Abstract
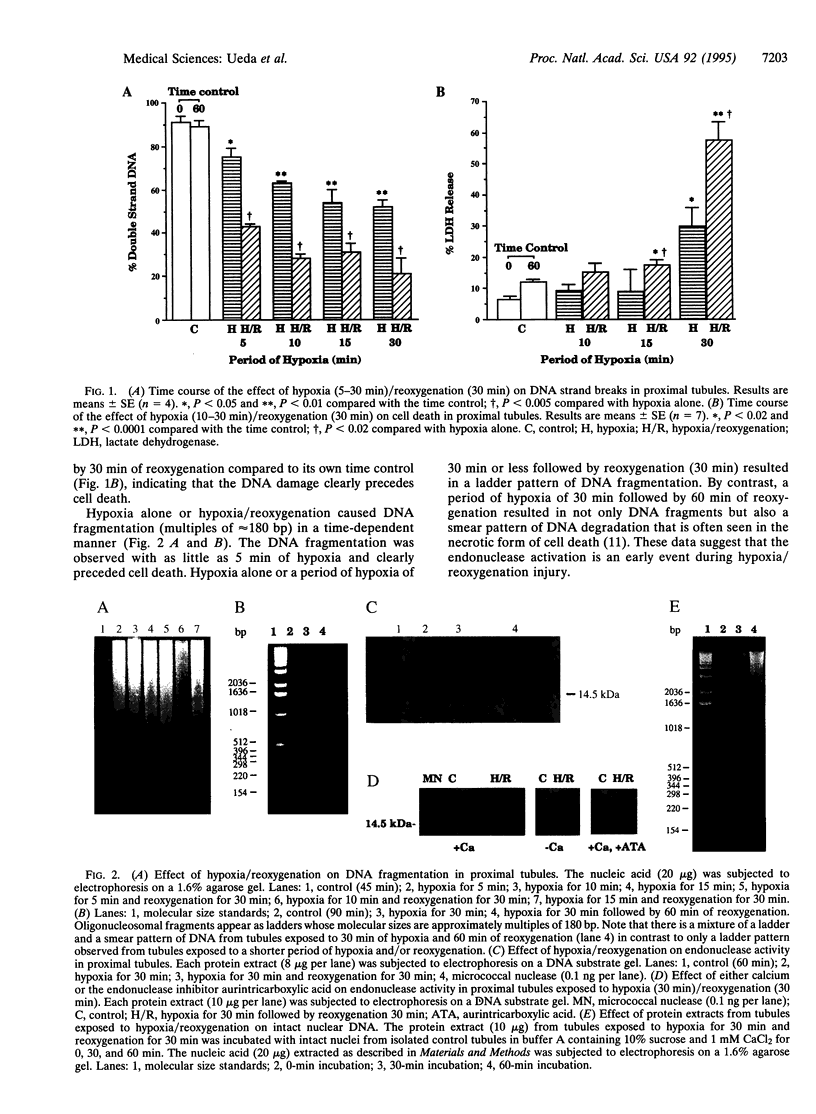
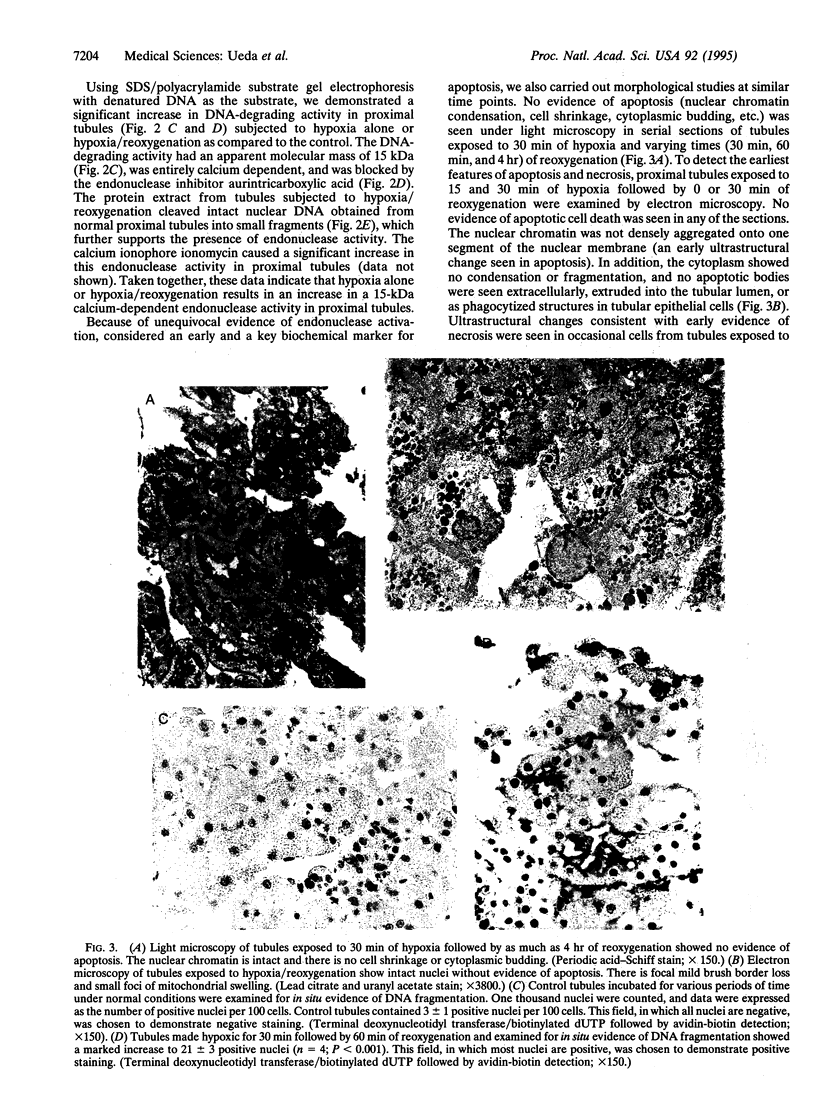
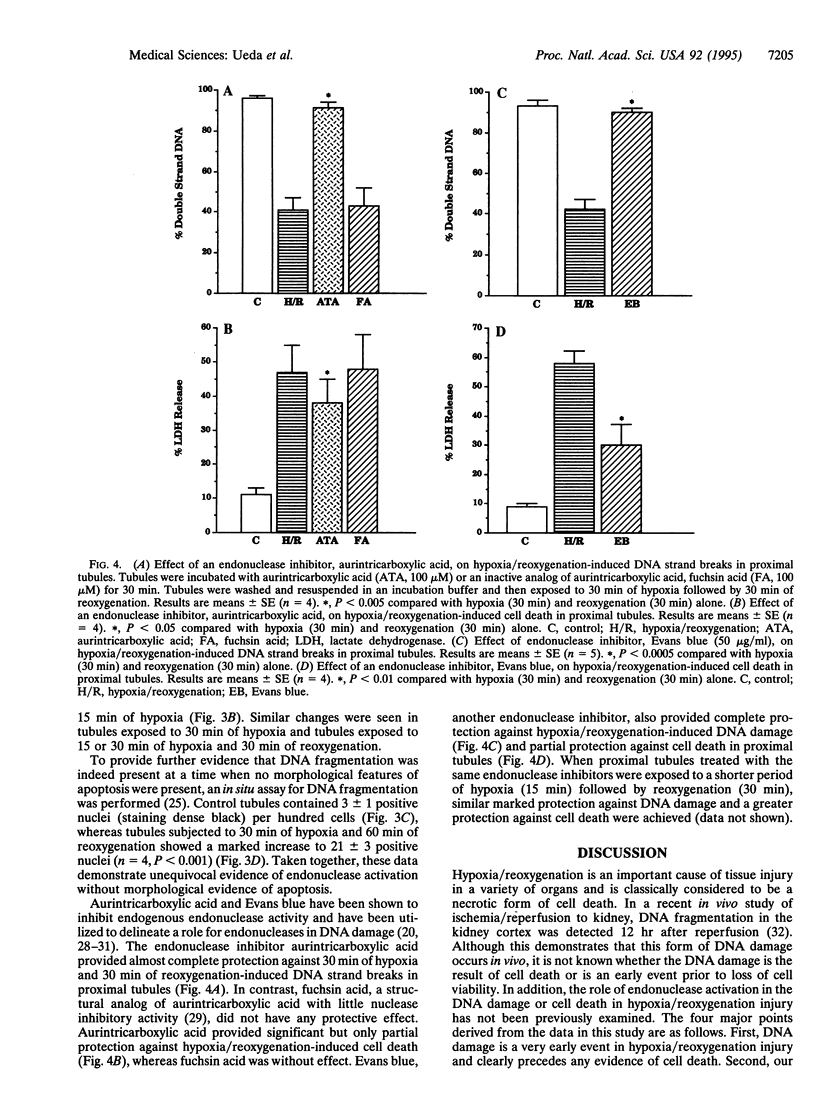
Hypoxia/reoxygenation is an important cause of tissue injury in a variety of organs and is classically considered to be a necrotic form of cell death. We examined the role of endonuclease activation, considered a characteristic feature of apoptosis, in hypoxia/reoxygenation injury. We demonstrate that subjecting rat renal proximal tubules to hypoxia/reoxygenation results in DNA strand breaks and DNA fragmentation (both by an in situ technique and by agarose gel electrophoresis), which precedes cell death. Hypoxia/reoxygenation resulted in an increase in DNA-degrading activity with an apparent molecular mass of 15 kDa on a substrate gel. This DNA-degrading activity was entirely calcium dependent and was blocked by the endonuclease inhibitor aurintricarboxylic acid. The protein extract from tubules subjected to hypoxia/reoxygenation cleaved intact nuclear DNA obtained from normal proximal tubules into small fragments, which further supports the presence of endonuclease activity. Despite unequivocal evidence of endonuclease activation, the morphologic features of apoptosis, including chromatin condensation, were not observed by light and electron microscopy. Endonuclease inhibitors, aurintricarboxylic acid and Evans blue, provided complete protection against DNA damage induced by hypoxia/reoxygenation but only partial protection against cell death. Taken together, our data provide strong evidence for a role of endonuclease activation as an early event, which is entirely responsible for the DNA damage and partially responsible for the cell death that