Abstract
Obesity and diabetes represent key healthcare challenges of our day, affecting upwards of one billion people worldwide. These individuals are at higher risk for cancer, stroke, blindness, heart and cardiovascular disease, and to date, have no effective long-term treatment options available. Recent and accumulating evidence has implicated the developmental morphogen Hedgehog and its downstream signalling in metabolic control. Generally thought to be quiescent in adults, Hedgehog is associated with several human cancers, and as such, has already emerged as a therapeutic target in oncology. Here, we attempt to give a comprehensive overview of the key signalling events associated with both canonical and non-canonical Hedgehog signalling, and highlight the increasingly complex regulatory modalities that appear to link Hedgehog and control metabolism. We highlight these key findings and discuss their impact for therapeutic development, cancer and metabolic disease.
Keywords: Non-canonical Hedgehog signaling, Metabolism, Development, Cancer
1. The Hedgehog signalling pathway
Proper control of development, tissue homeostasis and metabolism of higher multicellular organisms is a delicate and highly complex process that relies on the precise temporal, spatial and context-dependent regulation of molecular signalling pathways. The importance of controlled signal activation and termination is critical to the function of essentially all organisms. In higher organisms, the failure to induce, attenuate or stop signalling precisely can result in severe developmental anomalies, metabolic disorders or life threatening malignant diseases [1–6].
Despite decades of molecular genetic advances, our understanding of the cues governing embryonic development and health of adult higher organisms comprises only a handful of signalling pathways. These include, but are not limited to Hedgehog, Wnt, Notch, Hippo, Jak/Stat, receptor-tyrosine kinases, and Tgfβ signalling cascades [7]. While few, these signalling appear able to control a plethora of context dependent biological processes.
The Hedgehog signal transduction pathway can be regarded as a paradigm of how a single, and at first glance, simple molecular cue (i.e. secreted Hedgehog protein bound to its receptor) selectively translates into an array of distinct cellular reactions depending on context [8,9]. To date, a number of fundamental principles have been uncovered that link pathway activation and functionally distinct cellular response. For instance, Hedgehog can behave as a morphogen, that is, increasing concentrations of Hedgehog ligand can elicit distinct cellular responses [10,11]. Also, numerous studies have highlighted the importance of molecular cross-talk between the Hedgehog and other signalling cascades, interactions that can modulate signal strength and determine specificity of molecular and cellular phenotypes [12–23].
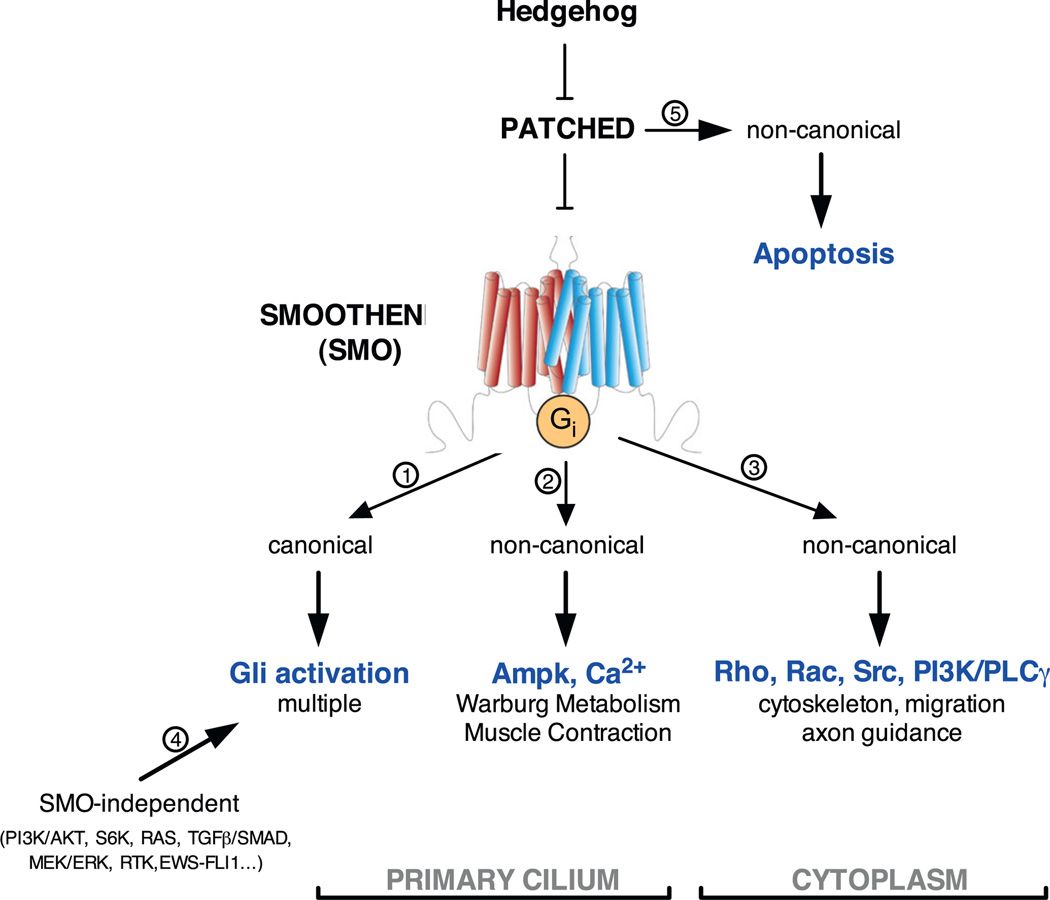
Somewhat unique to Hedgehog signalling are the number and variety of modes by which the Hedgehog signal can be stimulated and transduced to impact the behaviour and fate of the target cells [24]. For simplicity, the different modes of Hedgehog signalling are mostly commonly classified as either canonical or non-canonical. Canonical Hedgehog signalling in vertebrates is most similar to the Hedgehog signal transduction mechanisms originally identified in the fruit fly. They involve Hedgehog-dependent activation of the Gli zinc finger transcription factors [25,26]. Non-canonical Hedgehog signalling is less well defined; a multiplicity of different modes have been documented without any simple, over-arching theme. Simply put, non-canonical Hedgehog signalling currently refers to Hedgehog co-receptor dependent signals that do not clearly act via the canonical Hedgehog-to-Gli route (Fig. 1 and [24]). Non-canonical Hedgehog signalling pathways have been classified in two groups: Type I signals that stem from the peptide ligand receptor Ptch, and Type II signals that stem from the seven trans-membrane domain containing G-protein coupled receptor (GPCR) Smo. In addition, Smo-independent activation of Gli has also been referred to as non-canonical Hedgehog signalling.
Fig. 1.
Here, after a brief overview of canonical signalling, we will focus on Smo-dependent, Gli-independent non-canonical Hedgehog signalling. We will summarize recent findings on the role of Smo as a GPCR regulating cytoskeletal architecture, cell motility, and axon guidance, as well as highlighting a novel regulatory link to the maintenance of cellular and organismal energy homeostasis.
1.1. Canonical Hedgehog signalling
Canonical Hedgehog signalling was first discovered in Drosophila, where seminal work on embryonic patterning mutants led to the identification of the hedgehog gene. Loss of Hedgehog function in the fly results in a disorganized lawn of spiky processes and denticles on the surface of the fly larva, a Hedgehog-like phenotype that coined the name of the pathway [27]. While canonical Hedgehog signal transduction is highly conserved, several key differences have emerged since the divergence of flies and mammals. Included, are a critical negative regulatory function of vertebrate Sufu, and an expansion of the activator and repressor repertoire of the fly transcription factor Cubitus interruptus to three distinct zinc finger transcription factors, Gli1, Gli2 and Gli3, in vertebrates [8,28–30]. The primary cilium, commonly thought to be a prerogative of Hedgehog signalling in vertebrates, has also been shown to play a central role in flies [31,32].
Vertebrate canonical Hedgehog signalling is initiated by binding of proteolytically processed and lipid modified Hedgehog ligand to its receptor Patched (Ptch), a twelve-pass transmembrane protein that represses the pathway in the absence of ligand [33–37]. Three distinct co-receptors, Cdo, Boc, and Gas1, facilitate high-affinity binding of mature Hedgehog ligand to Ptch, thereby enhancing Hedgehog signal strength [38–42].
Ligand binding to Ptch abrogates its repressive effect on the seven-pass transmembrane protein Smo, a key effector essential for canonical Hedgehog signal transduction [43].The repressive role of ligand-free Ptch depends on its localization in the primary cilium, a single antenna-like structure that protrudes from the cell surface of most adherent cell types and functions as an organizer-like signal transduction compartment. Ciliary Ptch prevents pathway activation by blocking the entry of Smo into the primary cilium. Binding of Hedgehog protein to Ptch removes Ptch from the primary cilium, thereby allowing Smo to enter and, upon an unknown activation step, propagate the Hedgehog signal further downstream [28,44,45]. Despite intense efforts to understand Ptch function, the detailed mechanisms of how Ptch represses Smo in the absence of ligand is still elusive. Ptch contains a sterol-sensing domain and belongs to the family of RND (Resistance-Nodulation-cell Division) transporters [46]. Several functional studies support a model where Ptch prevents Smo activation eitherby removing Smo agonists such as oxysterols from the primary cilium or by increasing the influx of Smo antagonists into the cilium [47–50]. In addition, Ptch may also modify the lipid composition of Smo-containing endosomes and therefore negatively control Smo trafficking towards the primary cilium [51,52].
The key role of Smo in canonical Hedgehog signalling is to control the activation of the Gli zinc finger transcription factors [53]. Of note, the Gli family member Gli3, and to some extent also Gli2, exerts a dual function as transcriptional repressor (GliR) and activator (GliA) of Hedgehog target genes, where the two distinct functional states are controlled by proteolytic processing (reviewed in [2]). In the off-state of the Hedgehog pathway, Gli3 protein appears to continuously cycle through the primary cilium, where it is proteolytically cleaved into a C-terminally truncated repressor form lacking the transactivation domain. Gli3 repressor protein translocates to the nucleus, where it binds to the promoters of Hedgehog target genes to shut off transcription. The balance between Gli3 repressor and activator is tightly regulated by sequential phosphorylation and dephosphorylation events [54]. Kinases involved are PKA; Gsk-3β; and Ck1. The detailed mechanisms of how Smo activation in the primary cilium results in Gli activator formation are still unclear [55–61]. Recent studies suggest that binding of Hedgehog to Ptch removes the ciliary G-protein Coupled Receptor Gpr161 [62,63]. Gpr161 in the cilium inhibits Hedgehog signalling via PKA and Gli3 repressor formation [62].
Sufu is a critical negative pathway regulator in vertebrate Hedgehog signalling and prevents nuclear translocation of Gli proteins [29,64]. As a consequence of Sufu degradation and Gli-Sufu dissociation, Gli activator forms translocate to the nucleus and activate the expression of target genes, which includes a self-amplifying loop via induction of Gli1 itself [65–69].
1.2. Smo-dependent non-canonical Hedgehog signalling
Type II non-canonical Hedgehog signals are defined as Smo-dependent, Gli-independent cascades that respond to Smo agonists and elicit cellular responses, ranging from Ca2+ signalling and cytoskeletal rearrangement to metabolic rewiring (see Fig. 1).
