased serum progesterone level and significantly increased serum estradiol level as compared to acrylamide treated rats.
Table 1.
Effect of acrylamide and lipoic acid on male reproductive hormones of rats. Values are expressed as mean ± SE The values with different letter within the same raw significantly differ at P < 0.05
3.2. Antioxidant and oxidative stress indices
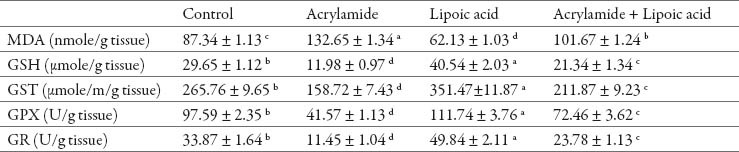
In relation to control rats, acrylamide treated rats had greater level of MDA in both testis and epididymis (P<0.05). Acrylamide caused a reduction in enzymatic activities of GST, GPX and GR and level of GSH in both organs (Table 2, 3). Alpha-lipoic acid treatment resulted in significant reduction in MDA level with induction in enzymatic and non enzymatic antioxidant status in examined tissues (P<0.05). The rats treated with lipoic acid and acrylamide had a significant reduction in MDA level and increased in GST, GPX and GR enzymatic activities and GSH content in testis and epididymis as compared to acrylamide treated rats (Table 2, 3).
Table 2.
Effect of acrylamide and lipoic acid on antioxidants and oxidative stress of rat’s testis. Values are expressed as mean ± SE. The values with different letter within the same raw significantly differ at P < 0.05
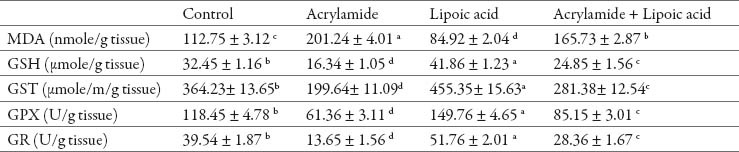
Table 3.
Effect of acrylamide and lipoic acid on antioxidants and oxidative stress of rat’s epididymis. Values are expressed as mean ± SE. The values with different letter within the same raw significantly differ at P < 0.05
3.3. Histopathological findings
Testicular sections of rats received lipoic acid had normal seminiferous tubules with complete spermatogenesis and normal interstitial connective tissue (Fig. 1a). However, testicular tissue of rats received acrylamide showed degenerative changes of majority of seminiferous tubules as incomplete spermatogenesis where seminiferous tubules were almost devoid of spermatids and spermatozoa and vacuolar degeneration of spermatogonia and Sertoli cells (Fig. 1b). Degenerated germinal epithelial cells were sloughed in the lumina of most seminiferous tubules and multiple giant cell formations (Fig. 1c). Other tubules showed coagulative necrosis and depletion of germinal epithelium with hyalinization of luminal contents (Fig. 1d). The majority of seminiferous tubules lost all germinal epithelium cells which evoked on cessation of spermatogenesis (Fig. 1e). Conversely, rats received acrylamide together with lipoic acid showed slight vacuolization of germinal epithelial cells. A few numbers of seminiferous tubules had sloughed necrotic germinal epithelium in their lumina in addition to marked improvement of spermatogenesis, evidenced by presence of elongated spermatids and spermatozoa in majority of seminiferous tubules (Fig. 1f). The caput and cauda epididymis of rats received lipoic acid showed normal histological structure with normal sperm density (Fig. 2a & b). Acrylamide treated rats exhibited histopathological alterations in the cauda epididymis as edema and moderate numbers of mononuclear cell infiltration of interstitial connective tissue beside the majority of epididymal ducts had no or low numbers of spermatozoa in their lumina (Fig. 2c). Sloughing of lining epithelial cells and giant cells was evident in some ducts of caput epididymis (Fig. 2d). Rats received acrylamide plus lipoic acid showed moderate enhancement in sperm density and epididymal structural integrity in caput (e) and cauda (f) epididymis.
Figure 1.
