Abstract
Structural congenital heart disease (CHD) has not previously been linked to autoimmunity. In our study, we developed an autoimmune model of structural CHD that resembles hypoplastic left heart syndrome (HLHS), a life-threatening CHD primarily affecting the left ventricle. Because cardiac myosin (CM) is a dominant autoantigen in autoimmune heart disease, we hypothesized that immunization with CM might lead to transplacental passage of maternal autoantibodies and a prenatal HLHS phenotype in exposed fetuses. Elevated anti-CM autoantibodies in maternal and fetal sera, and IgG reactivity in fetal myocardium were correlated with structural CHD that included diminished left ventricular cavity dimensions in the affected progeny. Further, fetuses that developed a marked HLHS phenotype had elevated serum titers of anti-β adrenergic receptor antibodies as well as increased protein kinase A activity, suggesting a potential mechanism for the observed pathological changes. Our maternal-fetal model presents a new concept linking autoimmunity against CM and cardiomyocyte proliferation with cardinal features of HLHS. This report shows the first evidence to support a novel immune-mediated mechanism for pathogenesis of structural CHD that may have implications in its future diagnosis and treatment.
Introduction
Congenital heart defects (CHD) are the most common cause of infant death resulting from birth defects (1). Hypoplastic left heart syndrome (HLHS), a severe and devastating congenital heart malformation, accounts for nearly 25% of all neonatal deaths from CHD (1-3). HLHS is uniformly fatal without intervention, and despite aggressive medical and surgical palliation many affected children experience a significant developmental delay and a decreased quality of life (4, 5). Although etiologic mechanisms leading to HLHS are largely unknown, both genetic and environmental insults are potential contributors (6-10). About one-fourth of HLHS cases occur in the context of recognized genetic disorders or syndromes; studies involving non-syndromic family members suggest that heritability is complex (9, 11) and environmental influences such as infection and autoimmunity might contribute to the phenotypic expression of certain subsets of HLHS (3, 6, 12, 13).
In some CHD, transplacental passage of maternal immunoglobulin G (IgG) has been reported to affect the fetus. For instance in congenital heart block, maternal autoantibodies in patients with systemic lupus erythematosus cause injury to the conduction system of the fetal heart (14-16). We had previously hypothesized that autoimmunity might play a role in a maternal-fetal model of structural left-sided CHD (12). Our hypothesis has been supported by the observation of high titers of anti-human cardiac myosin (CM) IgG autoantibodies in sera from mothers of babies with HLHS, but not other CHD or healthy controls, in an ongoing clinical study (Clinicaltrial.gov 201102410). Anti-cardiac myosin autoantibodies are linked to several autoimmune diseases of the heart including autoimmune myocarditis (17-22) and rheumatic carditis, the most serious manifestation of group A streptococcal induced rheumatic fever (23-25). In this study we determined whether maternal immunization with CM, a major autoantigen in human heart (22) could produce an HLHS-like phenotype in susceptible offspring following transplacental passage of anti-heart antibodies. Experiments conducted in the Lewis rat, an established model of CM-induced autoimmune heart disease (19, 20), led to an HLHS-like phenotype seen in human infants. Autoimmunity against the heart is a new concept in the pathogenesis of HLHS.
Materials and Methods
Antigen Preparation
Rat CM was purified from rat heart tissue according to previously described techniques with slight modifications (25, 26). Heart tissue was homogenized in a low-salt buffer (40 mM KCl, 20 mM imidazole, 5 mM EGTA, 5 mM DTT, 0.5 mM PMSF, 1 mcg of leupeptin/ml) for 15 sec on ice. The washed myofibrils were collected by centrifugation at 16,000 × g for 10 min. The pellets were then resuspended in high-salt buffer (0.3 M KCl, 0.15 M K2HPO4, 1 mM EGTA, 5 mM DTT, 0.5 mM PMSF, 1 mcg of leupeptin/ml) and homogenized for three 30 sec bursts on ice. The homogenized tissue was further incubated on ice with stirring for 30 min to facilitate actomyosin extraction. After clarification by centrifugation, actomyosin was precipitated by addition of 10 volumes of cold water, followed by a pH adjustment to 6.5. DTT was added to 5 mM, and the precipitation was allowed to proceed for 30 min. The actomyosin was then pelleted by centrifugation at 16,000 g. The actomyosin pellet was then resuspended in high-salt buffer, ammonium sulfate was increased to 33%, and the KCl concentration was increased to 0.5 M. After the actomyosin pellet and salts were dissolved, ATP was added to 10 mM and MgCl2 was added to 5 mM, and then the solution was centrifuged at 20,000 g for 15 min to remove actin filaments. The supernatant was removed and stored at 4°C in the presence of the following inhibitors: 0.5 mM PMSF, 5mcg/ml N-tosyl-L-lysine chloromethyl ketone, and 1 mcg of leupeptin/ml. The presence of CM was verified and quantitated by ELISA and Western immunoblot using monoclonal antibody specific for CM protein.
Immunization Protocol
Specific pathogen-free female Lewis rats (LEW-RT11) (~ 8 weeks old) were purchased from Charles River Laboratories (Raleigh, NC) and were maintained in a pathogen-free environment at Cincinnati Children’s Hospital animal facility. Rats were acclimated for 7 days prior to entering the immunization protocol. All animals were treated according to institutional guidelines and IACUC approved protocols. Female rats were immunized with either rat cardiac-myosin (CM; n=8) or saline (controls; n=5). A schematic of the immunization protocol is shown in Supplement Figure 1. The CM treatment rats were immunized on Day 0 by subcutaneous injection of 1 mg of rat CM extract emulsified in Complete Freund’s Adjuvant at a 1:1 ratio (v/v) in a total volume of 400 μl. On days 14, 28, and 42 after the initial immunization, all the rats were boosted intra-peritoneally with 500 μg of extract emulsified 1:1 with Incomplete Freund’s adjuvant in a total volume of 200 μl. Serum titers of CM antibodies were determined by ELISA assays every 7-14 days. Rats with no response (<1:100) exited the protocol, and rats with medium titers (<1:6400) were given up to a total of 3 additional boosters. In the control group CM extract was exchanged for saline in the presence of adjuvant. Control animals all received 3 booster injections. Breeding began 7 days after the final booster. No boosters were administered during gestation, which in the Lewis rat is 22 ± 0.2 days. Dams were left with males for 1-3 days and successful mating was confirmed by presence of spermatozoa on vaginal smear. Vaginal smears were preformed daily. Near term (estimated day of gestation 20 ± 1) cesarean section was performed under anesthesia (1.5% isofluorane) to deliver the progeny. Fetal animals were harvested following decapitation, and maternal animals after exsanguinations. Maternal and fetal blood was collected during harvest. Blood samples were centrifuged at 1,300g for 15 min in a fixed angle rotor. Serum was collected and stored at −20°C. Maternal and fetal hearts were immediately washed in PBS, fixed in 4% paraformaldehyde, paraffin embedded, sectioned at 7μm intervals, and analyzed for histology.