occurs during hypoxia/reoxygenation injury. Our data also indicate that in hypoxia/reoxygenation injury endonuclease activation and DNA fragmentation occur without the morphological features of apoptosis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Baba M., Schols D., Pauwels R., Balzarini J., De Clercq E. Fuchsin acid selectively inhibits human immunodeficiency virus (HIV) replication in vitro. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1404–1411. doi: 10.1016/s0006-291x(88)81297-x. [DOI] [PubMed] [Google Scholar]
- Batistatou A., Greene L. A. Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol. 1991 Oct;115(2):461–471. doi: 10.1083/jcb.115.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Borkan S. C., Schwartz J. H. Role of oxygen free radical species in in vitro models of proximal tubular ischemia. Am J Physiol. 1989 Jul;257(1 Pt 2):F114–F125. doi: 10.1152/ajprenal.1989.257.1.F114. [DOI] [PubMed] [Google Scholar]
- Cohen G. M., Sun X. M., Snowden R. T., Dinsdale D., Skilleter D. N. Key morphological features of apoptosis may occur in the absence of internucleosomal DNA fragmentation. Biochem J. 1992 Sep 1;286(Pt 2):331–334. doi: 10.1042/bj2860331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Doctor R. B., Mandel L. J. Minimal role of xanthine oxidase and oxygen free radicals in rat renal tubular reoxygenation injury. J Am Soc Nephrol. 1991 Jan;1(7):959–969. doi: 10.1681/ASN.V17959. [DOI] [PubMed] [Google Scholar]
- Enright H., Hebbel R. P., Nath K. A. Internucleosomal cleavage of DNA as the sole criterion for apoptosis may be artifactual. J Lab Clin Med. 1994 Jul;124(1):63–68. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golconda M. S., Ueda N., Shah S. V. Evidence suggesting that iron and calcium are interrelated in oxidant-induced DNA damage. Kidney Int. 1993 Dec;44(6):1228–1234. doi: 10.1038/ki.1993.373. [DOI] [PubMed] [Google Scholar]
