Abstract
Background and Aims
Consumption of foods that modulate inflammatory stress in genetically-prone individuals may influence development of cardiometabolic diseases. Isoflavones in soy-derived foods function as phytoestrogens, have antioxidant and anti-inflammatory activity, inhibit protein-tyrosine kinase activity, and may be atheroprotective. We examined the relationship between soy food consumption and inflammatory responses to endotoxemia, postprandial responses to oral lipid tolerance test (OLTT), and insulin sensitivity from frequently sampled intravenous tolerance tests (FSIGTT).
Methods and Results
We administered low-dose endotoxin (LPS 1 ng/kg) to induce transient endotoxemia in young, healthy volunteers (N=215) of African (AA), and European (EA) ancestry as part of the GENE Study. We further supported these findings in two independent samples: the MECHE Study and NHANES. Soy food consumption was a significant predictor of peak cytokine response following LPS. Individuals with moderate-high (>1.48mg/day, N=65) vs. low-no (<1.48mg/day, N=150) isoflavone consumption had significantly higher tumor necrosis factor alpha (TNFα) post-LPS (AUC, P=0.009). Further, high-isoflavone consumers were protected against inflammation-induced decline in insulin sensitivity (SI) in GENE. We observed significant differences by soy consumption in the interferon gamma (IFNγ) response to OLTT, and the insulin response to OGTT in MECHE, as well as significantly lower fasting insulin, and 2-hour glucose post-OGTT in EA NHANES subjects.
Conclusion
We demonstrate that soy consumption may influence inflammatory and metabolic responses. In research of nutritional exposures, measuring evoked phenotypes may be more informative than describing resting characteristics.
Clinical Trial Registry
The GENE Study was registered under NCT00953667 and the MECHE Study under NCT01172951, both at clinicaltrials.gov.
Keywords: Cardiometabolic disease, endotoxemia, LPS, isoflavone, soy, inflammation, insulin sensitivity
INTRODUCTION
Inflammation is a key component of several cardiometabolic diseases, including obesity, type 2 diabetes (T2D) and atherosclerotic cardiovascular disease (CVD)[1]. While many factors, including genetic, environmental and microbial, influence the development of a pro-inflammatory state, habitual diet may be a key inflammatory regulator[2]. We utilize evoked endotoxemia (LPS) in healthy individuals as a model of physiological responses to inflammatory stimuli, inflammation-induced insulin resistance, and cardiometabolic risk[3–5], with relevance to postprandial metabolic endotoxemia[6]. Dietary components which modulate response to LPS may influence transient postprandial inflammatory stress, affect ability to appropriately resolve inflammatory stimuli, and influence chronic cardiometabolic disease and diet-induced obesity.
Bioactive food compounds may have important health-modulating effects. Dietary plant-derived phytochemicals have anti-inflammatory and antioxidant properties that may be protective against disease development [7]. Isoflavones, primarily genistein (~50–60%), daidzein (~30–40%), and glycitein (~5–10%), are found in high concentrations in soy-derived foods[8]. Isoflavones function as phytoestrogens [9], inhibit protein-tyrosine kinase activity[10], and may have an anti-proliferative effect on cancer cells[11]. Although human epidemiological and interventional data remain inconclusive, mounting evidence suggests that isoflavones may be atheroprotective [12, 13].
As part of the Genetics of Evoked Responses to Niacin and Endotoxemia (GENE) Study [4], we administered a low dose of endotoxin (LPS 1 ng/kg) to induce a controlled inflammatory response. We found that dietary isoflavone intake was associated with the inflammatory response to endotoxemia, and with endotoxemia-induced changes in insulin sensitivity. The findings were supported by complementary analyses in two independent samples; the MECHE study (www.ucd.ie/jingo/) and NHANES (www.cdc.gov/nchs/nhanes.htm).