Antibody Quantification by Direct ELISA
The assays were conducted as described in previous publications (21, 22, 25). Immulon 4 (Dynatech) microtiter plates were coated at 4°C overnight with rat CM at 10 mg/mL in 0.1 mol/L carbonate-bicarbonate coating buffer (pH 9.6). Plates were washed with phosphate-buffered saline (PBS) containing 0.05% Tween 20, and blocked for 1 hr at 37°C with 1% bovine serum albumin (BSA, Fisher Scientific, Hanover Park, IL, USA). Plates were washed once again with PBS/Tween 20. To determine the rat anti-CM ELISA antibody titer, rat sera was titrated at an initial dilution of 1:500 in 1% BSA in PBS buffer and thereafter diluted two-fold, up to a final dilution of 1:12800. Fifty microliters of diluted serum were loaded into microtiter wells in duplicates and incubated overnight at 4°C. Plates were washed 5X with PBS/Tween 20, and 50 microliters of goat anti-rat IgG (Sigma, St Louis, MO, USA) conjugated with alkaline phosphatase (1/500 dilution) was added and incubated at 37°C for 1 hr. Plates were washed with PBS/Tween 20, and 50 microliters of substrate para-nitrophenyl-phosphate (Sigma) in 0.1 M diethanolamine buffer (pH 9.8) was added to each well. Optical density was measured at 405 nm in an ELISA plate reader (ELx800, Bio-Tek Instruments, Vermont, USA). Titers were determined at the highest dilution with an optical density value of 0.10 at approximately 60 min. Anti-rat CM antibody titers in the ELISA were standardized and controlled by using negative and positive control standard sera, obtained from previous experiments. Beta-adrenergic receptors 1 (β1-AR) and 2 (β2-AR) (Perkin-Elmer) were coated at 10 μg/ml onto microtiter plates for testing for rat IgG antibodies against the anti-β1-AR and anti-β2-AR antibodies in the serum. The ELISA was performed according to the same procedure stated above. Activation of serum protein kinase A (PKA) by the β-AR was performed as previously described (21, 22).
Histology and Morphometry studies
Sections from each fetal heart were stained with Movat’s pentachrome or Masson’s trichrome (both from American Mastertech Scientific, Inc.). All morphometric measurements were obtained using Image J software. Comparable apical four-chambered sections from each fetal heart were photographed (Nikon DS Ri1) and coded to eliminate bias. Two blinded observers obtained measurements. At the LV and RV lateral and apical free walls, three measurements were taken in 100μm intervals. Three area measurements of the LV and RV were also obtained using comparable apical four-chambered sections. Measurements were then averaged from each location for statistical analysis. Due to some cardiac damage during harvest that altered heart chamber dimensions, two affected and two unaffected fetal hearts were excluded from LV/RV lumen area measurements. Maternal hearts were processed as previously described (20). A veterinary pathologist evaluated maternal heart sections for presence of myocarditis and valvulitis.
Western blot analysis
Binding of maternal and fetal serum to lysates (10 mcg total protein) of adult rat heart, liver, lung, and spleen was determined by Western Blot analysis, as previously described (22). Serum from CM-injected maternal rats and affected fetal offspring, along with serum from control maternal rats and fetal offspring were analyzed at a dilution of 1:1000. Pre-incubation of the sera to porcine CM (20 mcg; Sigma) prior to incubation of the blots was performed to determine specificity.
Immunohistochemistry studies
The anti-rat IgG staining was performed as previously described with slight modifications (21) was gamma chain specific for rat IgG. Briefly, mounted tissues were baked at 60°C for 20 min and deparaffinized using a 3:1 ratio of Hemo-D (Fisher) to xylene. After rehydration in graded ethanol washes, tissues were washed twice with PBS, blocked with Protein Blocker (BioGenex, San Ramon, California, USA) for 30 min at room temperature, and washed twice with PBS. Biotin-conjugated goat anti-rat IgG antibodies (diluted 1:500; Jackson ImmunoResearch, West Grove, Pennsylvania, USA) or isotype control goat IgG biotin, were incubated on tissues overnight at 4°C in a humidity chamber, followed by three washes in PBS. Alkaline phosphatase-conjugated streptavidin was incubated with the tissues at 1 mg/mL for 30 minutes at room temperature. After three washes in PBS, antibody binding was detected with Fast Red substrate (BioGenex) against a counterstain of Mayer’s hematoxylin (BioGenex). Stained slides were mounted with crystal mount (Fisher), dried, and coverslipped using permount (Sigma Chemical Co.) The amount of IgG bound is indicated by scoring the amount and intensity of visual Fast Red staining in the heart tissue similar to previously described sections were deparaffinized, rehydrated and had antigenic sites unmasked using the Citrate-based solution (# H-3300) and high temperature-pressure protocol of Vector Laboratories. Sections were blocked for 1 hr at room temperature with 8% normal goat serum (Sigma-Aldrich, #G 9023), then incubated overnight at 40°C with primary antibodies PHH3 (Millipore, #06-570 [1:350]), to identify mitotic cells, and MF20 (Developmental Studies Hybridoma Bank, #MF 20 [1:200]), to identify myocytes. For immunofluorescent detection, sections were incubated with Alexa Fluor conjugated secondary antibodies (Invitrogen, goat anti-rabbit IgG #A11011 and goat anti-mouse IgG #A11001 [1:100]) for 1 hr at room temperature followed by a 30 min incubation with the nuclear stain TO-PRO-3 (Invitrogen, #T3605 [1:1000]). Samples were imaged using a Zeiss LSM 510 confocal microscope. The total number of nuclei, the number of nuclei in myocytes, the total number of pHH3 positive nuclei and the number of pHH3 positive nuclei in myocytes were counted for each image using Image J software. The total proliferative rate was calculated using total pHH3 positive nuclei divided by total nuclei. To determine the mitotic index, counts from four areas of the left ventricle, using comparable areas from each heart, were totaled and used to calculate the percentage of pHH3 positive nuclei. TUNEL staining (Roche Applied Science, Cat. No. 11 684 809 910) was performed according to manufacturer’s instruction. Cardiomyocytes were identified using MF-20 staining and the cardiomyocyte-specific proliferative rate was calculated using cardiomyocyte pHH3 positive nuclei divided by total cardiomyocyte nuclei.
Statistical Analysis
All statistics were completed using SAS version 9.2. Two variables were analyzed using two-sided, independent samples t-test and three variables were analyzed with two-way analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons.