The Hedgehog co-receptor Smo has 7-transmembrane (TM) domains. This topology resembles that of G protein-coupled receptors (GPCRs). The GPCR superfamily is subdivided into five families sharing homologous domains in the extracellular N-terminal region and intracellular C-terminal tail and having similar types of ligands, such as small molecules, peptides and glycosylated proteins [70]. Smo belongs to the F group, which also contains the Frizzled (Fz) isoforms, and has a characteristic extracellular cysteine-rich domain (CRD) and a long intracellular tail. Interestingly, the crystal structure of the 7TM bundle, despite a mere 10% sequence homology, highly resembles that of group A GPCRs, which use peptides as ligands [71] and also have an eighth short intracellular a-helix that lies parallel to the lipid bilayer. Smo is also characterized by large extracellular loops that make contacts with the CRD linker region and with TM helix III through a network of disulfide bonds [50]. The CRD is a compact globular domain, highly homologous to that of Fz receptors, containing four α-helices and 2 short β-barrel motifs. The CRD and the extracellular loops make a lid that partly occludes the binding pocket of one type of Smo small molecule modulators: the agonist SAG and the selective partial agonist/antagonist cyclopamine and its derivatives [50,72]. Oxysterols, small agonists of Smo, have been mapped to bind to the CRD, which allosterically modulates the cyclopamine-binding pocket [73,74]. Since some natural oxysterols bind to and activate Smo, they have been proposed as Smo endogenous ligands [47–49]. However, a recent study shows that oxysterol binding mutants, abrogating Smo activation by exogenous oxysterols, fail to suppress Shh or SAG ago-nism in vivo [50]. Thus, so far Smo is an orphan GPCR, making the “R” of GPCR potentially misleading.
Regardless of the orphan nature of Smo, there is clear evidence that it activates heterotrimeric G proteins, like all GPCRs. Biochemically, Smo catalyzes the GDP-GTP exchange of all members of the G inhibitory (Gi) family of G proteins (Gi1, Gi2, Gi3, Go, and Gz) but not of any other G protein families when expressed in the absence of Ptch or when activated by the small agonist purmorphamine [19]. Smo exists as a homodimer making contacts through both the N-terminus and the C-terminus domains, as reported for many GPCRs [75]. The long C-tail of Smo undergoes a conformational change upon activation when the strong positive charge of an Arg-rich stretch is neutralized by phosphorylation of a large number of Ser/Thr residues [75]. Interestingly, the C-tail partly precludes interaction with Gi proteins, since a C-tail deletion significantly increases GTPγ[S35] binding, thus suggesting that active Smo is in a less compacted quaternary structure that allows G protein interaction [19]. Supporting Smo-Gi coupling, the strong selectivity and potency of SMO towards the Gi family is similar to the prototypical serotonin receptor 5HT1aR Gi-GPCR. That said, Smo exhibits greater inhibitory potency for cAMP production [76]. Noteworthy, Smo-dependent Gi activation is not sufficient to trigger canonical Hedgehog signalling downstream of Smo, and in some cell types is not even necessary [19,77]. The long intracellular C-tail of Smo is required for phosphorylation by Grk2 and Ck1, for β-arrestin binding, trafficking to the primary cilium, and for interaction with Costal2 and Fused in Drosophila [78–84]. Therefore, it is possible that the conformational change of Smo allows interaction with Gi proteins regardless of plasma membrane vs. primary cilia membrane localization.
The capacity of Smo to act as a classic GPCR towards Gi proteins is evidenced in several contexts. Activation of Gli-dependent transcription is maximal in the presence SMO-Gi protein coupling in some cell types but not in all [77]. Specifically, the use of a Pertussis toxin (PTX), a small ADP-ribosylase that modifies an N-terminal Cysteine of Gαi thus preventing GPCR interaction, has served to demonstrate that Gli activation in NIH3T3 cells, CH10T1/2 cells, and cardiomyocytes is largely sensitive to inhibition of Gi protein activation ([19,76], N.A.R. unpublished data). Conversely, in mouse embryonic fibroblasts (MEFs) Smo-Gi coupling is not essential for canonical Hedgehog signalling, and the reason is unclear [77]. In Drosophila S2 cells, depletion of the single Gi family member by siRNA prevents activation of Ci and lack of Gi in the wing disc inhibits expression of the Hedgehog-target gene dpp [85]. PTX has also been used with conflicting results in in vivo studies in zebrafish and avian embryos. In zebrafish, PTX phenocopies many aspects of Shh deficiency including cyclopia, lack of forebrain ventral specification, and an expansion of the sclerotome at the expense of adaxial fates in the posterior somites [86]. In the chick embryo neural tube electroporation model, PTX did not affect gross dorso-ventral patterning and thus it was concluded that Gi is not required for Hedgehog-dependent patterning [87]. However, the nervous system expresses Gz, a member of the Gi family that couples to Smo but is insensitive to PTX because it lacks the Cys residue that is the substrate for ADP-ribosylation. Therefore, the results remain inconclusive with respect to the role of Gi proteins in neural tube patterning by Shh. Proliferation of mammalian cerebellar granule neuron precursors in response to Shh, mediated by Gli transcription, also depends on Gi proteins as it is inhibited by PTX and by siRNA-mediated depletion of Gαi1, Gαi2, or Gαi3. Interestingly, in the latter model, overexpressed Gαi1 and Gαi2 were detected in different regions of the primary cilium [88].
In the past five years, numerous pieces of evidence emerged supporting a key role of Smo/Gi coupling in non-canonical Hedgehog signalling. Recent studies reported that rapid activation of Rac1 and RhoA by Shh or a Smo agonist leads to migration in fibroblasts and cholangiocarcinoma cells and to tubulogenesis in endothelial cells [89–91]. This response is dependent upon Smo coupling to Gi, as it is sensitive to PTX and does not require the C-terminal domain of Smo nor Smo translocation to the primary cilium [90,92]. The use of PTX has also been essential to conclude that in spinal neuron precursors activation of Smo leads to increased calcium spike activity through a nifedipine-sensitive calcium channel [93]. Furthermore, the role of non-canonical Hh signalling utilizing Smo as a GPCR in the regulation of metabolism, as well as the pharmacology governing its function, have been recently reported [72].
Activation of Gi proteins by Smo has also been linked to activation of the NF-κB transcription factor in diffuse B-cell lymphoma cells [94]. In those cells, activation of Smo enhances its interaction with Gi proteins and promotes the recruitment of a signalling complex containing CARMA, Bcl10 and MALT1, which in turn initiate activation of p65/p50 NF-κB. The mechanism is rapid and independent of Gli-dependent transcription. Remarkably, this study and a previous one also provided evidence for G12 activation by Smo [95]. Our studies using the clean background of Sf9 cells and in HEK293 cells however indicate that Smo does not have the capacity to activate either G12 or G13 [19,76]. Maximizing stoichiometry and proximity of Smo and G13 via fusion of the C-tail to the α subunit of G13, a strategy commonly used when studying GPCRs, cannot induce G13 activation, while the same fusion to Gαi strongly promotes Gi activation [76]. A possibility is that Smo is activating G12 in those contexts via heterodimerization with another GPCR in a cell type specific manner, or that the interpretation was confounded by the fact that other GPCRs that stimulate G13 are able to increase Gli-transcriptional activity independently of SMO [96].
Smo is regulated by all tested inhibitors/agonists (cyclopamine, KAAD-cyclopamine, SANT-1, tomatidine, purmorphamine) at a similar concentration when Gli transcriptional activity and Gi protein activation are used as readouts [19,97–99].This evidence, thus, suggests that the active conformation of Smo for Gi protein coupling and Gli activation is the same. In addition, the lack of primary cilia in the vesicularized membranes of Sf9 cells (where the GTP binding assays were performed), as well as the finding of cilium-independent Gi protein-dependent Smo signalling in fibroblasts, suggests that Smo-Gi interactions are not confined to the primary cilium. However, Smo-Gi interaction within the primary cilium could occur in a cell type dependent manner. For instance, the metabolic switch induced by Smo activation in adipocytes and skeletal muscle is dependent on both Gi protein and translocation to the primary cilium [72]. In addition, Gαi2 and Gαi3 have been shown to localize to primary cilia in the context of Shh-induced proliferation of cerebellar granule precursor neurons [88].
Altogether, the burden of evidence indicates that Smo couples to heterotrimeric G proteins of the Gi family. The significance of that interaction is more obvious in non-canonical Hedgehog signalling, but it seems to be permissive for Gli activation in some cell types, possibly due to the consequent drop in cAMP levels and PKA activation.
2. Hedgehog signalling and metabolism
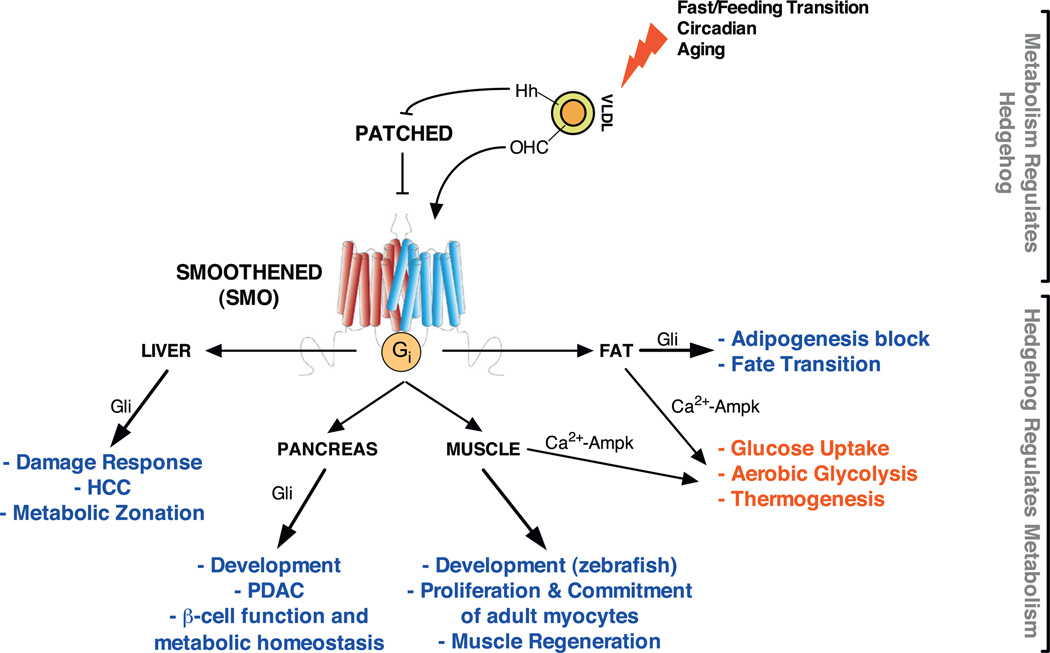
The connections between Hedgehog signalling and metabolism are multiple, and they span processes ranging from ligand maturation and secretion, to receptor activity and cellular and organismal responses (see Fig. 2). Work by ourselves and others suggest the connection is bi-directional: that organismal metabolic state modulates Hedgehog signalling and that Hedgehog signalling regulates cellular and organismal metabolism.
Fig. 2.