SUBJECTS AND METHODS
GENE Study Population
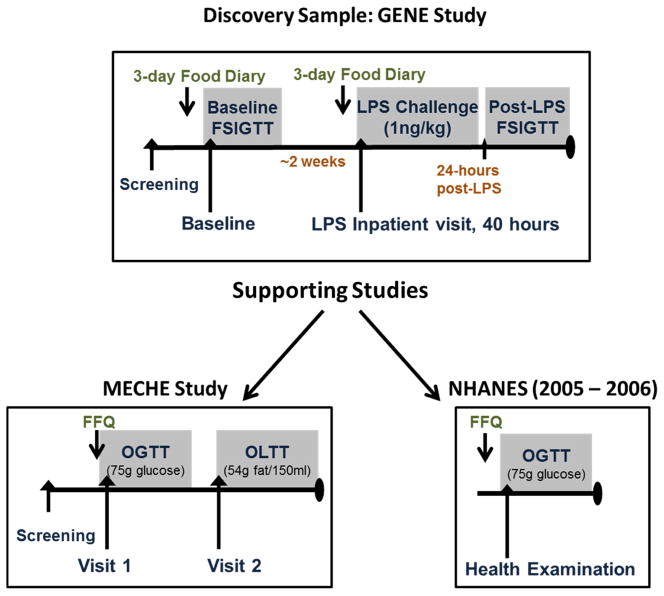
Details of the GENE Study have been published previously [4]. Briefly, healthy volunteers (N=294), non-smokers, age 18–45, BMI 18–30 kg/m2, African American (AA) or European (EA) ancestry were recruited to an inpatient endotoxin challenge (1ng/kg LPS), and frequently sampled intravenous glucose tolerance tests (FSIGTT) pre- and post-LPS at the University of Pennsylvania (UPenn) Clinical and Translational Research Center (CTRC). Subjects who completed dietary records (N=215) were analyzed here. An overview of the GENE-Diet Study is shown in Figure 1. The GENE study was conducted in accordance with UPenn’s IRB with regulatory oversight by the FDA (LPS: IND# 5984) and an NIH-appointed data-safety and monitoring board. All subjects provided informed written consent. The GENE Study was registered under NCT00953667 at Clinicaltrials.gov.
Figure 1. Overview of the Discovery and Validation/Extension studies.
The relationship between dietary isoflavone intake with inflammatory markers and insulin resistance was assessed in the GENE-LPS Diet Study sample (N=215). Findings were supported by complementary analyses in the MECHE Study (N=129) and the NHANES 2005–2006 sample (N=884).
GENE Dietary Analysis
Subjects received counseling from a CTRC dietician at study initiation to ensure adherence to AHA recommendations (55–60% carbohydrate; 10–15% protein; < 30% fat; <7–10% SFA; <300mg cholesterol/day), and were instructed in the use of food records. Subjects completed 3-day records on two separate occasions; before the LPS inpatient visit, and before a separate inpatient visit (±2–4 weeks). Results were averaged across the 6 days of records for each subject to obtain an estimate of habitual consumption, and these averages used in subsequent dietary analysis. Subjects did not receive any specific counseling related to soy food intake and there was no dietary intervention implemented. Subjects who completed all dietary records were included in the current analysis (N=215 completed). Nutrient data were analyzed using Food Processor 8.1 (ESHA Research, Salem, OR).
GENE LPS Inpatient visit
The inpatient LPS visit lasted ~40 hours, as described [4]. Following overnight acclimatization, LPS (1ng/kg) was administered intravenously by a licensed physician investigator. Anthropometric measurements were recorded and multiple clinical variables assessed regularly during the visit. Serial blood draws were taken, and serum and plasma isolated for measurement of cytokines. Samples were stored at −80°C prior to analysis.
GENE Frequently Sampled Intravenous Glucose Tolerance Testing (FSIGTT)
A 50% glucose bolus (0.3 g/kg) was administered at baseline over a one minute period as described[5]. Insulin (0.03U/kg) was injected 20 minutes post-glucose. Blood samples were obtained at 22 time-points throughout the 3-hour FSIGTT, and plasma aliquots stored at −80 °C prior to measurement of insulin and glucose. Data were modeled using MINMOD [14] a computational model which incorporates the insulin and glucose values obtained during FSIGTT to calculate parameters of glucose and insulin sensitivity including the insulin sensitivity index (SI), a measure of the capacity of tissues to take up glucose in response to insulin.
GENE Laboratory analyses
As described[4] plasma levels of Tumor necrosis factor alpha (TNF-α), Interleukin-6 (IL-6), and Interleukin-1 receptor agonist (IL-1RA) were measured by ELISA (Quantikine, R&D Systems; Minneapolis, MN). Plasma insulin levels were measured in duplicate (radio-immunoassays (RIA), Linco Research, St Charles, MO) according to manufacturers’ guidelines. A Hitachi 912 automated chemistry system was used to measure plasma glucose (Wako Diagnostics, Richmond VA). High-sensitivity C-reactive protein (CRP) was measured on a Behring Nephelometer II Analyzer (Siemens Diagnostics; Munich, Germany).
Supporting Studies
MECHE
The Metabolic Challenge (MECHE) Study (www.ucd.ie/jingo/) recruited healthy volunteers (N=200, age 18–60, European Ancestry) to a protocol at University College Dublin (UCD), Ireland. Subjects with complete data were analyzed for this study. Subjects completed one or both of two metabolic challenges; an oral lipid tolerance test (N=120; 54g Fat, 16g Glucose, 550kcal) and an oral glucose tolerance test (N=129; 75g glucose bolus) as described[15, 16]. Serial blood samples were taken for measurement of glucose, insulin and cytokines. Habitual diet was assessed using the validated EPIC-Norfolk 131-item food frequency questionnaire (FFQ)[17] and analyzed using the Compositional Analyses from Frequency Estimates (CAFE) program[18]. Ethical approval was obtained from the Research Ethics Committees in UCD. All subjects provided written informed consent. MECHE was registered under NCT01172951 at Clinicaltrials.gov.