Results
Heart defects correlated with elevated anti-CM titers
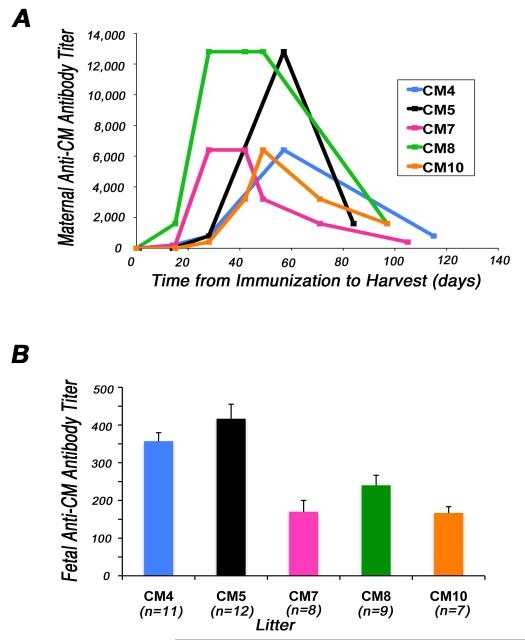
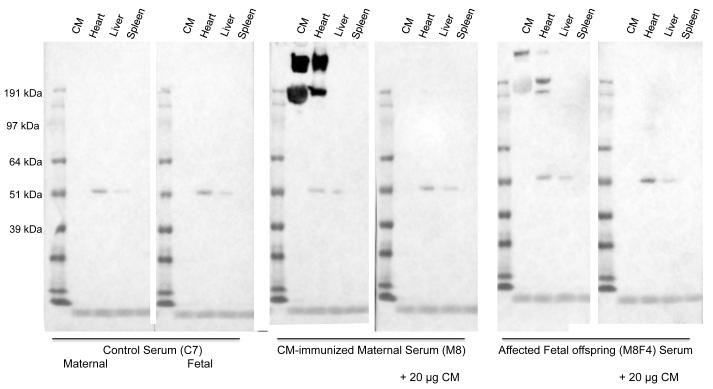
Female Lewis rats immunized with CM developed peak anti-CM autoantibody titers ranging from 1:6000 to >1:12800, prior to pregnancy (Fig. 1A). Most importantly, all maternal rats (n=8 mothers with 47 fetuses) that had elevated anti-CM antibody titers had at least one offspring with left-sided structural CHD as determined by histological and morphologic analyses. Fetal sera from offspring of CM-immunized mothers had elevated anti-CM antibody titers that ranged from > 1:100 to 1:800 (Fig. 1B). Fetal CM titers of ≥1:200 correlated with maternal peak CM titers of ≥1:6400 and/or maternal harvest CM titers of ≥1:800, confirming positive transplacental transfer of maternal anti-CM autoantibodies to their progeny. Serum titers of anti-CM antibodies in individual fetuses from each litter are shown in Supplement Figure 2. The highest anti-CM antibody titers were observed in maternal rat CM8 who also produced the largest number of progeny with structural congenital cardiac malformations (6 of 9) (Table 1). Control animals, which were injected with adjuvant, and their offspring (n=19), had undetectable (<100) anti-CM antibody titers (not shown). Western blot analyses showed specific binding of IgG autoantibodies in maternal and fetal serum to adult rat cardiac tissue lysates but not to rat kidney, lung or spleen (Fig. 2). Further, binding of this cardiac specific band at 200kD present in serum present in sera of a CM-immunized maternal rat and her affected fetus, but not in control maternal or fetal sera, was blocked by pre-incubation of the serum with CM.
Figure 1. Immunization with cardiac myosin (CM) induces elevated anti-CM antibody titers in adult rats and their fetal offspring.
(A) Serum titers measured in individual female Lewis rats (8 wks old) immunized with purified rat CM (n=8 mothers with 47 fetuses) followed by 3-4 booster injections administered at 2-week intervals are shown. Adjuvant was injected in control rats (data not shown). (B) Average fetal (harvested at estimated gestational day 20) anti-CM antibody titers in litters of individual adult animals with positive anti-CM antibody response (n=5 mothers) prior to mating, during pregnancy, and up to time of harvest. Bars shown are the mean ± s.e.m.
Table 1.
Maternal immune response against CM and associated left-side structural congenital cardiac abnormalities in the progeny.
| Fetal Litter | Litter Size | Total Maternal Antibody Burden |
Progeny with Heart Defects % (#affected) |
LV Cavity Hypoplasia % (#affected) |
Loss of Normal Valve Structure % (#affected) |
Severely Increased LV Myocardial Wall Thickness % (#affected) |
Moderately Increased LV Myocardial Wall Thickness % (#affected) |
|---|---|---|---|---|---|---|---|
| CM4 | 11 | 3.2 ×105 | 36% (4) | 27% (3) | 36% (4) | 9% (1) | 18% (2) |
| CM5 | 12 | 4.0 ×105 | 8% (1) | 8% (1) | 8% (1) | 8% (1) | 0 |
| CM7 | 8 | 2.6 ×105 | 25% (2) | 25% (2) | 25% (2) | 25% (2) | 0 |
| CM8 | 9 | 7.3 × 105 | 67% (6) | 55% (5) | 44% (4) | 55% (5) | 11% (1) |
| CM10 | 7 | 2.3 ×105 | 29% (2) | 29% (2) | 14% (1) | 29% (2) | 0 |
| C1 | 13 | <100 | 0% (0) | 0 | 0 | 0 | 0 |
| C2 | 3 | <100 | 0% (0) | 0 | 0 | 0 | 0 |
| C3 | 3 | <100 | 0% (0) | 0 | 0 | 0 | 0 |
Footnotes: The total maternal antibody burden was calculated using area under the curve of the maternal antibody titer graph (Fig.1). The values given are the mean ± s.e.m. of each liter. Neither maternal nor fetal adjuvant-injected control animals (C1-3) had elevated anti-CM antibody titers.
Abbreviations: CM=cardiac myosin immunized; C=adjuvant injected controls; D=day.
Figure 2. Western blot analysis of serum shows heart-specific binding.
Serum from a control maternal rat (C7) and a fetal offspring, and a CM-immunized maternal rat (M8) and an affected fetal offspring were incubated with CM and tissue extracts (10 mcg each) of adult heart, liver and spleen. The heart-specific binding by serum from the CM-immunized mother and her affected fetus, but not control sera, is blocked by pre-incubating the serum with CM (20 mcg).
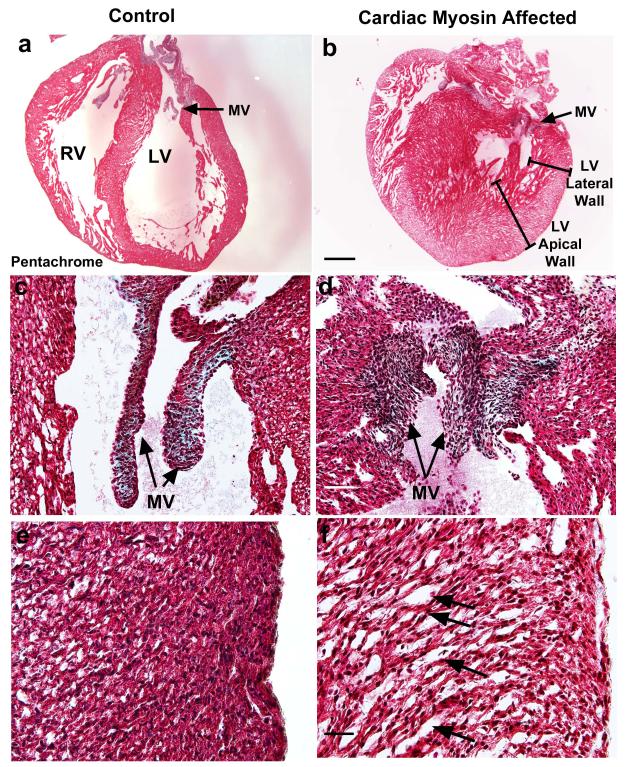
The HLHS-like pathology observed in fetuses from CM-immunized mother rats is illustrated in Fig. 3. We observed that 32% (15 of 47) of fetuses in the CM treatment group developed a left-sided structural CHD and 28% (13 of 47) of the fetuses had reduced or hypoplastic LV cavities (Figs. 3B and 3D), while none of the control fetuses (n = 19) had cardiac abnormalities (Figs. 3A and 3C). The congenitally malformed, affected fetal hearts with an HLHS phenotype had a thickened LV myocardium (30%; 14 of 47) and/or loss of normal mitral and aortic valve structure (26%; 12 of 47) (Fig. 3D). The left-sided valve structures displayed loss of smooth rounded edges and were foreshortened. The affected fetal hearts that were severely malformed (23%; 11 of 47) displayed a 50-160% increase in LV myocardial wall thickness, whereas, the moderately malformed hearts (6%; 3 of 47) displayed a 15-50% increase in LV wall thickness. In adjuvant controls, the fetal LV free wall myocardium displays normal compact myocardium (Fig. 3E). The severely malformed hearts also displayed a “spongy” LV myocardium (Fig. 3F). Examination of hematoxylin-eosin stained maternal heart sections from all CM-immunized adult maternal test rats and adult maternal control rats receiving only adjuvant appeared normal and showed no evidence of myocarditis or any abnormal histology in either maternal group. In addition, in the fetal hearts, there was also no myocarditis or cellular infiltration of the myocardium.