- González-Flecha B., Cutrin J. C., Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest. 1993 Feb;91(2):456–464. doi: 10.1172/JCI116223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H., Balzarini J., De Clercq E., Ono K. Differential inhibition of various deoxyribonucleic acid polymerases by Evans blue and aurintricarboxylic acid. Eur J Biochem. 1988 Oct 15;177(1):91–96. doi: 10.1111/j.1432-1033.1988.tb14348.x. [DOI] [PubMed] [Google Scholar]
- Nath K. A., Paller M. S. Dietary deficiency of antioxidants exacerbates ischemic injury in the rat kidney. Kidney Int. 1990 Dec;38(6):1109–1117. doi: 10.1038/ki.1990.320. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hoidal J. R., Ferris T. F. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984 Oct;74(4):1156–1164. doi: 10.1172/JCI111524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch M. C., Hesterkamp T., Polzar B., Mannherz H. G., Tschopp J. Functional characterisation of serum DNase I in MRL-lpr/lpr mice. Biochem Biophys Res Commun. 1992 Jul 31;186(2):739–745. doi: 10.1016/0006-291x(92)90808-x. [DOI] [PubMed] [Google Scholar]
- Peitsch M. C., Polzar B., Stephan H., Crompton T., MacDonald H. R., Mannherz H. G., Tschopp J. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death). EMBO J. 1993 Jan;12(1):371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A. L., Lacks S. A. Nuclease detection in SDS-polyacrylamide gel electrophoresis. Anal Biochem. 1977 May 15;80(1):76–90. doi: 10.1016/0003-2697(77)90627-3. [DOI] [PubMed] [Google Scholar]