NHANES
Publicly available data from the National Health and Nutrition Examination Survey (NHANES) 2005–2006 cohort was downloaded from http://wwwn.cdc.gov/nchs/nhanes/search/nhanes05_06.aspx. The NHANES FFQ questionnaire was developed by the NIH National Cancer Institute (NCI), and based on the widely-used 124-item NCI Diet History Questionnaire[19]. Data were analyzed and daily food frequency estimates produced using the NCI’s Diet*Calc software. Variables analyzed for the present study included dietary soy intake from FFQ, fasting glucose and insulin, 2-hour glucose post-OGTT, CRP, and demographic information (age, gender, race, BMI). Complete data for N=638 EA and N=246 AA subjects were analyzed here.
Statistical Analysis
We used linear regression to identify isoflavones as dietary predictors of the inflammatory cytokine response to LPS. To facilitate interpretation, individuals were grouped into moderate-high and low-no soy consumers based on tertiles of intake in GENE. Variables were assessed for normal distribution prior to analysis, and non-normally distributed variables analyzed by non-parametric tests. Group differences were analyzed by Kruskal-Wallis and Mann-Whitney U tests, or analysis of covariance (ANCOVA), adjusting for age, race, gender, BMI. Supplementary linear models in GENE adjusted for dietary macronutrients, micronutrients, vitamins, minerals and baseline CRP. Data were analyzed using SPSS 19 (IBM, Aramonk, NY). Values are presented as means, and standard deviation (SD).
RESULTS
Dietary isoflavone intake is a predictor of inflammatory response in the GENE study
Baseline anthropomorphic and inflammatory characteristics for GENE Study subjects (N=215, 50% female, 32% AA, 68% EA) are presented in Table 1 A, stratified by the upper vs. lower 2 tertiles of isoflavone intake. Dietary composition is presented in Table 1 B.
Table 1. Baseline characteristics and dietary macronutrient composition of soy consumers and soy non-consumers in the GENE Study.
EA participants had a higher proportion of soy consumers compared with AA. Dietary macronutrient composition was similar in soy consumers and non-consumers, however non-consumers had a lower intake of saturated fat.
| Low consumer (Isoflavone <= 1.48) N=150 Mean (SD) |
Moderate Consumer (Isoflavone >1.48) N=65 Mean (SD) |
P Value (K-W Test) | |
|---|---|---|---|
| A. Baseline Characteristics | |||
| Age (years) | 25.5 (6.5) | 26.0 (5.9) | 0.174 |
| Gender (male/female) | 78/72 | 32/33 | 0.711 |
| Race (EA/AA) | 94/56 | 53/12 | 0.006 |
| BMI (kg/m2) | 23.9 (2.9) | 23.2 (2.6) | 0.118 |
| C-Reactive Protein (mg/dL) | 1.6 (3.9) | 0.84 (1.4) | 0.01 |
| TNFα (pg/ml) Baseline | 1.3 (0.7) | 1.3 (0.8) | 0.955 |
| TNFα (pg/ml) Peak | 45.4 (41.3) | 63.8 (50.8) | 0.018 |
| IL-1RA (pg/ml) Baseline | 135.9 (99.6) | 123.0 (50.9) | 0.909 |
| IL-1RA (pg/ml) Peak | 39700 (27816) | 49030 (31840) | 0.048 |
| IL-6 (pg/ml) Baseline | 2.9 (1.9) | 2.5 (1.6) | 0.092 |
| IL-6 (pg/ml) Peak | 160.6 (144.1) | 208.2 (194.2) | 0.228 |
|
| |||
| B. Dietary Composition (% of total calories) | |||
| SFA (%) | 11.0 (3.1) | 9.7 (3.2) | 0.013 |
| MUFA (%) | 11.2 (2.6) | 11.2 (3.0) | 0.909 |
| PUFA (%) | 6.3 (2.0) | 6.8 (1.9) | 0.076 |
| Carbohydrate (%) | 51.2 (8.5) | 53.3 (8.9) | 0.148 |
| Protein (%) | 17.3 (3.9) | 16.9 (3.9) | 0.361 |
| Alcohol (%) | 1.5 (2.4) | 2.0 (2.9) | 0.142 |
| Genistien (mg/day) | 0.18 (0.17) | 6.9 (7.8) | <0.0001 |
| Daidzein (mg/day) | 0.19 (0.19) | 8.3 (9.3) | <0.0001 |
| Glycitein (mg/day) | 0.02 (0.04) | 1.7 (1.9) | <0.0001 |
| Total Isoflavone (mg/day) | 0.39 (0.39) | 16.9 (18.9) | <0.0001 |
EA: European Ancestry; AA: African Ancestry; BMI, body mass index; IL-6, interleukin 6; TNFα, tumor necrosis alpha; IL-1RA, interleukin 1 receptor agonist; SFA: Saturated fatty acids; MUFA: Monounsaturated fatty acids; PUFA: Polyunsaturated fatty acids.