Figure 3. Affected fetal rat hearts demonstrate left-sided structural cardiac malformations that are characteristic of HLHS-like congenital heart disease.
(A) Adjuvant control fetal rat heart with normal heart structures at estimated gestational day 20. (B) Affected fetal rat heart demonstrated hypoplastic left ventricular (LV) cavity with thickened LV free wall and septum at EGD20. Right ventricular (RV) free wall demonstrates similar dimensions to control. The arrow indicates mitral valve (MV). The brackets depict LV lateral and apical free walls. (C) Representative adjuvant control fetal heart shows MV with normal structure. (D) MV of affected fetal rat heart demonstrates loss of normal structure. The valve does not have smooth, rounded edges and appears foreshortened. (E) Adjuvant control fetal LV free wall myocardium displays normal compact myocardium. (F) Affected heart displays myocyte disarray and “spongy” myocardium of LV free wall. Arrows indicate areas of myocyte disarray with “spongy” myocardium. (A-B) 20x magnification. Scale bar, 600μm; (C-D) 200x magnification. Scale bar, 60μm; (E-F) 400x magnification. Scale bar, 30μm. All sections (A-F) were stained with Movat’s pentachrome.
Left ventricle primarily affected in rat model with HLHS phenotype
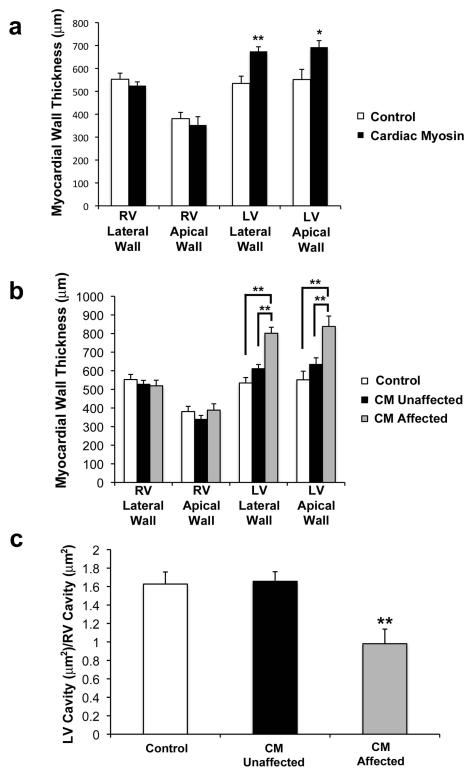
HLHS is characterized by a reduced, or hypoplastic, LV cavity that is unable to support the systemic circulation although there is anatomic variation within the classification of HLHS that yields a continuum of phenotypic heterogeneity (27, 28). To evaluate chamber-specific structural differences, comparable apical four-chamber sections of each heart were studied for cardiac morphometric measurements of the total CM treatment group (n = 47) compared to the adjuvant control group (n=19). Right ventricular (RV) myocardial thickness of the total CM treatment group was not significantly different from the adjuvant control group. In contrast, the total CM treatment group had significantly increased myocardial thickness of the LV lateral free wall (P < 0.001) and LV apical free wall (P < 0.05) compared to the adjuvant control group (Fig. 4A). Statistical analysis of the affected fetal hearts with the HLHS phenotype (n = 15) versus the unaffected fetal hearts (n = 32) and adjuvant controls (Fig. 4B) demonstrated no difference in RV myocardial thickness between the three groups. Further, the HLHS-like phenotype in fetal hearts had increased LV lateral free wall thickness compared to the unaffected fetal hearts (P < 0.0001) and adjuvant controls (P < 0.0001). Affected fetal hearts with the HLHS-like phenotype also demonstrated increased LV apical free wall thickness compared to the unaffected fetal hearts (P = 0.0009) and adjuvant controls (P = 0.0001). There was no significant difference in the LV wall thickness of the unaffected compared to adjuvant control fetal hearts. These findings indicate that the maternal immune response against CM was associated with increased LV, but not RV myocardial wall thickness.
Figure 4. Increased left ventricular (LV) myocardial thickness and LV cavity hypoplasia is present in affected hearts from CM immunized mothers.
(A) CM immunized group (n = 47 fetuses) had increased lateral free wall thickness (**P = 0.0009) and increased LV apical free wall thickness (*P = 0.02) compared to adjuvant controls (n = 19 fetuses). There was no difference in right ventricular (RV) thickness between groups. (B) Affected fetal hearts (n = 15) had increased LV lateral free wall thickness compared to unaffected fetal hearts (n = 32) (**P = <0.0001) and adjuvant controls (n=19) (**P = <0.0001). Affected fetal hearts had increased LV apical free wall thickness compared to unaffected fetal hearts (**P = 0.0009) and adjuvant controls (**P = 0.0001). There was no difference in RV lateral or apical myocardial thickness between groups. (C) Affected fetal hearts (n = 13) had decreased LV/RV lumen area ratio compared to unaffected fetal hearts (n = 30) (**P = 0.002) and adjuvant controls (n = 19) (**P = 0.007) indicating that affected hearts had reduced, or hypoplastic, LV cavity dimensions. Two variables (A) were analyzed using two-sided independent t-test. Three variables (B-C) were analyzed using two-way analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons. Bars shown are the mean ± s.e.m. All bars represent average of RV and LV dimensions for morphometric analysis of each group.
The significant impact of HLHS results from altered development of the LV and left-sided valve structures, characterized by a reduced or hypoplastic LV cavity rendering the heart unable to support the systemic circulation (29, 30). To determine ventricular chamber size in our model, the LV lumen area and RV lumen area of each specimen was measured, and an LV/RV lumen area ratio was used to determine relative LV chamber size. The RV served as an internal control for this comparison. The affected HLHS-like phenotype had a significantly decreased LV/RV lumen area ratio when compared to the unaffected normal fetal hearts (P = 0.002) and adjuvant controls (P = 0.007) (Fig. 4C). There was no significant difference in the LV/RV lumen area ratio between unaffected and adjuvant control fetal hearts. These findings indicate that the affected fetal hearts had a hypoplastic LV cavity reminiscent of HLHS in human infants.
Increased antibody binding in affected hearts
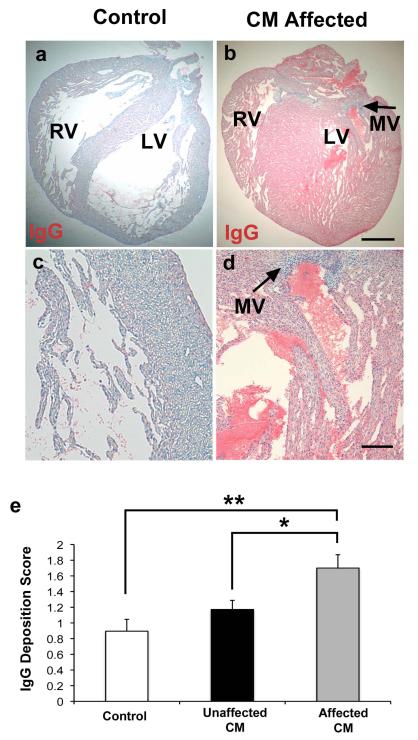
Since maternally acquired IgG is essential in newborn immunity, maternally transferred antibodies can mediate tissue injury (31, 32), we examined IgG binding to the fetal hearts in our study. The myocardium of affected fetal hearts with the HLHS phenotype had increased IgG deposition compared to unaffected fetal hearts (P = 0.03) and adjuvant controls (P = 0.002) (Fig. 5). Moreover, IgG deposition in the hearts of offspring of CM immunized mothers correlated with the observed cardiac malformations. The IgG deposition was principally found in the fetal myocardium with minimal staining on valve structures or atrioventricular cushions. There was no IgG deposition in maternal heart sections from CM treated or control groups.