2.1. Metabolism regulates Hedgehog
Active Hedgehog proteins (Shh, Dhh and Ihh) carry lipid moieties. They are fatty acid and cholesterol modified at the N- and C-termini respectively and these modifications are essential for their maturation, secretion and biological activity [39,100–107]. Lipid-modified Hedgehogs have high affinity for the cell membrane and localize to lipid-raft microdomains. The current model is that fatty acylation targets Hedgehog to the membrane of the secretory cell, while cholesterol anchors them to the rafts of both secretory and receiving cell, thus preventing inappropriate secretion and increasing stability at the target cell [108,109].
The Hedgehog pathway is also sensitive to intracellular and extracellular sterols. Specifically, hydroxylated cholesterol derivatives known as hydroxylsterols (OHCs) act as Smo agonists. Structure–Activity-Relationship (SAR) studies have highlighted two necessary features for OHCs to be effective Smo agonists: (i) stereoselectivity – only the (S) stereoisomer is active [110,111]; and (ii) hydroxyl-side chain substitution – long aliphatic chains and a free additional hydroxyl moiety on C6 increase activity [73,110]. Several of these hydroxysterols, including 20(S)OHC, 25OHC, 7-keto-25OHC and 7-keto-27OHC allosterically modulate Smo activity by binding either inside or outside of the SAG-binding pocket [49,50,73]. Also, pharmacological depletion of endogenous cellular sterols impacts Smo-mediated signalling [112] and cyclopamine, the prototypical competitive Smo inhibitor, is an alkaloid with a sterol-like structure [97]. Currently, the idea is that intracellular sterols inhibit, while extracellular sterols activate, Smo activity.
Sterol-dependent Smo modulation has been attributed thus far to the sterol-sensing domain present on Ptch1, which is essential for the Ptch1-dependent tonic inhibition of Smo [46,113–115]. That said, the Smo-CRD has been recently identified as the site of action for several oxysterols [73] and appears essential for oxysterol-dependent Smo modulatory activity. Interestingly, interaction events at the SAG-binding pocket and the CRD are independent. While resistant to Hedgehog-mediated pathway activation, CRD mutants are fully responsive to SAG and cyclopamine [50]. These results suggest physiologically independent, though equally important, sterol-sensing functions for both Ptch1 and Smo. Further supporting a sterol-sensing function for Smo, Lrp2 has been identified as a Smo co-receptor [116]. Lrp2, also known as Megalin, is a widely expressed 500KDa transmembrane protein that binds disparate substrates [117] and participates in their endocyto-sis and eventual lysosomal degradation [118,119]. Lrp2 acts as a Shh-binding protein in the forebrain where it sequesters Shh to its site of action and controls Shh-Ptch1 internalization and degradation [116]. LRP2 knockout mice phenocopy Hedgehog signalling mutants exhibiting anomalies in forebrain development [116].
In addition to sterols, intracellular lipids can also activate Smo, as is the case for phospatidyl-inositol-4-phosphate (PI4P) [120]. PI4P is a polar lipid mainly synthesized and localized to the Golgi membrane, where it contributes to vesicle budding and intracellular trafficking [121]. Recent findings also indicate roles for PI4P at the plasma membrane, where it contributes to the pool of polyanionic lipids that define plasma membrane identity [122]. In Drosophila melanogaster, PI4P accumulation facilitates Smo activation, most likely by promoting its trafficking to the plasma membrane [120], and indicative of a feeback system, PI4P levels are controlled by Ptch1 signalling [120]. Interestingly, PI4P synthesis and degradation are dynamically regulated by kinases and phosphatases whose loss of function leads respectively to inhibition or activation of Hedgehog signalling [120]. InD. melanogaster and mammals, Hedgehog proteins, hydroxysterols, and Smo modulatory lipids all travel on lipoproteins [123–127]. One hypothesis is that the Ptch1/Smo/Lrp2 receptor system anchors circulating lipoproteins to the cell surface, placing active Hedgehog ligands in close proximity their binding site on Ptch1. This “Lipoprotein bridge”, as well as the sterol-mediated Smo allosteric modifications, may modulate pathway activity by either facilitating the Hedgehog-dependent release of Ptch1 or by inhibiting Smo activity through lipoprotein-loaded inhibitory ligands. Noteworthy, Hedgehog proteins travel on VLDL in humans [128]. Intriguingly, VLDL dimensions (diameter 30–80 nm) are compatible with protein dimerization and membrane receptor clustering. We refer the reader to the review by S. Eaton et al. in this same issue for a detailed review of these ideas.
2.2. Hedgehog regulates metabolism
The Hedgehog signalling pathway is essential for proper embryonic development [11] and thought to be mostly quiescent in adults. Physiological reactivation of the pathway occurs in stem cell compartments as a response to tissue damage and drives cell fate transitions, trans-differentiation and metabolic reprogramming [129]. Also, genetic and pharmacological modulation of the pathway impacts adipose tissue development [4,130–133] and function [72,134,135]. Importantly, inappropriate reactivation of the pathway is associated with disparate human cancers [3,9,14,21,69,136–143] spanning almost every tissue type, including liver, pancreas, brain, stomach and intestine. Most of these effects have been associated with canonical, Gli-dependent, Hedgehog signalling pathway. Non-Canonical, Gli-independent, pathway(s) instead have been shown to play a prominent role in Ca2+ signalling, cytoskeletal rearrangement, axon guidance and in adipose tissue and skeletal muscle metabolism [72,77,89,90,93,144].
Below, we try to summarize these findings and put them into perspective. It is clear that while highly interesting, much work remains before we have clear understanding of the mechanisms and consequences of Hedgehog signalling activation in adult physiology and disease.
2.2.1. Liver
The liver is a vital organ for vertebrates. It participates in many physiological processes including detoxification, energy metabolism and digestion. The liver is composed of numerous cell types, each with its own specialized function. In addition to the parenchymal hepatocytes, the non-parenchymal compartment includes endothelial cells, Kupffer cells, cholangiocytes, hepatic stellate cells (HSC) and myofibroblasts.
The liver is the major source of the Hedgehog-carrying VLDL particles in mammals [145]. VLDL contain triglycerides, phospholipids, cholesterol and cholesteryl esters and transports them throughout the body [146,147]. Clinically, elevated triglycerides are associated with insulin resistance, non-alcoholic fatty liver disease (NAFLD), excess alcohol consumption, liver cirrhosis, hepatocellular carcinoma (HCC) and much more. Interestingly, upon liver damage, the secretion of Hedgehog ligands is upregulated in cholangiocytes and myofibroblasts and sustains proliferation and survival in both cell types [148,149]. In response to damage, HSCs undergo an Epithelial-to-Mesenchymal-Transition (EMT) and trans-differentiate into myofibroblasts (MF), resulting in liver fibrosis. Activation of the Hedgehog pathway in HSCs, for example downstream ofLeptin signalling [150], is responsible for HSC to MF trans-differentiation [150]. This cell fate transition is intriguingly dependent upon Gli transcription factors, Hif-1α and a metabolic switch towards aerobic glycolysis in HSCs [151]. These findings represent the first evidence of a direct implication of canonical Gli-dependent Hedgehog signalling in glycolytic reprogramming of cellular metabolism. Strikingly, this phenomenon seems to be a common feature of liver damage-induced Hedgehog signalling, as it has been shown in other forms of non-carcinogenic liver damage [149,152–156].
The HCC scenario is rather unique. Here, Hedgehog ligands are secreted by malignant hepatocytes, induce Gli-dependent Warburg reprogramming and lactate secretion in stromal cells (mainly myofibroblasts) and lactate signals back to malignant hepatocytes to sustain their growth and survival [137]. This metabolic reprogramming is known as the “Reverse Warburg”. First introduced in 2009 [157], a reverse Warburg effect describes the ability of cancer cells to induce a Warburg effect in neighbouring stromal cells and benefit from the secreted lactate to sustain their proliferation and survival [157–161]. Thus, damage-induced Hedgehog signalling in the liver leads to metabolic reprogramming and cell fate transition in HSCs and MFs, thus leading to conservative and/or pro-oncogenic consequences.
As a different form of liver damage, Hepatitis-C Virus (HCV) infection activates the Hedgehog signalling pathway in human hepatocytes [162], where Hedgehog activation has been shown to facilitate HCV secretion and replication [163]. Interestingly, the HCV replication-secretion cycle involves the intracellular pool of PI4P and takes advantage of the VLDL secretory machinery [164–166]. Besides damage-induced response, Hedgehog signalling is also active in healthy hepatocytes and contributes to Igf-1 homeostasis [167].
The findings presented above indicate a prominent role for Hedgehog in liver physiology and pathology. From a metabolic point of view, the liver features a so-called “Metabolic Zonation”. Different metabolic pathways are carried out in different zones of the hepatic lobules, allowing liver to progressively or selectively cope with the diversity of the metabolic challenges [154]. Molecular mechanisms of liver metabolic zonation are not clear. The Wnt/β-catenin signalling pathway has been proposed as the master regulator [168,169] and indirect evidence has led scientists hypothesize a prominent role for Hedgehog too [154].
2.2.2. Pancreas
The pancreas is a two-headed organ, which presents endocrine function (in relation to the control of blood glucose homeostasis) as well as gastrointestinal function as an exocrine organ. The endocrine function of the pancreas is achieved by four highly specialized cell types within the islet of Langerhans. These include: (i) alpha cells, which secrete glucagon, which stimulates hepatic glucose production and gluconeogenesis; (ii) beta cells, which secrete insulin, the master regulator of blood glucose homeostasis; (iii) delta cells, which secrete somatostatin, a hormonal brake on alpha and beta cell activity; and (iv) F-cells, which secrete pancreatic polypeptide in response to food intake to modulate endocrine and exocrine pancreatic functions.
Hedgehog signalling controls development of both endocrine and exocrine pancreas, as well as their function in adults. Furthermore, inappropriate activation of the pathway, alone or in combination with other oncogenes is associated with the development of pancreatic adenocarcinoma (PDAC) [139,170–172].
During embryonic development, the endodermal foregut gives rise to the anterior part of the digestive canal from the mouth to the duodenum. The pancreas develops from the pancreatic bud of the endodermal foregut and its surrounding mesoderm. Staining of embryos at E10.5 shows that Shh is expressed throughout the gut endoderm, with the exception of the pancreatic bud [173]. Interestingly, when Shh is constitutively overexpressed in the pancreatic bud under the control of the Ipf1/Pdx1 promoter the pancreatic mesoderm differentiates into smooth muscle and interstitial cajal cells typical of the developing intestine rather than into pancreatic mesenchyme and spleen [173]. These data indicate, therefore, that endodermal-derived Shh directs differentiation of the surrounding mesoderm and that tight spatial and temporal restriction of Hedgehog signalling is critical for proper development of the digestive tract.
The role of Hedgehog signalling in the pancreatic epithelium, though, is debated. Pancreatic epithelial cell-specific activation or inhibition of the pathway has only mild developmental effects, which suggest a role for Hedgehog signalling activation mainly in the pancreatic mesenchyme [170,173–177]. As an example, Pdx1-driven depletion of Smo in pancreatic epithelial progenitor cells results only in a transient delay in beta-cell development [177]. Interestingly, while beta-cell numbers recover after birth they are hypofunctional and Pdx1-Smo−/− mice develop a mild insulin-dependent diabetes, characterized by glucose intolerance, increased insulin sensitivity and reduced insulin secretion [177]. This work confirmed a previous study [178], which showed that canonical Hedgehog signalling controls insulin transcription and secretion.