Using multiple linear regression models, we assessed the relationship between macronutrients (carbohydrate, protein, saturated, monounsaturated and polyunsaturated fatty acids) as well as other bioactive dietary nutrients (beta-carotene, biochanin A, choline, coumestrol, daidzein, genistein, glycitein, lycopene, lutein) on peak cytokine responses (TNFα, IL-1RA, IL-6) post LPS. Additional models included all measured dietary variables. In all models employed, the three isoflavones, genistein, daidzein and glycitein emerged as the only significant predictors of peak cytokine response (e.g. P<0.001, 0.005, 0.05 respectively for peak TNFα, and similar for IL-1RA and IL-6). Intake of the three isoflavones was highly inter-correlated (r2=0.95–0.99, P<0.0001), and thus the sum of total isoflavone intake was used in subsequent analyses (P=0.0038). The effect of isoflavones on inflammatory response remained significant after Bonferroni correction for the number of independent tests (P<0.0042). There was no association between isoflavone intake and body composition, or baseline levels of cytokines. However there was a significant difference in baseline CRP (1.6 vs. 0.84 mg/dL CRP for low vs. moderate soy consumers, P=0.011 Mann-Whitney U non-parametric test). We ran secondary models to test for effects of potential covariates (e.g. race and SFA intake differed between soy groups); the association between total isoflavone intake and cytokine responses (TNFα, IL-1RA and IL-6) remained significant in models adjusted for age, gender, race and BMI, as well as in fully adjusted models which additionally adjusted for macronutrient and alcohol intake.
Moderate isoflavone intake is associated with a more robust cytokine response in GENE
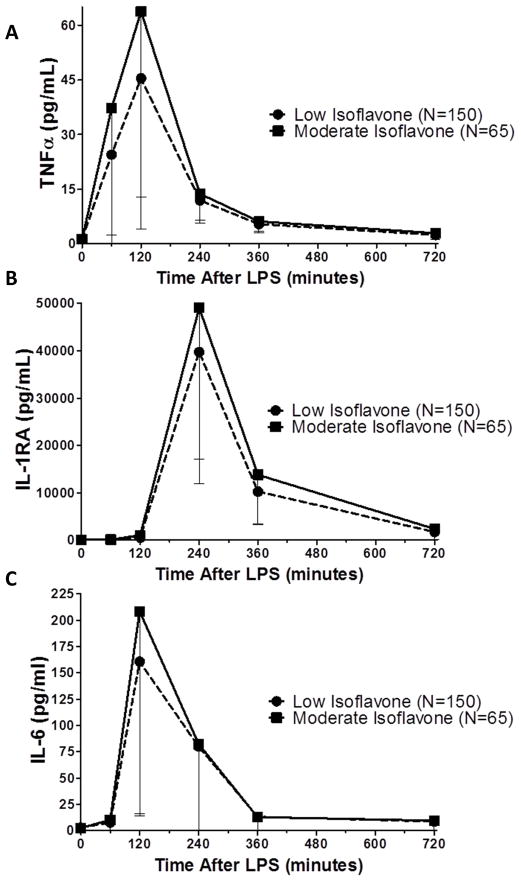
Isoflavone intake ranged from 0 to 80.47mg/day, with median intake of 0.45 mg/day. To facilitate further analysis and interpretation, we divided subjects into tertiles of isoflavone intake. We designated individuals in the highest tertile (≥1.48mg/day total isoflavones, N=65) as “moderate-high consumers”, and subjects in the lower 2 tertiles as “low-no consumers”. Soy food (tofu, tempeh, soy milk, miso) were the primary sources of isoflavones. We stress that the individuals in the highest tertile did not have a high intake of soy – the cut-point of 1.48mg/day isoflavone equates to an intake of approximately one portion of soy food a week. As shown in Figure 2 A, individuals with higher isoflavone intake had a significantly higher peak TNFα response (44 vs. 64 fold increase, P=0.018) compared with individuals consuming low or no amounts. This trend was also observed for IL-1RA (Fig. 2 B, 368 vs. 447 fold, P=0.048) and IL-6 (Fig. 2 C, 74 vs. 97 fold, P=0.2).
Figure 2. The cytokine response to LPS in individuals with moderate-high (>1.48mg/day, N=65) or low-no (<1.48mg/day, N=150) intake of dietary isoflavones in GENE.
Individuals with moderate-high isoflavone intake had a higher inflammatory response across multiple cytokines post-LPS, as measured by peak TNFα levels (A. P=0.009), peak IL-1RA levels (B. P=0.09) and peak IL-6 levels (C. P=0.2). P Value from ANCOVA analysis adjusted for age, sex, race, BMI.