Figure 5. Increased immunoglobulin (IgG) deposition in myocardium of affected hearts.
Both affected and unaffected hearts were observed in the CM immunized group (32% of fetal rats developed HLHS-like phenotype). Anti-rat IgG alkaline phosphatase conjugated was used to detect IgG as indicated by Fast Red substrate against a counterstain of Mayer’s hematoxylin. (A and C) Adjuvant control hearts demonstrated minimal anti-rat IgG staining. (B and D) Affected hearts demonstrated extensive anti-rat IgG binding, indicated by increased Fast Red substrate staining. Left-sided mitral valve (MV) identified by arrows on affected heart. There is more IgG deposited within the myocardium of the affected hearts compared with valve structures. PBS treated control sections did not stain red and were blue and negative for IgG (not shown). (E) Scored results for amount of anti-rat IgG deposition. The affected group (n = 15) had increased IgG deposition compared to the unaffected group (n = 32 fetuses) (*P = 0.03) and adjuvant control (n = 19 fetuses) (**P = 0.002) groups. (A-B) 20x magnification. Scale bar, 600μm. (c-d) 100x magnification. Scale bar 125 μm. Three variables (E) analyzed using twoway analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons. Average of data shown in bars are mean ± s.e.m.
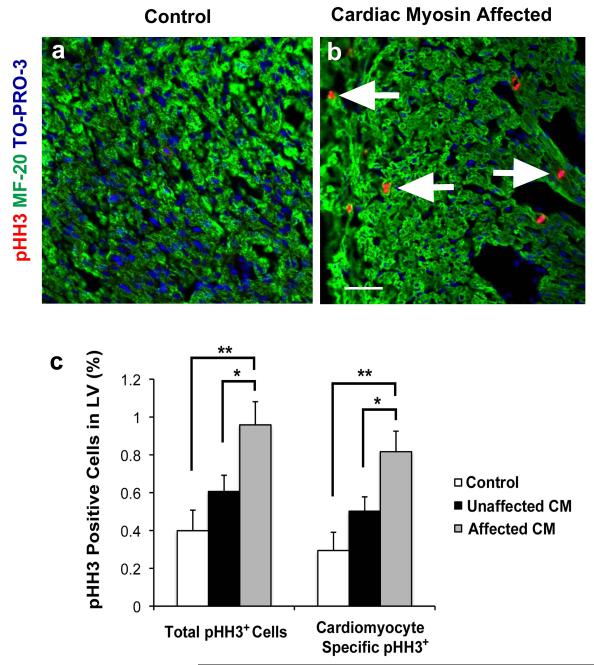
Cardiomyocyte proliferation increased in the HLHS phenotype
Although the etiologies of certain subtypes of HLHS may originate through primary valve defects (7, 28, 33) there is evidence that HLHS may result as a consequence of abnormal myocyte proliferation during development (34, 35). Further, to determine whether increased cardiomyocyte proliferation contributed to the thickening of the LV myocardium in our model both compact and trabeculated myocardium were examined as the pathologic specimens had increased thickness of both. Affected fetal hearts with the HLHS phenotype had an increased total LV proliferative rate compared to the unaffected fetal hearts (P = 0.05) and adjuvant controls (P = 0.003) (Figs. 6A and 6B). The affected fetal hearts with the HLHS phenotype had an increased cardiomyocyte-specific proliferative rate compared to the unaffected fetal hearts (P = 0.05) and adjuvant controls (P = 0.002) (Fig. 6C). In contrast, there were no significant differences between groups in the proliferative rate of non-myocyte nuclei or in apoptosis of the myocardium or valve structures (data not shown). Further, maternal hearts from CM treated groups did not display any abnormal histopathology or changes in cardiomyocyte proliferation.
Figure 6. Increased total proliferation and cardiomyocyte specific proliferation of the LV myocardium in affected hearts with the HLHS phenotype.
(A-B) Adjuvant control and affected hearts stained with pHH3, MF-20, and TO-PRO-3. There were more pHH3 positive nuclei in the affected heart compared with adjuvant control. Arrows indicate pHH3 positive nuclei. (C) Histogram demonstrating that the percentage of pHH3 positive total cells was greater in the affected group (n = 15) compared with the unaffected group (n = 32) (*P = 0.05) and adjuvant control (n = 19 fetuses) (**P = 0.003) groups. Myocytes were identified by MF-20 stain and manually counted. Myocyte specific percentage of pHH3 positive cells was greater in the affected group compared with the unaffected (*P = 0.05) and adjuvant control (**P = 0.002) groups. Both affected and unaffected groups were immunized with CM. There is no difference between the unaffected group and the adjuvant control group in either total proliferation or cardiomyocyte specific proliferation. (A-B) 400x magnification. Scale bar = 50μm. Three variables (C) analyzed using two-way analysis of variance (ANOVA) with Tukey-Kramer adjustment for multiple comparisons. The data shown are the mean ± s.e.m.
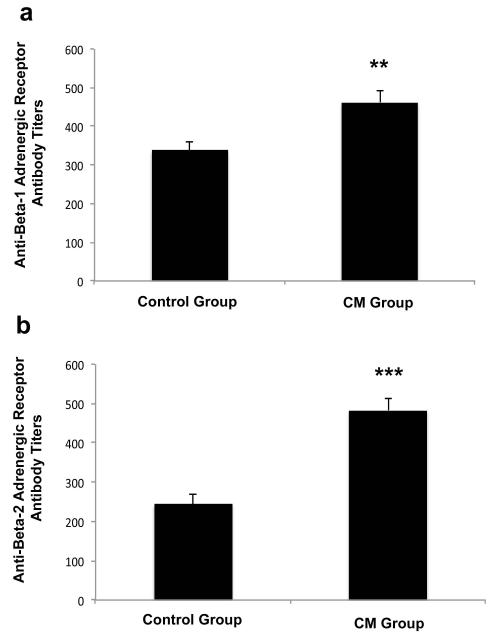
Increased anti-β-adrenergic receptor titers in affected fetus
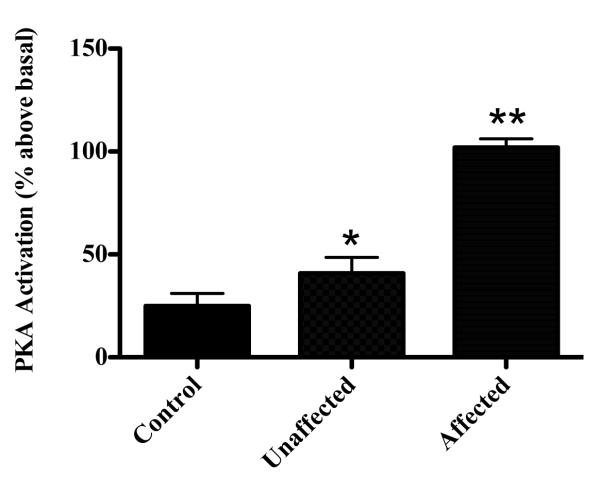
Our previous work has shown that anti-CM antibodies cross-react with the β-adrenergic (β-AR) receptor on the cardiomyocyte surface and induce cAMP-dependent protein kinase A activity in heart cells (21). Since the β-AR also plays a regulatory role in cardiomyocyte proliferation in early life (36), we measured anti-β-AR titers in affected fetus that had elevated anti-CM titers. We found increased anti-β1-AR (P = 0.007) (Fig. 7A) and anti-β2-AR (P < 0.0001) (Fig. 7B) antibody titers in fetal sera from the CM treatment group compared to adjuvant control sera. Since β-ARs on the heart cell surface stimulate cAMP-dependent protein kinase A (PKA) activity, we next incubated fetal sera with cultured rat heart cells (H9c2 primary cells) to determine if sera from CM treated animals could modulate PKA activity. We found a significant increase in PKA activity above basal levels only in fetal sera from the CM treatment group that developed heart pathology (affected group) compared with fetuses from the CM treatment group that were unaffected (p=0.00023) or controls (p=0.0005) (Fig. 8). There was no significant difference in PKA activity in sera from unaffected fetuses from the CM group or controls (p=0.1849).