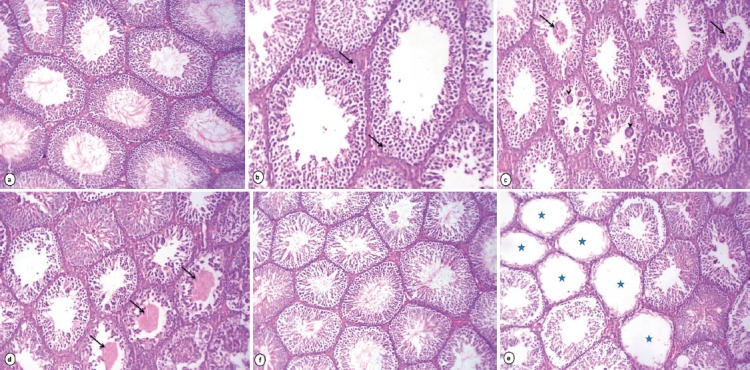
Photomicrograph of rat testes stained with HE: Rats received lipoic acid (1%) showing normal histoarchitecture of testes (a X.160). Rats received acrylamide (0.05%) exhibited vacuolar degeneration of the germinal epithelium and Sertoli cells (arrows) (b X.250), sloughing of the germinal epithelium (arrows) with giant cell formations (arrowheads) in the lumen of seminiferous tubules (c X.160), depletion of germinal cells and hyalinization of the luminal contents (arrows) (d X.160) and some seminiferous tubules loss of all germinal epithelium cells (stars) (e X.160). Improvement of spermatogenesis in testes of rats treated with acrylamide + lipoic acid (f X.160).
Figure 2.
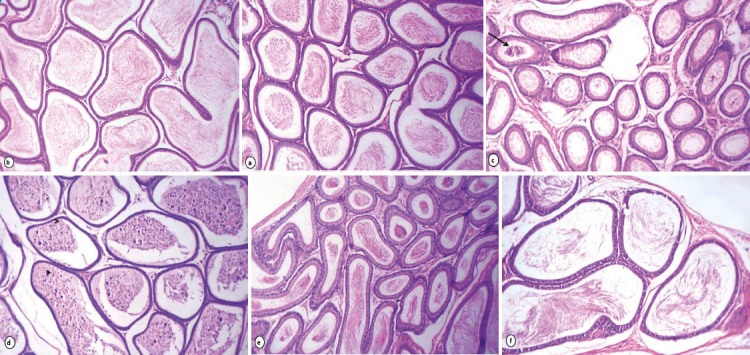
Photomicrograph of rat epididymis stained with HE (X. 160): caput and cauda epididymis of rats received lipoic acid showing normal histological structure with normal sperm density (a & b). Rats received acrylamide, the caput epididymis (c) showing the most of epididymal ductules were free from spermatozoa beside sloughed germ cells (arrows) while cauda epididymis (d) exhibited giant cells (arrowheads) and sloughed germ cells in their lumina. Rats treated with acrylamide plus lipoic acid nearly normal structure and sperm density (e & f).
4. DISCUSSION
The present study clearly demonstrates that alpha-lipoic acid has marked protective effect against the development of acrylamide induced testicular oxidative stress through its antioxidant properties. Our results were in accordance with (20, 21).
Furthermore, Yang et al. (6) proved that level of testosterone hormone in rats was severely affected in different doses of acrylamide and this led to the spermatogenic effects and decreased the Leydig cell viability. Also, Hamdy et al. (7) indicated that testis is a target organ of acrylamide action as it caused severe damage in seminiferous tubules and caused decrease in plasma free and total testosterone in a dose dependent manner. Song et al. (22) reported that the subchronic exposure of acrylamide could affect the normal development of sperm, cause changes of the activity of some enzymes in the testis and directly damage Leydig cells and affects the endocrine function of the testis, as confirmed by histopathological changes.
The inhibition of some members of the kinesin and dynein cytoskeletal motor protein families may be the common site of action of ACR and GA, which could explain both neurotoxicity and male reproductive toxicity observed in animals (23).
Rat’s Leydig cells utilize both serum uptake and Denovo synthesis of cholesterol from acetate to produce testosterone (24). It is possible that cytoskeletal inhibition by acrylamide could result in diminished uptake of cholesterol and consequent reduced testosterone levels. In addition, putative acrylamide-induced cytoskeletal dysfunction could inhibit the synthesis and/or transport of LH receptors to the cell membrane of Leydig cells, thereby indirectly decreasing testosterone biosynthesis (25).