The mild responsiveness of pancreatic epithelial cells to constitutive activation of Hedgehog has been suggested to be linked to the primary cilium, a structural requirement for signalling downstream of Smo [179]. Adult epithelial cell-specific over-activation of the pathway, together with primary cilium ablation, leads to pancreas dedifferentiation, with striking re-expression of progenitor markers and reduction of the endocrine area. Double transgenics are metabolically compromised and develop age-dependent insulin-negative undifferentiated pancreatic cancers [176,179]. So, while well-implicated in pancreatic form and function, a clear role for Hedgehog signalling in normal pancreatic physiology begs deeper investigation.
2.2.3. Muscle and adipose tissue
Until the 1980s adipose tissue was primarily viewed as a simple site of calorie storage. With the discovery of adipokines (adipocytes released cytokines), this view radically changed, and the concept was born of adipose tissue as an endocrine organ at the centre of energy homeostasis. The use of lipid droplet as a means of energy storage is conserved in all eukaryotes from worms to humans. In mammals, two grossly different adipocytes are recognized: (i) white adipocytes, which contain a single lipid droplet with hormone-sensing and lipid storing and releasing functions; and (ii) brown adipocytes, mitochondrial-dense, multilobular adipocytes, which burn lipids and produce heat via largely uncoupled respiration. White and brown adipocytes reside in different and differently distributed depots, namely white (WAT) and brown (BAT) adipose tissues and have largely different gene expression signatures and developmental origins [180].
The role of Hedgehog signalling in adipocyte differentiation and adipose tissue biology has long been debated. Since 1995, there have been indications sustaining a role for the pathway in stimulating and inhibiting adipogenesis [4,130,131,181–185]. The current notion, though, is that activation of the pathway in white adipocyte precursors has a general inhibitory role on adipogenesis from flies to mice [4,130].
Hedgehog acts upstream of PPARγ to channel preadipocytes fate away from adipogenesis towards osteogenesis. This is achieved via Gli-dependent induction of anti-adipogenic transcription factors, with concomitant inhibition of the pro-adipogenic ones [4,130]. Interestingly, Hedgehog-dependent adipogenesis block is restricted to white adipocyte progenitors. aP2-Sufu knockout mice (which feature constitutive activation of the pathway in both white and brown fat depots) display a white adipose tissue-specific lipoatrophy, with a fully developed and functionally intact brown adipose tissue depot [4]. Thus, Hedgehog was one of the first hormonal axes identified capable of differentially regulating white and brown adipogenesis.
Worthy of mention, aP2-Sufu−/− mice feature a unique healthy lipoatrophic phenotype with no signs of metabolic abnormalities. Even more surprisingly, the remaining white adipose tissue (roughly 10 per cent of a fully developed white fat mass) in these mice takes up 3-fold more glucose than that of wild-type animals and has morphological and genetic properties of the energy burning brown adipose tissue. Thus, constitutive, fat-specific activation of Hedgehog (i) selectively blocks white adipogenesis; (ii) activates metabolism in mature, fully developed, white adipocytes; and (iii) shifts white adipocytes towards brown-like functionality.
In an independent study using an oncogenic, constitutively active mutant of Smo, SmoM2, under control of aP2-Cre mediated expression, Hatley et al. observed aggressive head and neck tumours transcriptionally and histologically reminiscent of embryonic rhabdomyosarcoma (ERMS) [134]. ERMS is the most common soft tissue malignancy in children, commonly associated with hyper-activation of canonical Hedgehog signalling. Though a tumour of muscle-like appearance, the cell-of-origin for ERMS has not yet been identified. While speculated to be of brown adipocyte progenitors lineage in the case of the aP2Cre SmoM2 model, a caveat remains in that aP2-Cre has been shown on multiple occasions to be promiscuous in its expression. Activation of Hedgehog signalling is believed to be critical for muscle development in zebrafish [186–189]. In higher eukaryotes, Hedgehog promotes proliferation, commitment and differentiation of adult muscle cells [190–194] as well as muscle regeneration upon physical and ischaemic injury [195–198]. Surprisingly, SmoM2-driven Hedgehog activation in muscle satellite cells fails to induce ERMS [134,196,199].
So, with respect to a unified role in adipose, questions still remain: activation achieved by Su(fu) deletion leads to white-specific lipoatrophy with an apparent white-to-brown adipocyte transition [4]; upstream over-activation by SmoM2 using the same Cre driver leaves adipose tissue development unimpaired, but induces fat-to-muscle lineage transition and cancer [134]. Could unidentified Smo-dependent or SmoM2-specific secondary signalling pathways be involved? These questions remain open.
3. Non-canonical Hedgehog signalling regulates energy metabolism
The studies above indicate a clear role for canonical, Gli-dependent signalling in regulating metabolism and/or metabolically relevant tissues. Below we discuss the data that implicates non-canonical Hedgehog signalling as a novel rheostat linking acute nutrient status and energy homeostasis.
3.1. General features and molecular signature
As mentioned above, non-canonical Hedgehog pathways are defined as signalling originating from Ptch1 (Type I) and/or Smo (Type II) and independent from the Gli transcription factors [200].
We recently reported a novel Type II non-canonical pathway which rapidly rewires metabolism in vitro and in vivo [72]. Initially identified in differentiated 3T3L1 adipocytes, Smo activation by ligand binding, and/or Ptch1 inactivation, is coupled to a rapid L-type-dependent Ca2+ influx. Subsequent activation of Camkk2 then triggers phosphorylation and activation of Ampk, a sensor of cellular metabolic state and one of the master regulators of cellular metabolism (for a review see [201]). The result is a rapid, insulin-independent glucose uptake and a shift away from mitochondrial oxidative phosphorylation towards aerobic glycolysis -a metabolic signature reminiscent in many ways of the hallmark Warburg effect observed in many cancers [202,203]. Downstream signalling determinants of this glycolytic shift are the oncogenic slow-acting isoform of Pyruvate Kinase, Pkm2, and the rate-limiting enzyme of the Krebs cycle, Pdha1. Both Pkm2 and Pdha1 play important roles in aerobic glycolysis in cancer [204–209]. Tyrosine phosphorylation regulates their activities such that intracellular pyruvate accumulates and is then converted into lactate by the NAD+-producing Lactate Dehydrogenase.
For Hedgehog biologists, this novel non-canonical branch highlighted two noteworthy characteristics: (i) the notion of selective partial agonism; and (ii) a spatial restriction to the basal body of the primary cilium.
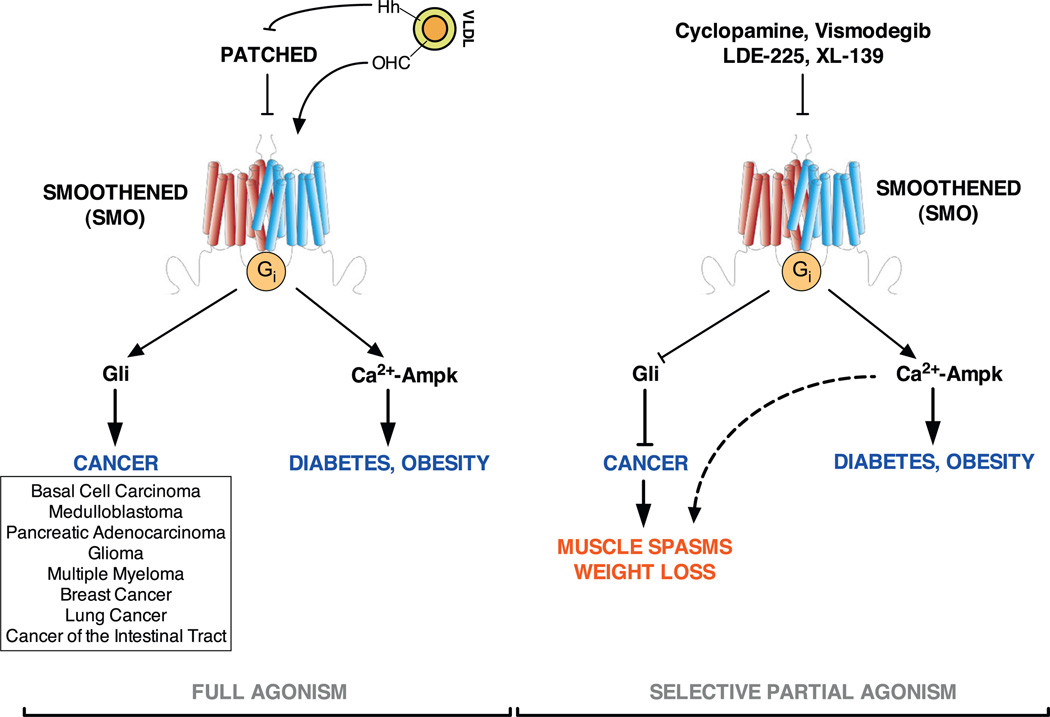
Inappropriate activation of the Hedgehog signalling pathway is associated with numerous disparate human cancers, ranging from haematological malignancies to brain and skin cancers and solid tumours of the lung and the intestinal tract [3,9,14,21,69,136–143]. At least seven pharmaceutical companies worldwide are developing Hedgehog antagonists and, many of them – those that target Smo – are in advanced clinical development. Among them, the Genentech-produced Vismodegib (or GDC-0449) was approved by the FDA in 2010 and is now the treatment of choice for locally advanced and metastatic basal cell carcinoma [138,210,211] – the most common non-melanoma skin cancer associated with constitutive activation of the Hedgehog signalling pathway [143,212,213].
Among Smo binding small molecules, several modes of inhibition exist. Cyclopamine and Vismodegib (among others), for example, allow Smo translocation to the primary cilium, but prevent its movement to the tip of the cilium and thus block signal transduction. Molecules of the SANT family (SANT-1, 2 and 3) block Smo at an earlier step, preventing translocation and entry to the primary cilium. Intriguingly, when probed against the ability to inhibit the metabolic non-canonical Hedgehog signalling, only molecules of the first class behave as agonists, inducing Ca2+ influx, Ampk phosphorylation and glucose uptake with a potency 10-fold higher on average than that required for their own inhibitory effects on canonical signalling. Smo agonists, which induce translocation to the base and subsequently to the tip of the cilium also induce the non-canonical signal, albeit at somewhat reduced amplitude [72]. These findings indicate first, that canonical and non-canonical signalling cascades can be uncoupled at the level of Smo, and second, that the metabolic non-canonical signalling arm requires Smo translocation to the base of the primary cilium. This interpretation is underscored by the fact that Smo-dependent Ampk activation requires a fully intact primary cilium, and that CLD-Smo mutant fails to induce it.
The primary cilium is a microtubule-based organelle with crucial roles in vertebrate development [31,214] and human genetic diseases [215,216]. Impaired cilium function or development is associated with a group of disorders known as ciliopathies and characterized by developmental defects and interestingly, obesity. Specifically, the AMPK activation step downstream of Smo is compartmentalized to the basal body of the primary cilium, where Ampk associates directly with its activating kinase Lkb1 [217]. The findings that Smo-CLD or other variants unable to localize to the cil-ium (e.g. SmoM2) are unable to activate the Smo-Ampk axis implies that the pathway, though compartmentalized to the basal body, still requires an intact primary cilium.