Figure 7. Increased anti-ß adrenergic receptor antibodies and PKA activity in fetal serum of CM-immunized rats with the HLHS phenotype.
Fetal serum from the CM immunized group (n = 29) had significantly increased (A) anti-ß1 AR antibodies (**P = 0.007) and (B) anti-β2 AR antibodies (***P < 0.0001) compared to adjuvant controls (n = 15).
Figure 8. Protein kinase A activity is increased in fetal serum from affected fetuses but not in unaffected offspring or controls.
Rat cardiac myocytes (H9c2 primary cell line) incubated with fetal serum show a significant increase in PKA activity above basal levels only in fetal sera from the CM treatment group that developed heart pathology (affected group) compared with fetuses from the CM treatment group that were unaffected (p=0.00023) or controls (p=0.0005). Two-variables analyzed by two-sided independent t-test. Averaged data shown in bars are mean ± s.e.m.
Discussion
This study presents data supporting a novel concept that defines an HLHS-like phenotype caused by a maternal autoimmune response against CM. Observations in our fetal rat model of elevated autoantibodies against CM, including heart-specific binding of CM-immunized maternal and affected fetal serum, IgG deposition in fetal rat hearts, and the appearance of an HLHS-like phenotype, support the hypothesis of an immune-mediated pathogenic mechanism in the development of congenital HLHS-like lesions in the fetal rat heart.
Up to 70% of human cases of HLHS have a reduced LV cavity surrounded by a thickened LV myocardium (4, 28). Affected fetal rat hearts from CM immunized mothers displayed an HLHS phenotype similar to human infants including the characteristic hypoplastic or decreased LV cavity dimensions although the RV dimensions were preserved. The affected fetal hearts also had an increased LV myocardial thickness, loss of normal structure of the mitral and aortic valves along with a disorganized myocardium as seen in HLHS on histopathology (3, 35, 37, 38). Moreover, cardiomyocyte proliferation was increased in the affected animal hearts that could contribute to the reduction of LV cavity size, as in HLHS. The severely malformed rat hearts also displayed a “spongy” LV myocardium, which has also has been described in histopathology reports of HLHS (38).
The development of the congenital HLHS-like phenotype in our model in association with elevated titers against CM occurred in approximately 32% of fetal rats. This rate is comparable to other autoimmune animal models, including experimental models of neonatal lupus where congenital heart block phenotype was observed in 20% to 30% of immunized pups (39). Although it is not clear why the disease process is primarily localized to left-sided heart structures, it is well known that in fetal circulation oxygen and antibody rich blood returning from the placenta will preferentially pass through the foramen ovale into the left side of the heart. Thus, fetal left-sided heart structures that are exposed to higher maternal antibody concentrations could be more susceptible to damage than right-sided structures. Further, maternal hearts in animals immunized against CM did not demonstrate any structural or inflammatory cardiac defects of the myocardium or valves when compared to adjuvant injected controls. Thus, our observations suggest that the developing fetal heart is more or uniquely susceptible to immune mediated injury than the mature adult heart, and that immune responses against CM led to malformations of the LV.
The amount of IgG deposition in the myocardium of adult rodents immunized with CM has been shown to correlate with autoimmune manifestations (17, 21). We found that the maternal immune response against CM was associated with IgG deposition coincident with left-sided congenital heart malformations in their progeny. The relative lack of antibody staining in the valves suggested that in our model of valvular abnormalities have occurred secondary to the initial myocardial insult. Elevated anti-CM autoantibody titers in both maternal and fetal serum of the CM immunized group indicated positive transplacental transfer of maternal anti-CM autoantibodies. Transplacental antibody-mediated injury to the fetal heart is the proposed mechanism for a variety of diseases of the fetus and newborn including erythroblastosis fetalis (or hemolytic disease of the newborn), hypothyroidism, lupus erythematosus, pemphigus vulgaris, and thyrotoxicosis (40). There is also precedence for such a mechanism leading to fetal heart disease, in congenital heart block. Cardiac injury in congenital heart block is presumed to arise from the active transplacental transport of maternal IgG antibodies into the fetal circulation. In this condition, injury to conduction tissue of the fetal heart by autoantibodies leads to destruction of normal pacing mechanisms (14-16).
Intrauterine and perinatal exposure of the fetus to maternal IgG during pregnancy when the IgG is transported from mother to fetus across the placenta, beginning at approximately 12 weeks gestation in humans (14, 32). Early findings of HLHS, as diagnosed via prenatal echocardiography, are appreciated between 14-24 weeks gestation in nearly all cases (41, 42). This gestational period correlates with the chronology of transplacental transfer of maternal IgG when maternally transferred antibodies can mediate tissue injury (14). Recent work has demonstrated that myocytes in HLHS are well differentiated (37), suggesting that HLHS results from an in-utero insult to the fetus after the completion of primary cardiac morphogenesis (i.e. after the first 8 weeks of human pregnancy) and corresponding to around gestation day 15.5 in the rat (34, 37, 43). IgG antibody distribution in our model suggests that the heart defects in affected animals were primarily myocardial in origin and that the valve abnormalities may be secondary in nature.
We examined a possible mechanism by which the observed anti-CM IgG response in the CM group could lead to the fetal heart pathology in our model. Passive transfer of cross-reactive anti-CM/anti-β-AR IgG autoantibodies into adult rats can cause myocardial injury (21). Further, immune-absorption of circulating autoantibodies improves cardiac function of patients with cardiomyopathy (44, 45). In animal models, antibody-induced cardiomyopathy induced by stimulation of the β1-AR agonist, can be prevented by pharmacological neutralization of functionally active anti-β1-AR antibodies or by the elimination of antibodies by anti-β1-AR-selective immune-absorption (46). Moreover, blocking the β-AR inhibits cardiomyocyte proliferation (36) suggesting a key role for the β-AR in the heart. In addition, studies in rats and humans have shown that removal of IgG or removal of specific anti-CM and anti-BAR antibodies from the sera depletes the PKA activation properties of the sera (21, 22). Studies in our rat model showed that fetal sera contained elevated IgG autoantibody titers against CM as well as the β-AR, and furthermore, only sera from affected fetuses stimulated PKA activity in rat heart cells in culture. These data strengthen our hypothesis that functional signaling autoantibodies reactive against both the CM and the β-AR are associated with the observed HLHS-like phenotype in the Lewis rat. Further, the known cross-reactivity between CM and the β-AR may mediate the increased cardiomyocyte proliferation contributing to the thickening of the LV myocardium, and subsequently to a reduction in LV cavity size. Other antigenic targets are also plausible as etiologic in abnormal fetal cardiomyocyte development resulting in an HLHS-like phenotype. Pathogenesis of certain CHD such as HLHS may be influenced, either wholly or in part, by alterations in fetal cardiomyocyte proliferation or differentiation caused by autoantibodies directed to CM or related antigens.