Estradiol is secreted by the ovarian follicles although there is evidence that the adrenal gland is believed to secrete minute quantities of estrogen (26). The decreased levels of estradiol and progesterone reported in the ACR treated group may indicate that ACR affects the ovarian follicles directly and/or indirectly through the effects on the pituitary gland and decrease its secretion of FSH which stimulates follicle growth and regulates the conversion of androstendione to estradiol (27) as confirmed by Mannaa et al. (28) who reported that acrylamide induced significant decrease in the levels of estrogen and progesterone in rats.
Treatment with lipoic acid improved the changes in serum male sex hormones induced by acrylamide. These findings correlated with Othman et al. (29) who demonstrated that lipoic acid sustained the levels of cholesterol and sex hormone binding globulin (SHBG) to those seen in the control rats. This finding might be attributed to antioxidant milieu of LA which maintained a fine tuning of signal transduction mechanisms necessary for normal function of hypothalamus-testicular axis leading to normal secretion of testosterone and sperm production.
Our results revealed regarding oxidative stress and antioxidant status were in agreement with Alturfan et al. (30) who mentioned that rats treated with acrylamide 40 mg/kg b.wt/day for 10 days exhibited an increased tissue MDA level with decreased level of GSH.
Enhancement of lipid peroxidation is a consequence of depletion of GSH to certain critical levels. There was a significant decrease in thiol groups in lungs, kidney, brain, testis and liver homogenates in male rats treated with acrylamide in a dose dependent manner (31).
ACR is capable of interacting with vital cellular nucleophiles possessing -SH, -NH2 or –OH group. Therefore, it reacts with GSH in a similar manner and forms glutathione S-conjugates, which is the initial step in the biotransformation of electrophiles into mercapturic acids (32). In the present study, decreased GSH content and increased lipid peroxidation in tissues can be explained by the reaction of ACR with GSH, which in turn causes the depletion of GSH and the enhancement of lipid peroxidation.
Mannaa et al. (28) showed that acrylamide caused a significant decrease in total antioxidant and significant increase in lipid peroxidation in brain homogenate in female rats. The levels of oxidative stress indices and lipid peroxidation (MDA) in the plasma of rats treated with acrylamide were significantly increased while total antioxidant activity was significantly decreased compared with their levels in the controls (5).
Treatment of rats with LA showed an increase in the activities of antioxidant enzymes and reduced glutathione level with a decrease in lipid peroxidation which might be attributed to the antioxidant and oxidative damage repairing ability of LA (8). Also, Othman et al. (29) reported a decrease in the levels of TBARS and protein carbonyl with normalized levels of endogenous antioxidants and protection of reproductive markers in the lipoic acid octylphenol treated rats compared to the control rats. El-Beshbishy et al. (33) reported that co-administration of lipoic acid 20 mg/kg orally for 14 days together with bisphenol resulted in decreasing lipid peroxidation and increasing enzymatic and non-enzymatic antioxidants in rats.
The current study confirms the results of other studies (34) who demonstrated that LA regulated the GST activity in the hypothalamus and epididymal sperm and corrected their deficient thiol status by increasing the level of GSH. Because LA is a dithiol it can scavenge several ROS and also bind ferrous ions to inhibit the generation of hydroxyl radicals (9). In addition, LA can stimulate the recycling of tocopherol and ascorbic acid (35) and in combination with its scavenging ability, maintain levels of protein thiol and therefore modulated endogenous antioxidants in the hypothalamus and epididymal sperm.
The maintenance of reproductive capacity requires proper integrity of blood–testis barrier. Exposure to environmental toxicants can lead to impairment of this barrier and, in turn, can induce the generation of ROS leading to testicular oxidative DNA damage and oxidative infertility. Since LA is water and fat soluble antioxidant, therefore, it is distributed in both the cellular membranes and the cytosol. Our study is compatible with the earlier findings by others who reported that LA has protective effect against adriamycin and lipopolysaccharide-induced oxidative disruption of blood–testis barrier and testicular histological changes (8).
5. CONCLUSION
In conclusion, our results demonstrate for the first time that in ACR induced oxidative stress, alpha-lipoic acid by inhibiting the changes in male sex hormones and balancing oxidant-antioxidant status protected the tissues and thus, supplementation of alpha-lipoic acid may be useful in individuals who are at risk to ACR toxicity.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED.