Soy consumption is associated with increased inflammatory response to oral lipid load in an independent study (MECHE)
We sought additional independent studies to examine effects of isoflavones on other evoked phenotypes. As calculated isoflavone intake was not available in other studies, we used soy food consumption as a surrogate of isoflavone intake. The Metabolic Challenge (MECHE) Study [15, 16] administered an oral lipid challenge to 120 healthy subjects. We hypothesized that postprandial metabolic endotoxemia in response to a lipid load would differ by habitual soy intake. Subjects were dichotomized into soy consumers (N=20) and soy non-consumers (N=100) based on their reported intake of soy foods in the FFQ [18]. Soy consumers had significantly higher plasma interferon gamma (IFNγ) 4-hours post-OLTT (P=0.048), with a significantly greater change in IFNγ in response to OLTT (P=0.001) (Supplementary Figure 1). This thus provided indirect support for the role of soy isoflavones in the inflammatory response to endotoxemia.
Dietary isoflavone intake is associated with protection against inflammation-induced insulin resistance in GENE
We have previously demonstrated that LPS increases markers of insulin resistance in healthy individuals [3].We examined markers of insulin sensitivity obtained from FSIGTT’s at baseline, and 24 hours after LPS in a subset of GENE study participants (N=118). There were no baseline differences in insulin sensitivity (SI) between the moderate and low isoflavone consumers. Following LPS, SI decreased in both groups, however there was a significant difference in SI post-LPS (P=0.008), with moderate consumers (N=40) experiencing less of a decrease in SI than low consumers (N=78), suggesting that soy intake may be protective against inflammation-induced insulin resistance (Supplementary Figure 2). This effect was stronger in EA individuals, with no effect in AA alone; however due to small sample size (N=30 AA) and low numbers of AA soy-consumers (N=3), we lacked power to assess race differences in the GENE FSIGTT sample.
The relationship between soy intake and glucose homeostasis is validated in independent samples (MECHE and NHANES)
We further examined the effects of soy intake on glucose homeostasis using OGTT data from the MECHE Study, as well as the US-based National Health and Nutrition Examination Survey (NHANES). In MECHE, we observed a significant difference in the insulin response to glucose load, with lower AUC for insulin in soy consumers (N=20) compared with non-consumers (N=109) (Figure 3 A, P=0.03) despite very similar glucose curves (Figure 3 B), suggesting more efficient insulin-mediated glucose disposal. We examined fasting insulin and glucose, and 2-hour glucose post-OGTT in the NHANES 2005–2006 sample. EA soy consumers had significantly lower fasting insulin and trended towards lower fasting glucose compared with soy non-consumers (Insulin P=0.028; Glucose P=0.091), as well as significantly lower glucose levels post-OGTT (P<0.0001) (Figure 4 A and B). Further, EA soy consumers had significantly lower baseline CRP than non-consumers (0.55 vs. 0.36 mg/dL CRP, P<0.001 Mann-Whitney U). We did not observe any differences in glucose traits or CRP in NHANES AA subjects (Supplementary Figure 3A,B).
Figure 3. Insulin response to Oral Glucose Tolerance Test (OGTT) differs by soy consumption in MECHE.
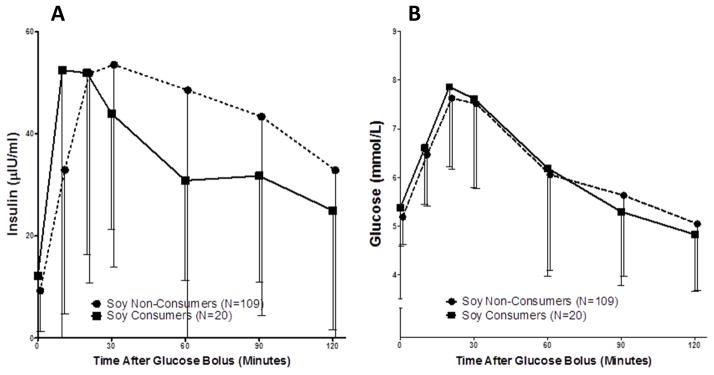
In the MECHE study, soy consumers (N=20) had a significantly lower overall insulin response to glucose bolus during OGTT (A) (ΔAUC P=0.03), despite similar glucose curves (B) to soy non-consumers (N=109).
Figure 4. Fasting insulin and glucose, and 2-hour glucose post-OGTT in NHANES are lower in EA soy consumers than soy non-consumers.