- Russell S. W., Rosenau W., Lee J. C. Cytolysis induced by human lymphotoxin. Am J Pathol. 1972 Oct;69(1):103–118. [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Walczak H., Dröge W., Krammer P. H. Cell nucleus and DNA fragmentation are not required for apoptosis. J Cell Biol. 1994 Oct;127(1):15–20. doi: 10.1083/jcb.127.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M., Colombel M. C., Sawczuk I. S., Gobé G., Connor J., O'Toole K. M., Olsson C. A., Wise G. J., Buttyan R. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992 Apr;140(4):831–838. [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Smith S. W., Jones M. E., Osborne B. A. Do all programmed cell deaths occur via apoptosis? Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Sun D. Y., Jiang S., Zheng L. M., Ojcius D. M., Young J. D. Separate metabolic pathways leading to DNA fragmentation and apoptotic chromatin condensation. J Exp Med. 1994 Feb 1;179(2):559–568. doi: 10.1084/jem.179.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda N., Shah S. V. Endonuclease-induced DNA damage and cell death in oxidant injury to renal tubular epithelial cells. J Clin Invest. 1992 Dec;90(6):2593–2597. doi: 10.1172/JCI116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. D., Kaushal G. P., Shah S. V. Presence of a distinct extracellular matrix-degrading metalloproteinase activity in renal tubules. J Am Soc Nephrol. 1994 Jul;5(1):55–61. doi: 10.1681/ASN.V5155. [DOI] [PubMed] [Google Scholar]
- Weinberg J. M. The cell biology of ischemic renal injury. Kidney Int. 1991 Mar;39(3):476–500. doi: 10.1038/ki.1991.58. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]