REFERENCES
- 1.Doerge DR, Young JF, Chen JJ, DiNovi MJ, Henry SH. Using dietary exposure and physiologically based pharmacokinetic/pharmacodynamic modeling in human risk extrapolations for acrylamide toxicity. J Agric Food Chem. 2008;56:6031–6038. doi: 10.1021/jf073042g. [DOI] [PubMed] [Google Scholar]
- 2.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–449. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 3.Hand MS, Thatcher CD, Remillard RL, Roudebush P. 4th Edition. Topeka, KS: Mark Morris Institute; 2002. Eds Small Animal Clinical Nutrition. [Google Scholar]
- 4.Ghanayem BI, McDaniel LP, Churchwell MI, Twaddle NC, Snyder R, Fennell TR, Doerge DR. Role of CYP2E1 in the epoxidation of acrylamide to glycidamide and formation of DNA and hemoglobin adducts. Toxicol Sci. 2005;88:311–318. doi: 10.1093/toxsci/kfi307. [DOI] [PubMed] [Google Scholar]
- 5.Paulsson B, Granath F, Grawe J, Ehrenberg L, Tornqvist M. The multiplicative model for cancer risk assessment: application of acrylamide. Carcinogen. 2001;22:816–819. doi: 10.1093/carcin/22.5.817. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Lee S, Jin Y, Choi J, Han D, Chae C, Lee M, Han C. Toxicological effects of acrylamide on rat testicular gene expression profile. Reprod Toxicol. 2005;19:527–534. doi: 10.1016/j.reprotox.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Hamdy SM, Bakeer HM, Eskande EF, Sayed ON. Effect of acrylamide on some hormones and endocrine tissues in male rats. Hum Exp Toxicol. 2011;5:1–9. doi: 10.1177/0960327111417267. [DOI] [PubMed] [Google Scholar]
- 8.Prahalathan C, Selvakumar E, Varalakshmi P. Lipoic acid modulates adriamycin-induced testicular toxicity. Reprod Toxicol. 2006;211:54–59. doi: 10.1016/j.reprotox.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid Free Radic. Biol Med. 1997;221-222:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki YJ, Tsuchiya M, Packer L. Thioctic acid and dihydrolipoic acid are novel antioxidants which interact with reactive oxygen species. Free Radic. Res. Commun. 1991;155:255–263. doi: 10.3109/10715769109105221. [DOI] [PubMed] [Google Scholar]
- 11.Guimaraes SB, Santos JM, Aragao AA, de Sandes Kimura O, Barbosa PH, de Vasconcelos PR. Protective effect of alpha-lipoic acid in experimental spermatic cord torsion. Nutrition. 2007;231:76–80. doi: 10.1016/j.nut.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Aly HA, Lightfoot DA, El-Shemy HA. Modulatory role of lipoic acid on lipopolysaccharide-induced oxidative stress in adult rat Sertoli cells in vitro. Chem. Biol Interact. 2009;1822-1823:112–118. doi: 10.1016/j.cbi.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Korkmaz A, Ahbab MA, Kolankaya D, Barlas N. Influence of vitamin C on bisphenol A, nonylphenol and octylphenol induced oxidative damages in liver of male rats. Food Chem. Toxicol. 2010;4810:2865–2871. doi: 10.1016/j.fct.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Ohkawa H, Wakatsuki A, Kaneda C. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1982;951:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 15.Sedlak J, Lindsay RH. Estimation of total, protein bound and non-protein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 16.Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 17.Paglia D, Valentine W. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- 18.Horn D. Methods in enzymatic analysis. In: Bergmayer H, editor. London: Academic Press; 1965. pp. 75–879. [Google Scholar]