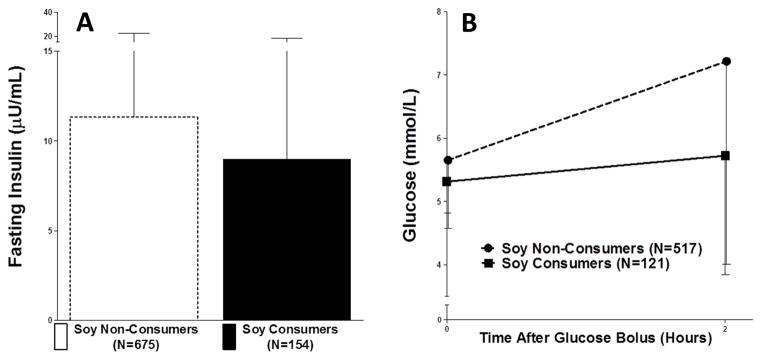
Fasting insulin was significantly lower in soy consumers (A. P=0.028 N=121 consumer, N=517 non-consumer), while fasting glucose trended towards being lower (B. P=0.091) and glucose 2-hours post-OGTT was significantly lower (C. P<0.0001).
DISCUSSION
We investigated the effects of dietary isoflavone consumption on evoked phenotypes of relevance to inflammatory cardiometabolic disease. Isoflavone consumption was associated with an increased cytokine response to evoked endotoxemia in young healthy subjects and an increased inflammatory response to an oral lipid load in a general population sample. Isoflavone consumers were somewhat protected against inflammation-induced insulin resistance, and soy consumers had lower fasting glucose and insulin, as well as improved glucose homeostasis in response to an oral glucose load. Consistent effects highlighting a role for soy/isoflavone consumption in inflammation and insulin resistance were observed across the three independent samples.
Evoked endotoxemia has been used extensively as a powerful experimental model of inflammatory cardiometabolic disease [3, 4], with a low dose approximating the inflammatory state seen in chronic metabolic disease. A higher inflammatory response to experimental LPS may be indicative of enhanced acute immune responses and rapid resolution of infection. Thus, a phenotype characterized by lower resting levels of inflammation, coupled with higher response to inflammatory provocation, may be protective. Meals high in fat, or fat and simple carbohydrates, are known to induce metabolic endotoxemia[6], characterized by increased circulating markers of inflammation, hypothesized to be linked to transient bacteremia from reduced gut barrier function[20]. Dietary factors that improve gut barrier function and protect against metabolic endotoxemia may improve the capacity to mount an effective immune response to pathogens. Isoflavones have been shown to improve intestinal barrier integrity[21] and reduce colitis in animal models [22], potentially through modulation of the microbiome[23]. High soy consumption has been associated with reduced levels of circulating cytokines in women [24, 25]. Thus, regular consumption of soy foods may confer protection against diet-induced metabolic dysfunction, and reduce the development of insulin resistance. We observed a significantly increased IFNγ response to oral lipid load in soy consumers in the MECHE study, indicative of greater immune response, suggesting that soy consumption may have an effect on metabolic endotoxemia. Although there are no official dietary recommendations relating to isoflavone intake, intake of 40–80mg/day have been suggested for cancer prevention or modulation of lipoproteins in hyperlipidemia[26]. We note that we did not observe any differences in cholesterol (total, LDL or HDL) or triglycerides by isoflavone consumption in GENE. Our findings, while not informing ideal dosage, suggest that relatively low intake of isoflavones may be sufficient to obtain benefits in healthy individuals. In support of this, habitual soy intake in healthy US women was associated with lower CRP, with comparable intakes (median intake 0.55mg.day compared with 0.45mg/day in our study) [25], highlighting the potential value of “low” intake.
Elevated fasting levels of glucose and insulin, as well as increased AUC following OGTT are risk factors for development of insulin resistance, T2D and atherosclerosis[27]. We observed interesting effects of soy consumption on markers of glucose homeostasis and insulin resistance in the GENE, MECHE and NHANES study samples. Soy consumption 1) conferred protection against LPS-induced decline in insulin sensitivity; 2) was associated with improved insulin-mediated glucose disposal as evidenced by reduced insulin secretion required to normalize glucose post-OGTT in MECHE, and 3) was associated with lower fasting insulin and lower 2-hour glucose post-OGTT in NHANES. We did not observe differences in AA. Race differences in diabetes prevalence and glucose homeostasis are known[28], and as-yet poorly understood race differences in immune response and maintenance of normoglycemia may explain the failure of soy consumption to modulate parameters of insulin sensitivity in AA. However, smaller sample size, and lower levels of soy consumption may have hampered the ability to detect effects of soy consumption in AA. The soy isoflavone genistein has been found to protect against cytokine-mediated dysfunction of pancreatic beta cells[29], while daidzein has been found to promote glucose uptake and reduce cytokine expression[30], suggesting that soy consumption may protect against inflammation-induced insulin resistance in humans through modulation of cytokine signaling[31].