3.2. Therapeutic and physiological relevance
Hedgehog signalling is a pro-oncogenic pathway. Therefore, the use of Hedgehog agonists in therapy has always been disregarded, despite beneficial potential in a series of diseases [110]. The finding that canonical antagonists were partial agonists of the metabolic pathway, prompted us to further investigate the glucose uptake effects. Notably, Ampk activation and glucose uptake is conserved in primary human myocytes [72]. Perhaps more important the effect is preserved in vivo. Mice under constant cyclopamine infusion show robust, insulin-independent glucose uptake in skeletal muscle and BAT [72]. A single Cyclopamine bolus can equally clear blood glucose in overt, insulin-deficient, type 1 diabetic animals [72], highlighting potential for Hedgehog selective partial agonists as glucose lowering agents. In the context of cancer, marked muscle spasms and weight loss marred the first successes of BCC patient therapy with Vismodegib [138]. Recent evidence suggests that these prominent and unforeseen side-effects of Vismodegib treatment are largely prevented by combined therapy with L-Type Ca2+ channel inhibitors, strong indication that the side-effects are non-canonical derived (personal communication). These findings provide tools and a platform for reassessing and validating optimal Smo-targeting strategies (Fig. 3).
Fig. 3.
4. Conclusions and future directions
In the context of metabolic control, the next big question certainly remains physiological relevance. Our own preliminary data suggest that a fully functional endogenous ligand-receptor axis is active in vivo, one that does indeed link substrate utilization and whole body energy homeostasis (unpublished). The findings suggest Hedgehog indeed comprises a novel endocrine regulator of metabolic homeostasis.
The studies above suggest that much is still to be learned. In particular, we may have just struck the tip of an iceberg of regulatory potential. We would predict that while not all cells will respond with glucose uptake, every Smo expressing cell with a primary cilium likely has its own physiological output for rapid acute Smo-coupled non-canonical signalling. If true, there are some exciting discoveries to come.
Abbreviations
- GLI1/2/3
glioma-associated oncogene homologue 1/2/3
- PTCH
patched
- SMO
smoothened
- SUFU
suppressor of fused
- CDO
CAM-related/downregulated by oncogenes
- BOC
brother of CDO
- GAS1
growth arrest-specific 1
- PKA
protein kinase A
- GSK3β
glycogen synthase kinase 3β
- CK1
casein kinase 1
- SAG
smoothened agonist
- SHH
sonic Hedgehog
- DHH
desert Hedgehog
- IHH
Indian Hedgehog
- GRK2
G-protein coupled receptor kinase 2
- PTX
pertussis toxin
- RAC1
Ras-relatedC3 botulinum toxin substrate 1
- RHOA
Ras-homolog family member A
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- CARMA1
CARD-containing MAGUK protein 1
- BCL10
B-cell CLL/lymphoma 10
- MALT1
mucosa associated lymphoid tissue lymphoma translocation gene 1
- LRP2
LDL-receptor-related-protein 2
- VLDL
very low-density lipoprotein
- HIF1α
hypoxia-inducible factor 1α
- IGF1
insulin-like growth factor 1
- IPF1/PDX1
insulin promoter factor 1/pancreatic and duodenal homeobox 1
- PPARγ
peroxisome proliferator-activated receptor gamma
- CAMKK2
calcium-calmodulin-dependent kinase 2
- AMPK
AMP-dependent kinase
- PKM2
pyruvate kinase M2 isoform
- PDHa1
pyruvate dehydrogenase a1
- CLD-Smo
ciliary localization deficient Smo
- LKB1
liver kinase B1
References
- 1.Anderson E, Peluso S, Lettice LA, Hill RE. Human limb abnormalities caused by disruption of Hedgehog signaling. Trends Genet. 2012;28:364–373. doi: 10.1016/j.tig.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Hui CC. Gli proteins in development and disease. Annu Rev Cell Dev Biol. 2011;27:513–537. doi: 10.1146/annurev-cellbio-092910-154048. [DOI] [PubMed] [Google Scholar]
- 3.Jiang J, Hui CC. Hedgehog signaling in development and cancer. Dev Cell. 2008;15:801–812. doi: 10.1016/j.devcel.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pospisilik JA, Schramek D, Schnidar H, Cronin SJ, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui CC, Esterbauer H, Penninger JM. Drosophila genome-wide obesity screen reveals Hedgehog as a determinant of brown versus white adipose cell fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 5.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 6.Shyh-Chang N, Daley GQ, Cantley LC. Stem cell metabolism in tissue development and aging. Development. 2013;140:2535–2547. doi: 10.1242/dev.091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pires-daSilva A, Sommer RJ. The evolution of signalling pathways in animal development. Nat Rev Genet. 2003;4:39–49. doi: 10.1038/nrg977. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz i Altaba A, Mas C, Stecca B. The Gli code: an information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stecca B, Ruiz A, Altaba I. Context-dependent regulation ofthe GLI code in can-cerby HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen M, Briscoe J, Blassberg R. Morphogen interpretation: the transcriptional logic of neural tube patterning. Curr Opin Genet Dev. 2013;23:423–8. doi: 10.1016/j.gde.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Vincent JP, Briscoe J. Morphogens. Curr Biol. 2001;11:R851–R854. doi: 10.1016/s0960-9822(01)00514-0. [DOI] [PubMed] [Google Scholar]
- 12.Beauchamp E, Bulut G, Abaan O, Chen K, Merchant A, Matsui W, Endo Y, Rubin JS, Toretsky J, Uren A. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennler S, André J, Alexaki I, Li A, Magnaldo T, ten Dijke P, Wang XJ, Verrecchia F, Mauviel A. Induction of sonic Hedgehog mediators by transforming growth factor-beta: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6986. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 14.Eberl M, Klingler S, Mangelberger D, Loipetzberger A, Damhofer H, Zoidl K, Schnidar H, Hache H, Bauer HC, Solca F, Hauser-Kronberger C, Ermilov AN, Verhaegen ME, Bichakjian CK, Dlugosz AA, Nietfeld W, Sibilia M, Lehrach H, Wierling C, Aberger F. Hedgehog-EGFR cooperation response genes determine the oncogenic phenotype of basal cell carcinoma and tumour-initiating pancreatic cancer cells. EMBO Mol Med. 2012;4:218–233. doi: 10.1002/emmm.201100201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogarty MP, Emmenegger BA, Grasfeder LL, Oliver TG, Wechsler-Reya RJ. Fibroblast growth factor blocks Sonic Hedgehog signaling in neuronal precursors and tumor cells. Proc Natl Acad Sci U S A. 2007;104:2973–2978. doi: 10.1073/pnas.0605770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauth M, Bergström A, Toftgård R. Phorbol esters inhibit the Hedgehog signalling pathway downstream of Suppressor of Fused, but upstream of Gli. Oncogene. 2007;26:5163–8. doi: 10.1038/sj.onc.1210321. [DOI] [PubMed] [Google Scholar]
- 17.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernández-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riobo NA, Haines GM, Emerson CP. Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in Hedgehog signaling. Cancer Res. 2006;66:839–845. doi: 10.1158/0008-5472.CAN-05-2539. [DOI] [PubMed] [Google Scholar]
- 19.Riobó NA, Lu K, Ai X, Haines GM, Emerson CP. Phosphoinositide 3-kinase and Akt are essential for Sonic Hedgehog signaling. Proc Natl Acad Sci U S A. 2006;103:4505–10. doi: 10.1073/pnas.0504337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R, Moriggl R, Sibilia M, Aberger F. Epidermal growth factor receptor signaling synergizes with Hedgehog/GLI in onco-¥ genic transformation via activation ofthe MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stecca B, Mas C, Clement V, Zbinden M, Correa R, Piguet V, Beermann F, Ruiz A, Altaba I. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A. 2007;104:5895–5900. doi: 10.1073/pnas.0700776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Davidow L, Arvanites AC, Blanchard J, Lam K, Xu K, Oza V, Yoo JW, Ng JM, Curran T, Rubin LL, McMahon AP. Glucocorticoid compounds modify smoothened localization and Hedgehog pathway activity. Chem Biol. 2012;19:972–982. doi: 10.1016/j.chembiol.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whisenant TC, Ho DT, Benz RW, Rogers JS, Kaake RM, Gordon EA, Huang L, Baldi P, Bardwell L. Computational prediction and experimental verification of new MAP kinase docking sites and substrates including Gli transcription factors. PLoS Comput Biol. 2010:6. doi: 10.1371/journal.pcbi.1000908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan D, Chen X, Cheng L, Mahoney M, Riobo NA. Noncanonical Hedgehog signaling. Vitam Horm. 2012;88:55–72. doi: 10.1016/B978-0-12-394622-5.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, McMahon AP, Allen BL. Shifting paradigms in Hedgehog signaling. Curr Opin Cell Biol. 2007;19:159–165. doi: 10.1016/j.ceb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 28.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates Hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 29.Svärd J, Heby-Henricson K, Henricson KH, Persson-Lek M, Rozell B, Lauth M, Bergström A, Ericson J, Toftgård R, Teglund S. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Varjosalo M, Li SP, Taipale J. Divergence of Hedgehog signal transduction mechanism between Drosophila and mammals. Dev Cell. 2006;10:177–186. doi: 10.1016/j.devcel.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuzhandaivel A, Schultz SW, Alkhori L, Alenius M. Cilia-mediated Hedgehog signaling in Drosophila. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Goodrich LV, Milenković L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 34.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations ofthe human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo A, Ingham P. Cell patterning inthe Drosophila segment: spatial regulation ofthe segment polarity gene patched. Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- 36.Ingham PW, Taylor AM, Nakano Y. Role ofthe Drosophila patched gene in positional signalling. Nature. 1991;353:184–187. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, Quinn AG, Myers RM, Cox DR, Epstein EH, Scott MP. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 38.Allen BL, Tenzen T, McMahon AP. The Hedgehog-binding proteins Gas1 and Cdo cooperate to positively regulate Shh signaling during mouse development. Genes Dev. 2007;21:1244–1257. doi: 10.1101/gad.1543607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beachy PA, Hymowitz SG, Lazarus RA, Leahy DJ, Siebold C. Interactions between Hedgehog proteins and their binding partners come into view. Genes Dev. 2010;24:2001–2012. doi: 10.1101/gad.1951710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinelli DC, Fan CM. Gas1 extends the range of Hedgehog action by facilitating its signaling. Genes Dev. 2007;21:1231–1243. doi: 10.1101/gad.1546307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McLellan JS, Zheng X, Hauk G, Ghirlando R, Beachy PA, Leahy DJ. The mode of Hedgehog binding to Ihog homologues is not conserved across different phyla. Nature. 2008;455:979–983. doi: 10.1038/nature07358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 43.van den Heuvel M, Ingham PW. smoothened encodes a receptor-like serpentine protein required for Hedgehog signalling. Nature. 1996;382:547–551. doi: 10.1038/382547a0. [DOI] [PubMed] [Google Scholar]