- 19.Wanga H, Huanga P, Lie T, Li J, Hutzc R, Li K, Shia F. Reproductive toxicity of acrylamide-treated male rats. Reprod Toxicol. 2010;29:225–230. doi: 10.1016/j.reprotox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Camacho L, Latendresse JR, Muskhelishvili L, Patton R, Bowyer JF, Thomas M, Doerge DR. Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicology Letters. 2012;211:135–143. doi: 10.1016/j.toxlet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Yue W, Ren Y, Zhang C. Enhanced role of eliadic acid on acrylamide-induced oxidative stress in epididymis and epididymal sperm that contributed to the impairment of spermatogenesis in mice. Toxicol Ind Health. 2010;26:469–477. doi: 10.1177/0748233710373084. [DOI] [PubMed] [Google Scholar]
- 22.Song H, Wang R, Geng Z, Cao S, Liu T. Subchronic exposure to acrylamide affects reproduction and testis endocrine function of rats. Zhonghua Nan Ke Xue. 2008;14:406–410. [PubMed] [Google Scholar]
- 23.Friedman MA, Zeiger E, Marroni DE, Sickles DW. Inhibition of rat testicular nuclear kinesins (krp2;KIFC5A) by acrylamide as a basis for establishing a genotoxicity threshold. J Agric Food Chem. 2008;56:6024–6030. doi: 10.1021/jf703746f. [DOI] [PubMed] [Google Scholar]
- 24.Latendresse JR, Azhar S, Brooks CL, Capen CC. Pathogenesis of cholesteryl lipidosis of adrenocortical and ovarian interstitial cells in F344 rats caused by tricresyl phosphate and butylated triphenyl phosphate. Toxicol Appl Pharmacol. 1993;122:281–289. doi: 10.1006/taap.1993.1197. [DOI] [PubMed] [Google Scholar]
- 25.Laws AO, Kerr JB, de Kretser DM. The role of the microtubular system in LH/hCG receptor downregulation in rat Leydig cells. Mol Cell Endocrinol. 1984;38(1):39–51. doi: 10.1016/0303-7207(84)90143-6. [DOI] [PubMed] [Google Scholar]
- 26.Carr BR. Disorders of the ovary and female reproductive tract. In: Wilson JD, Foster DW, editors. Williams Textbook of Endocrinology. 8th edn. Philadelphia: W. B. Saunders; 1992. pp. 733–798. [Google Scholar]
- 27.Channing CP, Schaerf FW, Anderson LD. Ovarian follicular and luteal physiology. In: Greep RO, editor. Reproductive Physiology III. Baltimore: University Park Press; 1980. p. 117. [PubMed] [Google Scholar]
- 28.Mannaa F, Abdel-Wahhab MA, Ahmed HH, Park MH. Protective role of Panax ginseng extract standardized with ginsenoside Rg3 against acrylamide induced neurotoxicity in rats. J Appl Toxicol. 2006;26:198–206. doi: 10.1002/jat.1128. [DOI] [PubMed] [Google Scholar]
- 29.Othman AI, El-Missiry MA, Koriem KM, El-Sayed AA. Alfa-Lipoic acid protects testosterone secretion pathway and sperm quality against 4-tert-octylphenol induced reproductive toxicityEcotoxicology and Environmental Safety. 2012;81:76–83. doi: 10.1016/j.ecoenv.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Alturfan EI, Beceren A, Şehirli AO, Demiralp ZE, Şener G, Omurtag GZ. Protective effect of N-acetyl-L-cysteine against acrylamide-induced oxidative stress in rats. Turk J Vet Anim Sci. 2012;36(4):438–445. [Google Scholar]
- 31.Yousef MI, El-Demerdash FM. Acrylamide-induced oxidative stress and biochemical perturbations in rats. Toxicology. 2006;219:133–141. doi: 10.1016/j.tox.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Awad ME, Abdel-Rahman MS, Hassan SA. Acrylamide toxicity in isolated rat hepatocytes. Toxicol In Vitro. 1998;12:699–704. doi: 10.1016/s0887-2333(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 33.El-Bishbishy HA, Aly HA, ElShafey M. Lipoic acid mitigates bisphenol A induced testicular mitochondrial toxicity in rats. Environmental and Occupational Health. 2013;29(10):875–887. doi: 10.1177/0748233712446728. [DOI] [PubMed] [Google Scholar]
- 34.Han D, Handelman G, Marcocci L, Sen CK, Roy S, Kobuchi H, Tritschler HJ, Flohe L, Packer L. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;63:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 35.Navari-Izzo F, Quartacci MF, Sgherri C. Lipoic acid: a unique antioxidant in the detoxification of activated oxygen species. Plant Physiol. Biochem. 2002;406-8:463–470. [Google Scholar]