Our study had unique strengths. To our knowledge, this is the first study to look at the association of habitual soy food or isoflavone consumption on multiple evoked phenotypes in humans, including inflammatory responses in the setting of evoked endotoxemia, as well as metabolic and insulin responses to high lipid or sugar meals. As reported by our group and others, an evoked phenotype model allows for improved ability to investigate dynamic data[4, 32–34], and this may be particularly important in nutritional investigations. This study also had certain limitations; no dietary soy supplementation was implemented, and we relied on self-reported estimates of isoflavone/soy intake in all studies. While 3-day food records and FFQs are validated and widely-used dietary assessment methods, all self-reported data is subject to bias[35]. We did not have estimates of isoflavone consumption in the 2 supporting studies, and thus relied on soy food intake as a surrogate. Further, we cannot exclude a role for other flavonoids or phytonutrients[36]. Soy/isoflavone intake was generally low, as expected in North American/European populations, but was comparable to the intake recently shown to be associated with lower CRP in healthy women[25]. Despite the limitations, the present study demonstrated a significant effect of moderate soy/isoflavone consumption on metabolic health. These effects are seen with low isoflavone intake, equivalent to approximately one portion a week of isoflavone-rich soy food. Thus, these intakes are very achievable, even in populations without a high traditional intake of soy food. Measuring evoked phenotypes may be more informative than describing resting characteristics, and may increase power to detect effects of nutrition on biomarkers of cardiometabolic health. Future intervention studies, designed to assess the effect of soy/isoflavone supplementation on evoked phenotypes in both healthy and disease populations of different ancestry, may help to determine subsets of individuals who would benefit from increased soy consumption.
Supplementary Material
Acknowledgments
We would like to thank the participants of the GENE, MECHE, and NHANES studies, the staff at the UPenn Clinical Translational Research Center and the support of the UPenn Nutrition Core Dietary Assessment Unit.
Funding: The GENE Study was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003 as well as a NIH-NHLBI SCCOR Project grant (P50-HL-083799) to MPR. JFF is supported by a postdoctoral fellowship grant from the American Heart Association (12POST11840017). MPR is also supported by R01-HL-111694, R01-HL-113147, R01-DK-090505, U01-HL-108636 and K24-HL-107643. HMR is supported by SFI PI Programme (11/PI/1119). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The MECHE Study was funded under the Food for Health Research Initiative (NDP 2007–2012; 07FHRIUCD1) by the Irish Department of Agriculture, Food & the Marine.
Abbreviations
- LPS
Lipopolysaccharide
- FSIGTT
Frequently sampled intravenous glucose tolerance test
- OGTT
Oral glucose tolerance test
- OLTT
Oral lipid tolerance test
- GENE
Genetics of Evoked responses to Niacin and Endotoxemia Study
- MECHE
Metabolic Challenge Study
- NHANES
National Health and Nutrition Examination Survey
- SFA
saturated fatty acids
- MUFA
monounsaturated fatty acids
- PUFA
polyunsaturated fatty acids
Footnotes
Authors’ contributions to manuscript: JFF and MPR designed the research. JFF conducted research in the GENE Study. MFR, ERG, LB and HMR conducted research in the MECHE Study. JFF analyzed data and wrote the paper. All authors read and approved the final manuscript.
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. The British journal of nutrition. 2011;106 (Suppl 3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- 3.Mehta NN, Heffron SP, Patel PN, Ferguson J, Shah RD, Hinkle CC, et al. A human model of inflammatory cardio-metabolic dysfunction; a double blind placebo-controlled crossover trial. J Transl Med. 2012;10:124. doi: 10.1186/1479-5876-10-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferguson JF, Patel PN, Shah RY, Mulvey CK, Gadi R, Nijjar PS, et al. Race and gender variation in response to evoked inflammation. J Transl Med. 2013;11:63. doi: 10.1186/1479-5876-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, et al. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–81. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira AP, Texeira TF, Ferreira AB, do Peluzio MC, de Alfenas RC. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. The British journal of nutrition. 2012;108:801–9. doi: 10.1017/S0007114512001213. [DOI] [PubMed] [Google Scholar]
- 7.Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113(Suppl 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Agriculture. ARSU-ISUDotICoF, Release 1.3 - 2002. Nutrient Data Laboratory Web site. http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html.