- 44.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohatgi R, Milenkovic L, Corcoran RB, Scott MP. Hedgehog signal transduction by smoothened: pharmacologic evidence for a 2-step activation process. Proc Natl Acad Sci U S A. 2009;106:3196–3201. doi: 10.1073/pnas.0813373106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched acts catalytically to suppress the activity of smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 47.Corcoran RB, Scott MP. Oxysterols stimulate sonic Hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dwyer JR, Sever N, Carlson M, Nelson SF, Beachy PA, Parhami F. Oxysterols are novel activators of the Hedgehog signaling pathway in pluripotent mesenchymal cells. J Biol Chem. 2007;282:8959–8968. doi: 10.1074/jbc.M611741200. [DOI] [PubMed] [Google Scholar]
- 49.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–220. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nachtergaele S, Whalen DM, Mydock LK, Zhao Z, Malinauskas T, Krishnan K, Ingham PW, Covey DF, Siebold C, Rohatgi R. Structure and function of the smoothened extracellular domain in vertebrate Hedgehog signaling. Elife. 2013;2:e01340. doi: 10.7554/eLife.01340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Callejo A, Culi J, Guerrero I. Patched, the receptor of Hedgehog, is a lipoprotein receptor. Proc Natl Acad Sci U S A. 2008;105:912–917. doi: 10.1073/pnas.0705603105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khaliullina H, Panáková D, Eugster C, Riedel F, Carvalho M, Eaton S. Patched regulates smoothened trafficking using lipoprotein-derived lipids. Development. 2009;136:4111–4121. doi: 10.1242/dev.041392. [DOI] [PubMed] [Google Scholar]
- 53.Kasper M, Regl G, Frischauf AM, Aberger F. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer. 2006;42:437–445. doi: 10.1016/j.ejca.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 54.Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R. Gli protein activity is controlled by multisite phosphorylation in vertebrate Hedgehog signaling. Cell Rep. 2013 doi: 10.1016/j.celrep.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Humke EW, Dorn KV, Milenkovic L, Scott MP, Rohatgi R. The output of Hedgehog signaling is controlled by the dynamic association between suppressor of fused and the gli proteins. Genes Dev. 2010;24:670–682. doi: 10.1101/gad.1902910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proc Natl Acad Sci U S A. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Price MA, Kalderon D. Proteolysis of Cubitus interruptus in Drosophila requires phosphorylation by protein kinase A. Development. 1999;126:4331–4339. doi: 10.1242/dev.126.19.4331. [DOI] [PubMed] [Google Scholar]
- 58.Liu A, Wang B, Niswander LA. Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development. 2005;132:3103–3111. doi: 10.1242/dev.01894. [DOI] [PubMed] [Google Scholar]
- 59.Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 60.Tukachinsky H, Lopez LV, Salic A. A mechanism for vertebrate Hedgehog signaling: recruitment to cilia and dissociation of SuFu-Gli protein complexes. J Cell Biol. 2010;191:415–428. doi: 10.1083/jcb.201004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen X, Lai CK, Evangelista M, Hongo JA, de Sauvage FJ, Scales SJ. Kinetics of Hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Mol Cell Biol. 2010;30:1910–1922. doi: 10.1128/MCB.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The ciliary G-protein-coupled receptor Gpr161 negatively regulates the Sonic Hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 63.Barzi M, Berenguer J, Menendez A, Alvarez-Rodriguez R, Pons S. Sonic-Hedgehog-mediated proliferation requires the localization of PKA to the cilium base. J Cell Sci. 2010;123:62–69. doi: 10.1242/jcs.060020. [DOI] [PubMed] [Google Scholar]
- 64.Kogerman P, Grimm T, Kogerman L, Krause D, Undén AB, Sandstedt B, Toftgård R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 65.Dai P, Akimaru H, Tanaka Y, Maekawa T, Nakafuku T, Ishii S. Sonic Hedgehog-induced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem. 1999;274:8143–8152. doi: 10.1074/jbc.274.12.8143. [DOI] [PubMed] [Google Scholar]
- 66.Ikram MS, Neill GW, Regl G, Eichberger T, Frischauf AM, Aberger F, Quinn A, Philpott M. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol. 2004;122:1503–1509. doi: 10.1111/j.0022-202X.2004.22612.x. [DOI] [PubMed] [Google Scholar]
- 67.Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F. Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene. 2002;21:5529–5539. doi: 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz i Altaba A. Gli proteins and Hedgehog signaling: development and cancer. Trends Genet. 1999;15:418–425. doi: 10.1016/s0168-9525(99)01840-5. [DOI] [PubMed] [Google Scholar]
- 69.Ruiz i Altaba A, Sánchez P, Dahmane N. Gli and Hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 70.Kristiansen K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther. 2004;103:21–80. doi: 10.1016/j.pharmthera.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Wang C, Wu H, Katritch V, Han GW, Huang XP, Liu W, Siu FY, Roth BL, Cherezov V, Stevens RC. Structure of the human smoothened receptor bound to an antitumour agent. Nature. 2013;497:338–343. doi: 10.1038/nature12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, Dalgaard K, Selvaraj M, Gaster M, Lee-Young RS, Febbraio MA, Knauf C, Cani PD, Aberger F, Penninger JM, Pospisilik JA, Esterbauer H. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell. 2012;151:414–426. doi: 10.1016/j.cell.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Myers BR, Sever N, Chong YC, Kim J, Belani JD, Rychnovsky S, Bazan JF, Beachy PA. Hedgehog pathway modulation by multiple lipid binding sites on the smoothened effector of signal response. Dev Cell. 2013 doi: 10.1016/j.devcel.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nedelcu D, Liu J, Xu Y, Jao C, Salic A. Oxysterol binding to the extracellular domain of Smoothened in Hedgehog signaling. Nat Chem Biol. 2013;9:557–564. doi: 10.1038/nchembio.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao Y, Tong C, Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450:252–8. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 76.Shen F, Cheng L, Douglas AE, Riobo NA, Manning DR. Smoothened is a fully competent activator of the heterotrimeric G protein G(i) Mol Pharmacol. 2013;83:691–7. doi: 10.1124/mol.112.082511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem. 2011;286:19589–19596. doi: 10.1074/jbc.M110.197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen W, Ren XR, Nelson CD, Barak LS, Chen JK, Beachy PA, de Sauvage F, Lefkowitz RJ. Activity-dependent internalization of smoothened mediated by beta-arrestin 2 and GRK2. Science. 2004;306:2257–2260. doi: 10.1126/science.1104135. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y, Li S, Tong C, Zhao Y, Wang B, Liu Y, Jia J, Jiang J. G protein-coupled receptor kinase 2 promotes high-level Hedgehog signaling by regulating the active state of Smo through kinase-dependent and kinase-independent mechanisms in Drosophila. Genes Dev. 2010;24:2054–2067. doi: 10.1101/gad.1948710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF. Vertebrate smoothened functions at the primary cilium. Nature. 2005;437:1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 81.Kovacs JJ, Whalen EJ, Liu R, Xiao K, Kim J, Chen M, Wang J, Chen W, Lefkowitz RJ. Beta-arrestin-mediated localization of smoothened to the primary cilium. Science. 2008;320:1777–1781. doi: 10.1126/science.1157983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lum L, Zhang C, Oh S, Mann RK, von Kessler DP, Taipale J, Weis-Garcia F, Gong R, Wang B, Beachy PA. Hedgehog signal transduction via smoothened association with a cytoplasmic complex scaffolded by the atypical kinesin, Costal-2. Mol Cell. 2003;12:1261–1274. doi: 10.1016/s1097-2765(03)00426-x. [DOI] [PubMed] [Google Scholar]
- 83.Shi Q, Li S, Jia J, Jiang J. The Hedgehog-induced smoothened conformational switch assembles a signaling complex that activates fused by promoting its dimerizationand phosphorylation. Development. 2011;138:4219–31. doi: 10.1242/dev.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilbanks AM, Fralish GB, Kirby ML, Barak LS, Li YX, Caron MG. Beta-arrestin 2 regulates zebrafish development through the Hedgehog signaling pathway. Science. 2004;306:2264–7. doi: 10.1126/science.1104193. [DOI] [PubMed] [Google Scholar]
- 85.Ogden SK, Fei DL, Schilling NS, Ahmed YF, Hwa J, Robbins DJ. G protein Galphai functions immediately downstream of smoothened in Hedgehog signalling. Nature. 2008;456:967–970. doi: 10.1038/nature07459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hammerschmidt M, McMahon AP. The effect of pertussis toxin on zebrafish development: a possible role for inhibitory G-proteins in Hedgehog signaling. Dev Biol. 1998;194:166–171. doi: 10.1006/dbio.1997.8796. [DOI] [PubMed] [Google Scholar]
- 87.Low WC, Wang C, Pan Y, Huang XY, Chen JK, Wang B. The decoupling of smoothened from Galphai proteins has little effect on Gli3 protein processing and Hedgehog-regulated chick neural tube patterning. Dev Biol. 2008;321:188–196. doi: 10.1016/j.ydbio.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barzi M, Kostrz D, Menendez A, Pons S. Sonic Hedgehog-induced proliferation requires specific Gα inhibitory proteins. J Biol Chem. 2011;286:8067–8074. doi: 10.1074/jbc.M110.178772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chinchilla P, Xiao L, Kazanietz MG, Riobo NA. Hedgehog proteins activate pro-angiogenic responses in endothelial cells through non-canonical signaling pathways. Cell Cycle. 2010;9:570–579. doi: 10.4161/cc.9.3.10591. [DOI] [PubMed] [Google Scholar]