In conclusion, our evidence suggests a potential novel autoimmune mechanism that may contribute in part to the pathogenesis of CHD such as HLHS. However, the immune response genes and factors that can impact such a response, in particular as seen in other autoimmune and cardiac diseases (47), including autoimmune myocarditis (17), remain to be elucidated. The presence of abundant IgG deposition primarily in the myocardium of affected fetuses, in addition to the absence of cellular inflammatory changes in the CM immunized mothers and their offspring, may suggest an antibody-mediated alteration in susceptible individuals. The binding of IgG to the β-AR and activation of signaling by increasing PKA activity could be a potential plausible mechanism that stimulates myocyte proliferation, leading to downstream morphologic and histologic pathology seen in the affected fetuses. The potential role of alterations in regulatory mechanisms of cardiomyocyte proliferation in the fetal myocardium in response to autoantibodies directed to CM, the β-AR, or other potential antigenic targets, deserves further scrutiny.
Supplementary Material
Acknowledgments
We gratefully acknowledge the technical assistance of Mitali Basu and Christopher Lam with the immunization experiments, R. Scott Baker and Danielle Herbert, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH and Heidi Wagner, Washington University Medical Center, St Louis, MO for tissue sectioning and morphological measurements. We thank Adita Mascaro-Blanco, Kathy Alvarez and Stanley Kosanke, DVM, PhD, University of Oklahoma Health Sciences Center, Oklahoma City, OK for excellent technical support for the immunoassays, phosphohistone and immunohistochemistry. We also thank Dennis Hanseman, University of Cincinnati, OH for statistical support. Madeleine Cunningham is Chief Scientific Officer for Moleculera Labs. The other authors have no conflict of interest to report.
This work was supported in part by NIH grants R21-HL104391 (to PE) NIH F32- HL103054 (to CRC), NIH R01-HL56267 (to MWC) and NIH R37-HL35280 (to MWC) and funds from the Saving Tiny Hearts Society. MWC is the recipient of an NHLBI MERIT Award.
2: Non-standard abbreviations used
- β-AR
β adrenergic receptor
- CHD
congenital heart disease
- CM
cardiac myosin
- HLHS
hypoplastic left heart syndrome
- LV
left ventricle
- MV
mitral valve
- RV
right ventricle
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2011;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Sedmera D, Cook AC, Shirali G, McQuinn TC. Current issues and perspectives in hypoplasia of the left heart. Cardiol Young. 2005;15:56–72. doi: 10.1017/S1047951105000132. [DOI] [PubMed] [Google Scholar]
- 4.Mahle WT, Spray TL, Wernovsky G, Gaynor JW, Clark BJ., 3rd Survival after reconstructive surgery for hypoplastic left heart syndrome: A 15-year experience from a single institution. Circulation. 2000;102:III136–141. doi: 10.1161/01.cir.102.suppl_3.iii-136. [DOI] [PubMed] [Google Scholar]
- 5.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106:I82–89. [PubMed] [Google Scholar]
- 6.Eghtesady P, Brar A, Hall M. Seasonality of hypoplastic left heart syndrome in the United States: a 10-year time-series analysis. J Thorac Cardiovasc Surg. 2010;141:432–438. doi: 10.1016/j.jtcvs.2010.06.060. [DOI] [PubMed] [Google Scholar]
- 7.Hinton RB, Martin LJ, Rame-Gowda S, Tabangin ME, Cripe LH, Benson DW. Hypoplastic left heart syndrome links to chromosomes 10q and 6q and is genetically related to bicuspid aortic valve. J Am Coll Cardiol. 2009;53:1065–1071. doi: 10.1016/j.jacc.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinton RB, Jr., Martin LJ, Tabangin ME, Mazwi ML, Cripe LH, Benson DW. Hypoplastic left heart syndrome is heritable. J Am Coll Cardiol. 2007;50:1590–1595. doi: 10.1016/j.jacc.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 9.Grossfeld PD. Hypoplastic left heart syndrome: it is all in the genes. J Am Coll Cardiol. 2007;50:1596–1597. doi: 10.1016/j.jacc.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 11.Grossfeld P. Hypoplastic left heart syndrome: new insights. Circ Res. 2007;100:1246–1248. doi: 10.1161/01.RES.0000268192.20525.c2. [DOI] [PubMed] [Google Scholar]
- 12.Eghtesady P. Hypoplastic left heart syndrome: Rheumatic heart disease of the fetus? Med Hypotheses. 2006;66:554–565. doi: 10.1016/j.mehy.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Ni J, Bowles NE, Kim YH, Demmler G, Kearney D, Bricker JT, Towbin JA. Viral infection of the myocardium in endocardial fibroelastosis. Molecular evidence for the role of mumps virus as an etiologic agent. Circulation. 1997;95:133–139. doi: 10.1161/01.cir.95.1.133. [DOI] [PubMed] [Google Scholar]
- 14.Chameides L, Truex RC, Vetter V, Rashkind WJ, Galioto FM, Jr., Noonan JA. Association of maternal systemic lupus erythematosus with congenital complete heart block. N Engl J Med. 1977;297:1204–1207. doi: 10.1056/NEJM197712012972203. [DOI] [PubMed] [Google Scholar]
- 15.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, Lee LA, Provost TT, Reichlin M, Rider L, Rupel A, Saleeb S, Weston WL, Skovron ML. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 16.Scott JS, Maddison PJ, Taylor PV, Esscher E, Scott O, Skinner RP. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983;309:209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- 17.Liao L, Sindhwani R, Rojkind M, Factor S, Leinwand L, Diamond B. Antibody-mediated autoimmune myocarditis depends on genetically determined target organ sensitivity. J Exp Med. 1995;181:1123–1131. doi: 10.1084/jem.181.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neu N, Rose NR, Beisel KW, Herskowitz A, Gurri-Glass G, Craig SW. Cardiac myosin induces myocarditis in genetically predisposed mice. J Immunol. 1987;139:3630–3636. [PubMed] [Google Scholar]
- 19.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of giant cell myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Cryptic epitope identified in rat and human cardiac myosin S2 region induces myocarditis in the Lewis rat. J Immunol. 2004;172:3225–3234. doi: 10.4049/jimmunol.172.5.3225. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Heuser JS, Cunningham LC, Kosanke SD, Cunningham MW. Mimicry and antibody-mediated cell signaling in autoimmune myocarditis. J Immunol. 2006;177:8234–8240. doi: 10.4049/jimmunol.177.11.8234. [DOI] [PubMed] [Google Scholar]