- 9.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262:5592–5. [PubMed] [Google Scholar]
- 11.Qi W, Weber CR, Wasland K, Savkovic SD. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer. 2011;11:219. doi: 10.1186/1471-2407-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann GE, Bonacasa B, Ishii T, Siow RC. Targeting the redox sensitive Nrf2-Keap1 defense pathway in cardiovascular disease: protection afforded by dietary isoflavones. Curr Opin Pharmacol. 2009;9:139–45. doi: 10.1016/j.coph.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, et al. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–62. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 14.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes technology & therapeutics. 2003;5:1003–15. doi: 10.1089/152091503322641060. [DOI] [PubMed] [Google Scholar]
- 15.Ryan MF, Grada CO, Morris C, Segurado R, Walsh MC, Gibney ER, et al. Within-person variation in the postprandial lipemic response of healthy adults. The American journal of clinical nutrition. 2013;97:261–7. doi: 10.3945/ajcn.112.047936. [DOI] [PubMed] [Google Scholar]
- 16.Morris C, Grada CO, Ryan M, Roche HM, De Vito G, Gibney MJ, et al. The relationship between aerobic fitness level and metabolic profiles in healthy adults. Mol Nutr Food Res. 2013;57:1246–54. doi: 10.1002/mnfr.201200629. [DOI] [PubMed] [Google Scholar]
- 17.Bingham SA, Welch AA, McTaggart A, Mulligan AA, Runswick SA, Luben R, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public health nutrition. 2001;4:847–58. doi: 10.1079/phn2000102. [DOI] [PubMed] [Google Scholar]
- 18.Welch AA, Luben R, Khaw KT, Bingham SA. The CAFE computer program for nutritional analysis of the EPIC-Norfolk food frequency questionnaire and identification of extreme nutrient values. Journal of human nutrition and dietetics : the official journal of the British Dietetic Association. 2005;18:99–116. doi: 10.1111/j.1365-277X.2005.00593.x. [DOI] [PubMed] [Google Scholar]
- 19.Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–99. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 20.Pendyala S, Walker JM, Holt PR. A high-fat diet is associated with endotoxemia that originates from the gut. Gastroenterology. 2012;142:1100–1. e2. doi: 10.1053/j.gastro.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki T, Hara H. Role of flavonoids in intestinal tight junction regulation. The Journal of nutritional biochemistry. 2011;22:401–8. doi: 10.1016/j.jnutbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Moussa L, Bezirard V, Salvador-Cartier C, Bacquie V, Lencina C, Leveque M, et al. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS ONE. 2012;7:e49547. doi: 10.1371/journal.pone.0049547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutrition and cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 24.Wu SH, Shu XO, Chow WH, Xiang YB, Zhang X, Li HL, et al. Soy food intake and circulating levels of inflammatory markers in Chinese women. Journal of the Academy of Nutrition and Dietetics. 2012;112:996–1004. e1–4. doi: 10.1016/j.jand.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filiberto AC, Mumford SL, Pollack AZ, Zhang C, Yeung EH, Perkins NJ, et al. Habitual dietary isoflavone intake is associated with decreased C-reactive protein concentrations among healthy premenopausal women. The Journal of nutrition. 2013;143:900–6. doi: 10.3945/jn.112.173187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soy Henkel J. Health claims for soy protein, questions about other components. FDA consumer. 2000;34:13–5. 8–20. [PubMed] [Google Scholar]
- 27.Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR) Diabetes Care. 2008;31:2056–61. doi: 10.2337/dc08-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall MC., Jr Diabetes in African Americans. Postgraduate medical journal. 2005;81:734–40. doi: 10.1136/pgmj.2004.028274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim EK, Kwon KB, Song MY, Seo SW, Park SJ, Ka SO, et al. Genistein protects pancreatic beta cells against cytokine-mediated toxicity. Mol Cell Endocrinol. 2007;278:18–28. doi: 10.1016/j.mce.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Cheong SH, Furuhashi K, Ito K, Nagaoka M, Yonezawa T, Miura Y, et al. Daidzein promotes glucose uptake through glucose transporter 4 translocation to plasma membrane in L6 myocytes and improves glucose homeostasis in Type 2 diabetic model mice. The Journal of nutritional biochemistry. 2014;25:136–43. doi: 10.1016/j.jnutbio.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. The Journal of nutrition. 2009;139:1431–8. doi: 10.3945/jn.109.107029. [DOI] [PubMed] [Google Scholar]
- 32.Franco LM, Bucasas KL, Wells JM, Nino D, Wang X, Zapata GE, et al. Integrative genomic analysis of the human immune response to influenza vaccination. eLife. 2013;2:e00299. doi: 10.7554/eLife.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen R, Mias GI, Li-Pook-Than J, Jiang L, Lam HY, Miriami E, et al. Personal omics profiling reveals dynamic molecular and medical phenotypes. Cell. 2012;148:1293–307. doi: 10.1016/j.cell.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson JF, Mulvey CK, Patel PN, Shah RY, Doveikis J, Zhang W, et al. Omega-3 PUFA supplementation and the response to evoked endotoxemia in healthy volunteers. Mol Nutr Food Res. 2013 doi: 10.1002/mnfr.201300368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. American journal of physiology. 2001;281:E891–9. doi: 10.1152/ajpendo.2001.281.5.E891. [DOI] [PubMed] [Google Scholar]
- 36.Babu PV, Liu D, Gilbert ER. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. The Journal of nutritional biochemistry. 2013;24:1777–89. doi: 10.1016/j.jnutbio.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.