- 90.Polizio AH, Chinchilla P, Chen X, Manning DR, Riobo NA, Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal. 2011;4:pt7. doi: 10.1126/scisignal.2002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Razumilava N, Gradilone SA, Smoot RL, Mertens JC, Bronk SF, Sirica AE, Gores GJ. Non-canonical Hedgehog signaling contributes to chemotaxis in cholan-giocarcinoma. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bijlsma MF, Damhofer H, Roelink H. Hedgehog-stimulated chemotaxis is mediated by smoothened located outside the primary cilium. Sci Signal. 2012;5:ra60. doi: 10.1126/scisignal.2002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Belgacem YH, Borodinsky LN. Sonic Hedgehog signaling is decoded by calcium spike activity in the developing spinal cord. Proc Natl Acad Sci U S A. 2011;108:4482–7. doi: 10.1073/pnas.1018217108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qu C, Liu Y, Kunkalla K, Singh RR, Blonska M, Lin X, Agarwal NK, Vega F. Trimeric G protein-CARMA1 axis links smoothened, the Hedgehog receptor transducer, to NF-κB activation in diffuse large B-cell lymphoma. Blood. 2013;121:4718–4728. doi: 10.1182/blood-2012-12-470153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, Arnheiter H. The G12 family of heterotrimeric G proteins and RhoGTPase mediate Sonic Hedgehog signalling. Genes Cells. 2004;9:49–58. doi: 10.1111/j.1356-9597.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- 96.Douglas AE, Heim JA, Shen F, Almada LL, Riobo NA, Fernández-Zapico ME, Manning DR. The alpha subunit of the G protein G13 regulates activity of one or more Gli transcription factors independently of smoothened. J Biol Chem. 2011;286:30714–30722. doi: 10.1074/jbc.M111.219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine tosmoothened. Genes Dev. 2002;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of smoothened activity. Proc Natl Acad Sci U S A. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- 100.Aikin R, Cervantes A, D’Angelo G, Ruel L, Lacas-Gervais S, Schaub S, Thérond P. A genome-wide RNAi screen identifies regulators of cholesterol-modified Hedgehog secretion in Drosophila. PLoS ONE. 2012;7:e33665. doi: 10.1371/journal.pone.0033665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beug ST, Parks RJ, McBride HM, Wallace VA. Processing-dependent trafficking of Sonic Hedgehog to the regulated secretory pathway in neurons. Mol Cell Neurosci. 2011;46:583–596. doi: 10.1016/j.mcn.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Chamoun Z, Mann RK, Nellen D, von Kessler DP, Bellotto M, Beachy PA, Basler K. Skinny Hedgehog, an acyltransferase required for palmitoylation and activity of the Hedgehog signal. Science. 2001;293:2080–2084. doi: 10.1126/science.1064437. [DOI] [PubMed] [Google Scholar]
- 103.Creanga A, Glenn TD, Mann RK, Saunders AM, Talbot WS, Beachy PA. Scube/You activity mediates release of dually lipid-modified Hedgehog signal in soluble form. Genes Dev. 2012;26:1312–1325. doi: 10.1101/gad.191866.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kornberg TB. Barcoding Hedgehog for intracellular transport. Sci Signal. 2011;4:pe44. doi: 10.1126/scisignal.2002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li YX, Yang HT, Zdanowicz M, Sicklick JK, Qi Y, Camp TJ, Diehl AM. Fetal alcohol exposure impairs Hedgehog cholesterol modification and signaling. Lab Invest. 2007;87:231–240. doi: 10.1038/labinvest.3700516. [DOI] [PubMed] [Google Scholar]
- 106.Mann RK, Beachy PA. Novel lipid modifications of secreted protein signals. Annu Rev Biochem. 2004;73:891–923. doi: 10.1146/annurev.biochem.73.011303.073933. [DOI] [PubMed] [Google Scholar]
- 107.Thérond PP. Release and transportation of Hedgehog molecules. Curr Opin Cell Biol. 2012;24:173–180. doi: 10.1016/j.ceb.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 108.Levi B, James AW, Nelson ER, Li S, Peng M, Commons GW, Lee M, Wu B, Longaker MT. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev. 2011;20:243–257. doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mao H, Diehl AM, Li YX. Sonic Hedgehog ligand partners with caveolin-1 for intracellular transport. Lab Invest. 2009;89:290–300. doi: 10.1038/labinvest.2008.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hadden MK. Hedgehog pathway agonism: therapeutic potential and small-molecule development. ChemMedChem. 2013 doi: 10.1002/cmdc.201300358. [DOI] [PubMed] [Google Scholar]
- 111.Kha HT, Basseri B, Shouhed D, Richardson J, Tetradis S, Hahn TJ, Parhami F. Oxysterols regulate differentiation of mesenchymal stem cells: pro-bone and anti-fat. J Bone Miner Res. 2004;19:830–840. doi: 10.1359/JBMR.040115. [DOI] [PubMed] [Google Scholar]
- 112.Cooper MK, Wassif CA, Krakowiak PA, Taipale J, Gong R, Kelley RI, Porter FD, Beachy PA. A defective response to Hedgehog signaling in disorders of cholesterol biosynthesis. Nat Genet. 2003;33:508–513. doi: 10.1038/ng1134. [DOI] [PubMed] [Google Scholar]
- 113.Martín V, Carrillo G, Torroja C, Guerrero I. The sterol-sensing domain of patched protein seems to control smoothened activity through patched vesicular trafficking. Curr Biol. 2001;11:601–607. doi: 10.1016/s0960-9822(01)00178-6. [DOI] [PubMed] [Google Scholar]
- 114.Strutt H, Thomas C, Nakano Y, Stark D, Neave B, Taylor AM, Ingham PW. Mutations in the sterol-sensing domain of patched suggest a role for vesicular trafficking in smoothened regulation. Curr Biol. 2001;11:608–613. doi: 10.1016/s0960-9822(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 115.Johnson RL, Zhou L, Bailey EC. Distinct consequences of sterol sensor mutations in Drosophila and mouse patched homologs. Dev Biol. 2002;242:224–235. doi: 10.1006/dbio.2001.0524. [DOI] [PubMed] [Google Scholar]
- 116.Christ A, Christa A, Kur E, Lioubinski O, Bachmann S, Willnow TE, Hammes A. LRP2 is an auxiliary SHH receptor required to condition the forebrain ventral midline for inductive signals. Dev Cell. 2012;22:268–78. doi: 10.1016/j.devcel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 117.Saito A, Pietromonaco S, Loo AK, Farquhar MG. Complete cloning and sequencing of rat gp330/”megalin,” a distinctive member of the low density lipoprotein receptor gene family. Proc Natl Acad Sci U S A. 1994;91:9725–9729. doi: 10.1073/pnas.91.21.9725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Christensen EI, Birn H. Megalinand cubilin: multifunctional endocytic receptors. Nat Rev Mol Cell Biol. 2002;3:256–266. doi: 10.1038/nrm778. [DOI] [PubMed] [Google Scholar]
- 119.May P, Woldt E, Matz RL, Boucher P. The LDL receptor-related protein (LRP) family: an old family of proteins with new physiological functions. Ann Med. 2007;39:219–228. doi: 10.1080/07853890701214881. [DOI] [PubMed] [Google Scholar]
- 120.Yavari A, Nagaraj R, Owusu-Ansah E, Folick A, Ngo K, Hillman T, Call G, Rohatgi R, Scott MP, Banerjee U. Role of lipid metabolism in smoothened derepression in Hedgehog signaling. Dev Cell. 2010;19:54–65. doi: 10.1016/j.devcel.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santiago-Tirado FH, Bretscher A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011;21:515–525. doi: 10.1016/j.tcb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hammond GR, Fischer MJ, Anderson KE, Holdich J, Koteci A, Balla T, Irvine RF. PI4P and PI(4,5)P2 are essential but independent lipid determinants of membrane identity. Science. 2012;337:727–730. doi: 10.1126/science.1222483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Panáková D, Sprong H, Marois E, Thiele C, Eaton S. Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature. 2005;435:58–65. doi: 10.1038/nature03504. [DOI] [PubMed] [Google Scholar]
- 124.Swierczynska MM, Lamounier-Zepter V, Bornstein SR, Eaton S. Lipoproteins and Hedgehog signalling - possible implications for the adrenal gland function. EurJ Clin Invest. 2013;43:1178–1183. doi: 10.1111/eci.12145. [DOI] [PubMed] [Google Scholar]
- 125.Willnow TE, Hammes A, Eaton S. Lipoproteins and their receptors in embryonic development: more than cholesterol clearance. Development. 2007;134:3239–3249. doi: 10.1242/dev.004408. [DOI] [PubMed] [Google Scholar]
- 126.Eaton S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat Rev Mol Cell Biol. 2008;9:437–445. doi: 10.1038/nrm2414. [DOI] [PubMed] [Google Scholar]
- 127.Palm W, Swierczynska MM, Kumari V, Ehrhart-Bornstein M, Bornstein SR, Eaton S. Secretion and signaling activities of lipoprotein-associated Hedgehog and non-sterol-modified Hedgehog in flies and mammals. PLoS Biol. 2013;11:e1001505. doi: 10.1371/journal.pbio.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Queiroz KC, Tio RA, Zeebregts CJ, Bijlsma MF, Zijlstra F, Badlou B, de Vries M, Ferreira CV, Spek CA, Peppelenbosch MP, Rezaee F. Human plasma very low density lipoprotein carries Indian Hedgehog. J Proteome Res. 2010;9:6052–6059. doi: 10.1021/pr100403q. [DOI] [PubMed] [Google Scholar]
- 129.Briscoe J, Thérond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013 doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 130.Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006;3:25–34. doi: 10.1016/j.cmet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 131.Todoric J, Strobl B, Jais A, Boucheron N, Bayer M, Amann S, Lindroos J, Teperino R, Prager G, Bilban M, Ellmeier W, Krempler F, Müller M, Wagner O, Patsch W, Pospisilik JA, Esterbauer H. Cross-talk between interferon-7 and Hedgehog signaling regulates adipogenesis. Diabetes. 2011;60:1668–1676. doi: 10.2337/db10-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wu X, Ding S, Ding Q, Gray NS, Schultz PG. A small molecule with osteogenesis-inducing activity in multipotent mesenchymal progenitor cells. J Am Ceram Soc. 2002;124:14520–14521. doi: 10.1021/ja0283908. [DOI] [PubMed] [Google Scholar]
- 133.Zehentner BK, Leser U, Burtscher H. BMP-2 and sonic Hedgehog have contrary effects on adipocyte-like differentiation of C3H10T1/2 cells. DNA Cell Biol. 2000;19:275–281. doi: 10.1089/10445490050021186. [DOI] [PubMed] [Google Scholar]
- 134.Hatley ME, Tang W, Garcia MR, Finkelstein D, Millay DP, Liu N, Graff J, Galindo RL, Olson EN. A mouse model of rhabdomyosarcoma originating from the adipocyte lineage. Cancer Cell. 2012;22:536–546. doi: 10.1016/j.ccr.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Marion V, Mockel A, De Melo C, Obringer C, Claussmann A, Simon A, Messad-deq N, Durand M, Dupuis L, Loeffler JP, King P, Mutter-Schmidt C, Petrovsky N, Stoetzel C, Dollfus H. BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell Metab. 2012;16:363–377. doi: 10.1016/j.cmet.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 136.Blotta S, Jakubikova J, Calimeri T, Roccaro AM, Amodio N, Azab AK, Foresta U, Mitsiades CS, Rossi M, Todoerti K, Molica S, Morabito F, Neri A, Tagliaferri P, Tassone P, Anderson KC, Munshi NC. Canonical and noncanonical Hedgehog pathway in the pathogenesis of multiple myeloma. Blood. 2012;120:5002–5013. doi: 10.1182/blood-2011-07-368142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan IS, Guy CD, Chen Y, Lu J, Swiderska-Syn M, Michelotti GA, Karaca G, Xie G, Krüger L, Syn WK, Anderson BR, Pereira TA, Choi SS, Baldwin AS, Diehl AM. Paracrine Hedgehog signaling drives metabolic changes in hepatocellular carcinoma. Cancer Res. 2012;72:6344–6350. doi: 10.1158/0008-5472.CAN-12-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ng JM, Curran T. The Hedgehog’s tale: developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pasca di Magliano M, Sekine S, Ermilov A, Ferris J, Dlugosz AA, Hebrok M. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Scales SJ, de Sauvage FJ. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 141.Teglund S, Toftgård R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 142.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancertumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vorechovský I, Tingby O, Hartman M, Strömberg B, Nister M, Collins VP, Toftgård R. Somatic mutations in the human homologue of Drosophila patched in primitive neuroectodermal tumours. Oncogene. 1997;15:361–366. doi: 10.1038/sj.onc.1201340. [DOI] [PubMed] [Google Scholar]