- 22.Mascaro-Blanco A, Alvarez K, Yu X, Lindenfeld J, Olansky L, Lyons T, Duvall D, Heuser JS, Gosmanova A, Rubenstein CJ, Cooper LT, Kem DC, Cunningham MW. Consequences of unlocking the cardiac myosin molecule in human myocarditis and cardiomyopathies. Autoimmunity. 2008;41:442–453. doi: 10.1080/08916930802031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krisher K, Cunningham MW. Myosin: a link between streptococci and heart. Science. 1985;227:413–415. doi: 10.1126/science.2578225. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham MW, Antone SM, Gulizia JM, McManus BM, Fischetti VA, Gauntt CJ. Cytotoxic and viral neutralizing antibodies crossreact with streptococcal M protein, enteroviruses, and human cardiac myosin. Proc Natl Acad Sci U S A. 1992;89:1320–1324. doi: 10.1073/pnas.89.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvin JE, Hemric ME, Ward K, Cunningham MW. Cytotoxic mAb from rheumatic carditis recognizes heart valves and laminin. The Journal of clinical investigation. 2000;106:217–224. doi: 10.1172/JCI7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tobacman LS, Adelstein RS. Enzymatic comparisons between light chain isozymes of human cardiac myosin subfragment-1. J Biol Chem. 1984;259:11226–11230. [PubMed] [Google Scholar]
- 27.Sinha SN, Rusnak SL, Sommers HM, Cole RB, Muster AJ, Paul MH. Hypoplastic left ventricle syndrome. Analysis of thirty autopsy cases in infants with surgical considerations. Am J Cardiol. 1968;21:166–173. doi: 10.1016/0002-9149(68)90316-0. [DOI] [PubMed] [Google Scholar]
- 28.Hickey EJ, Caldarone CA, McCrindle BW. Left ventricular hypoplasia: a spectrum of disease involving the left ventricular outflow tract, aortic valve, and aorta. J Am Coll Cardiol. 2012;59:S43–54. doi: 10.1016/j.jacc.2011.04.046. [DOI] [PubMed] [Google Scholar]
- 29.Allan LD, Sharland G, Tynan MJ. The natural history of the hypoplastic left heart syndrome. Int J Cardiol. 1989;25:341–343. doi: 10.1016/0167-5273(89)90226-x. [DOI] [PubMed] [Google Scholar]
- 30.Tchervenkov CI, Jacobs JP, Weinberg PM, Aiello VD, Beland MJ, Colan SD, Elliott MJ, Franklin RC, Gaynor JW, Krogmann ON, Kurosawa H, Maruszewski B, Stellin G. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young. 2006;16:339–368. doi: 10.1017/S1047951106000291. [DOI] [PubMed] [Google Scholar]
- 31.Gill HK, Splitt M, Sharland GK, Simpson JM. Patterns of recurrence of congenital heart disease: an analysis of 6,640 consecutive pregnancies evaluated by detailed fetal echocardiography. J Am Coll Cardiol. 2003;42:923–929. doi: 10.1016/s0735-1097(03)00853-2. [DOI] [PubMed] [Google Scholar]
- 32.Buyon JP, Friedman DM. Autoantibody-associated congenital heart block: the clinical perspective. Curr Rheumatol Rep. 2003;5:374–378. doi: 10.1007/s11926-003-0024-6. [DOI] [PubMed] [Google Scholar]
- 33.Ludman P, Foale R, Alexander N, Nihoyannopoulos P. Cross sectional echocardiographic identification of hypoplastic left heart syndrome and differentiation from other causes of right ventricular overload. Br Heart J. 1990;63:355–361. doi: 10.1136/hrt.63.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.deAlmeida A, McQuinn T, Sedmera D. Increased ventricular preload is compensated by myocyte proliferation in normal and hypoplastic fetal chick left ventricle. Circ Res. 2007;100:1363–1370. doi: 10.1161/01.RES.0000266606.88463.cb. [DOI] [PubMed] [Google Scholar]
- 35.Sedmera D, Hu N, Weiss KM, Keller BB, Denslow S, Thompson RP. Cellular changes in experimental left heart hypoplasia. Anat Rec. 2002;267:137–145. doi: 10.1002/ar.10098. [DOI] [PubMed] [Google Scholar]
- 36.Tseng YT, Kopel R, Stabila JP, McGonnigal BG, Nguyen TT, Gruppuso PA, Padbury JF. Beta-adrenergic receptors (betaAR) regulate cardiomyocyte proliferation during early postnatal life. FASEB J. 2001;15:1921–1926. doi: 10.1096/fj.01-0151com. [DOI] [PubMed] [Google Scholar]
- 37.Bohlmeyer TJ, Helmke S, Ge S, Lynch J, Brodsky G, Sederberg JH, Robertson AD, Minobe W, Bristow MR, Perryman MB. Hypoplastic left heart syndrome myocytes are differentiated but possess a unique phenotype. Cardiovasc Pathol. 2003;12:23–31. doi: 10.1016/s1054-8807(02)00127-8. [DOI] [PubMed] [Google Scholar]
- 38.O’Connor WN, Stahr BJ, Cottrill CM, Todd EP, Noonan JA. Ventriculocoronary connections in hypoplastic right heart syndrome: autopsy serial section study of six cases. J Am Coll Cardiol. 1988;11:1061–1072. doi: 10.1016/s0735-1097(98)90066-3. [DOI] [PubMed] [Google Scholar]
- 39.Clancy RM, Buyon JP. More to death than dying: apoptosis in the pathogenesis of SSA/Ro-SSB/La-associated congenital heart block. Rheum Dis Clin North Am. 2004;30:589–602. doi: 10.1016/j.rdc.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 40.Chang C. Neonatal autoimmune diseases: a critical review. J Autoimmun. 2012;38:J223–238. doi: 10.1016/j.jaut.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Marshall AC, Tworetzky W, Bergersen L, McElhinney DB, Benson CB, Jennings RW, Wilkins-Haug LE, Marx GR, Lock JE. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535–539. doi: 10.1016/j.jpeds.2005.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Tworetzky W, Wilkins-Haug L, Jennings RW, van der Velde ME, Marshall AC, Marx GR, Colan SD, Benson CB, Lock JE, Perry SB. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125–2131. doi: 10.1161/01.CIR.0000144357.29279.54. [DOI] [PubMed] [Google Scholar]
- 43.Neu N, Craig SW, Rose NR, Alvarez F, Beisel KW. Coxsackievirus induced myocarditis in mice: cardiac myosin autoantibodies do not cross-react with the virus. Clin Exp Immunol. 1987;69:566–574. [PMC free article] [PubMed] [Google Scholar]
- 44.Felix SB, Staudt A, Friedrich GB. Improvement of cardiac function after immunoadsorption in patients with dilated cardiomyopathy. Autoimmunity. 2001;34:211–215. doi: 10.3109/08916930109007387. [DOI] [PubMed] [Google Scholar]
- 45.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 46.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, Lohse MJ. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. The Journal of clinical investigation. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feinstein JA, Benson DW, Dubin AM, Cohen MS, Maxey DM, Mahle WT, Pahl E, Villafane J, Bhatt AB, Peng LF, Johnson BA, Marsden AL, Daniels CJ, Rudd NA, Caldarone CA, Mussatto KA, Morales DL, Ivy DD, Gaynor JW, Tweddell JS, Deal BJ, Furck AK, Rosenthal GL, Ohye RG, Ghanayem NS, Cheatham JP, Tworetzky W, Martin GR. Hypoplastic left heart syndrome: current considerations and expectations. J Am Coll Cardiol. 2012;59:S1–42. doi: 10.1016/j.jacc.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.