- 144.Yam PT, Langlois SD, Morin S, Charron F. Sonic Hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–62. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 145.Gruffat D, Durand D, Graulet B, Bauchart D. Regulation ofVLDL synthesis and secretion in the liver. Reprod Nutr Dev. 1996;36:375–389. doi: 10.1051/rnd:19960404. [DOI] [PubMed] [Google Scholar]
- 146.Tiwari S, Siddiqi SA. Intracellular trafficking and secretion of VLDL. Arte-riosclerThromb Vasc Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab (Lond) 2010;7:35. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Omenetti A, Yang L, Li YX, McCall SJ, Jung Y, Sicklick JK, Huang J, Choi S, Suzuki A, Diehl AM. Hedgehog-mediated mesenchymal-epithelial interactions modulate hepatic response to bile duct ligation. Lab Invest. 2007;87:499–514. doi: 10.1038/labinvest.3700537. [DOI] [PubMed] [Google Scholar]
- 149.Omenetti A, Syn WK, Jung Y, Francis H, Porrello A, Witek RP, Choi SS, Yang L, Mayo MJ, Gershwin ME, Alpini G, Diehl AM. Repair-related activation of Hedgehog signaling promotes cholangiocyte chemokine production. Hepa-tology. 2009;50:518–527. doi: 10.1002/hep.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choi SS, Syn WK, Karaca GF, Omenetti A, Moylan CA, Witek RP, Agboola KM, Jung Y, Michelotti GA, Diehl AM. Leptin promotes the myofibroblastic pheno-type in hepatic stellate cells by activating the Hedgehog pathway. J Biol Chem. 2010;285:36551–36560. doi: 10.1074/jbc.M110.168542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, Moylan CA, Garibaldi F, Premont R, Suliman HB, Piantadosi CA, Diehl AM. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319.e1–1329.e11. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chan IS, Guy CD, Machado MV, Wank A, Kadiyala V, Michelotti G, Choi S, Swiderska-Syn M, Karaca G, Pereira TA, Yip-Schneider MT, Max Schmidt C, Diehl AM. Alcohol activates the Hedgehog pathway and induces related procarcinogenic processes in the alcohol-preferring rat model of hepatocar-cinogenesis. Alcohol Clin Exp Res. 2013 doi: 10.1111/acer.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Cal-abro SR, Dwyer BJ, Tirnitz-Parker JE, Neil Watkins D, Warner FJ, Shackel NA, McCaughan GW. The intrahepatic signalling niche of Hedgehog is defined by primary cilia positive cells during chronic liver injury. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 154.Matz-Soja M, Hovhannisyan A, Gebhardt R. Hedgehog signalling pathway in adult liver: a major new player in hepatocyte metabolism and zonation? Med Hypotheses. 2013;80:589–594. doi: 10.1016/j.mehy.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 155.Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM. Sonic Hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol. 2008;48:98–106. doi: 10.1016/j.jhep.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Krüger L, Premont R, Yang L, Syn WK, Metzger D, Diehl AM. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013 doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 158.Sotgia F, Whitaker-Menezes D, Martinez-Outschoorn UE, Flomenberg N, Birbe RC, Witkiewicz AK, Howell A, Philp NJ, Pestell RG, Lisanti MP. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11:1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guido C, Whitaker-Menezes D, Capparelli C, Balliet R, Pestell Z, Pestell RG, Howell A, Aquila S, Andò S, Martinez-Outschoorn U, Sotgia F, Lisanti MP. Metabolic reprogramming of cancer-associated fibroblasts by TGF-β drives tumor growth: connecting TGF-β signaling with “Warburg-like” cancer metabolism and L-lactate production. Cell Cycle. 2012;11:3019–3035. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiava-rina B, Frank PG, Flomenberg N, Howell A, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. Ketones and lactate “fuel” tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism. Cell Cycle. 2010;9:3506–14. doi: 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pereira Tde A, Witek RP, Syn WK, Choi SS, Bradrick S, Karaca GF, Agboola KM, Jung Y, Omenetti A, Moylan CA, Yang L, Fernandez-Zapico ME, Jhaveri R, Shah VH, Pereira FE, Diehl AM. Viral factors induce Hedgehog pathway activation in humans with viral hepatitis, cirrhosis, and hepatocellular carcinoma. Lab Invest. 2010;90:1690–1703. doi: 10.1038/labinvest.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi SS, Bradrick S, Qiang G, Mostafavi A, Chaturvedi G, Weinman SA, Diehl AM, Jhaveri R. Up-regulation of Hedgehog pathway is associated with cellular permissiveness for hepatitis C virus replication. Hepatology. 2011;54:1580–1590. doi: 10.1002/hep.24576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Targett-Adams P, Boulant S, Douglas MW, McLauchlan J. Lipid metabolism and HCV infection. Viruses. 2010;2:1195–217. doi: 10.3390/v2051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rojas Á, del Campo JA, Maraver M, Aparcero R, García-Valdecasas M, Diago M, Carmona I, Andrade RJ, Solà R, Romero-Gómez M. Hepatitis C virus infection alters lipid metabolism depending on IL28B polymorphism and viral genotype and modulates gene expression in vivo and in vitro. J Viral Hepat. 2014;21:19–24. doi: 10.1111/jvh.12209. [DOI] [PubMed] [Google Scholar]
- 166.Schaefer EA, Chung RT. HCV and host lipids: an intimate connection. Semin Liver Dis. 2013;33:358–368. doi: 10.1055/s-0033-1358524. [DOI] [PubMed] [Google Scholar]
- 167.Matz-Soja M, Aleithe S, Marbach E, Böttger J, Arnold K, Schmidt-Heck W, Kratzsch J, Gebhardt R. Hepatic Hedgehog signaling contributes to the regulation of IGF1 and IGFBP1 serum levels. Cell Commun Signal. 2014;12:11. doi: 10.1186/1478-811X-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Loeppen S, Schneider D, Gaunitz F, Gebhardt R, Kurek R, Buchmann A, Schwarz M. Overexpression of glutamine synthetase is associated with beta-catenin-mutations in mouse liver tumors during promotion of hepatocarcinogenesis by phenobarbital. Cancer Res. 2002;62:5685–5688. [PubMed] [Google Scholar]
- 169.Benhamouche S, Decaens T, Godard C, Chambrey R, Rickman DS, Moinard C, Vasseur-Cognet M, Kuo CJ, Kahn A, Perret C, Colnot S. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. DevCell. 2006;10:759–70. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 170.Kawahira H, Scheel DW, Smith SB, German MS, Hebrok M. Hedgehog signaling regulates expansion of pancreatic epithelial cells. Dev Biol. 2005;280:111–121. doi: 10.1016/j.ydbio.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 171.Lauth M, Bergström A, Shimokawa T, Tostar U, Jin Q, Fendrich V, Guerra C, Barbacid M, Toftgård R. DYRK1B–dependent autocrine-to-paracrine shift of Hedgehog signaling by mutant RAS. Nat Struct Mol Biol. 2010;17:718–725. doi: 10.1038/nsmb.1833. [DOI] [PubMed] [Google Scholar]
- 172.El-Zaatari M, Daignault S, Tessier A, Kelsey G, Travnikar LA, Cantu EF, Lee J, Plonka CM, Simeone DM, Anderson MA, Merchant JL. Plasma Shh levels reduced in pancreatic cancer patients. Pancreas. 2012;41:1019–1028. doi: 10.1097/MPA.0b013e31824a0eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Apelqvist A, Ahlgren U, Edlund H. Sonic Hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7:801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 174.Mfopou JK, Bouwens L. Hedgehog signals in pancreatic differentiation from embryonic stem cells: revisiting the neglected. Differentiation. 2008;76:107–117. doi: 10.1111/j.1432-0436.2007.00191.x. [DOI] [PubMed] [Google Scholar]
- 175.Mfopou JK, Baeyens L, Bouwens L. Hedgehog signals inhibit postnatal beta cell neogenesis from adult rat exocrine pancreas in vitro. Diabetologia. 2012;55:1024–1034. doi: 10.1007/s00125-011-2434-8. [DOI] [PubMed] [Google Scholar]
- 176.Landsman L, Parent A, Hebrok M. Elevated Hedgehog/Gli signaling causes beta-cell dedifferentiation in mice. Proc Natl Acad Sci U S A. 2011;108:17010–17015. doi: 10.1073/pnas.1105404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lau J, Hebrok M. Hedgehog signaling in pancreas epithelium regulates embryonic organ formation and adult beta-cell function. Diabetes. 2010;59:1211–1221. doi: 10.2337/db09-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Thomas MK, Rastalsky N, Lee JH, Habener JF. Hedgehog signaling regulation of insulin production by pancreatic beta-cells. Diabetes. 2000;49:2039–2047. doi: 10.2337/diabetes.49.12.2039. [DOI] [PubMed] [Google Scholar]
- 179.Cervantes S, Lau J, Cano DA, Borromeo-Austin C, Hebrok M. Primary cilia regulate Gli/Hedgehog activation in pancreas. Proc Natl Acad Sci U S A. 2010;107:10109–10114. doi: 10.1073/pnas.0909900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Spinella-Jaegle S, Rawadi G, Kawai S, Gallea S, Faucheu C, Mollat P, Courtois B, Bergaud B, Ramez V, Blanchet AM, Adelmant G, Baron R, Roman-Roman S. Sonic Hedgehog increases the commitment of pluripotent mesenchymal cells into the osteoblastic lineage and abolishes adipocytic differentiation. J Cell Sci. 2001;114:2085–2094. doi: 10.1242/jcs.114.11.2085. [DOI] [PubMed] [Google Scholar]
- 182.Sweet HO, Bronson RT, Donahue LR, Davisson MT. Mesenchymal dysplasia: a recessive mutation on chromosome 13 ofthe mouse. J Hered. 1996;87:87–95. doi: 10.1093/oxfordjournals.jhered.a022981. [DOI] [PubMed] [Google Scholar]
- 183.van der Horst G, Farih-Sips H, Löwik CW, Karperien M. Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone. 2003;33:899–910. doi: 10.1016/j.bone.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 184.Wang LC, Nassir F, Liu ZY, Ling L, Kuo F, Crowell T, Olson D, Davidson NO, Burkly LC. Disruption of Hedgehog signaling reveals a novel role in intestinal morphogenesis and intestinal-specific lipid metabolism in mice. Gastroenterology. 2002;122:469–482. doi: 10.1053/gast.2002.31102. [DOI] [PubMed] [Google Scholar]
- 185.Wu X, Walker J, Zhang J, Ding S, Schultz PG. Purmorphamine induces osteogenesis by activation of the Hedgehog signaling pathway. Chem Biol. 2004;11:1229–1238. doi: 10.1016/j.chembiol.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 186.Jackson HE, Ingham PW. Control of muscle fibre-type diversity during embryonic development: the zebrafish paradigm. Mech Dev. 2013;130:447–457. doi: 10.1016/j.mod.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 187.Elworthy S, Hargrave M, Knight R, Mebus K, Ingham PW. Expression of multiple slow myosin heavy chain genes reveals a diversity of zebrafish slow twitch muscle fibres with differing requirements for Hedgehog and Prdm1 activity. Development. 2008;135:2115–2126. doi: 10.1242/dev.015719. [DOI] [PubMed] [Google Scholar]
- 188.Ingham PW, Kim HR. Hedgehog signalling and the specification of muscle cell identity in the zebrafish embryo. Exp Cell Res. 2005;306:336–342. doi: 10.1016/j.yexcr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 189.Lewis KE, Currie PD, Roy S, Schauerte H, Haffter P, Ingham PW. Control of muscle cell-type specification in the zebrafish embryo by Hedgehog signalling. Dev Biol. 1999;216:469–480. doi: 10.1006/dbio.1999.9519. [DOI] [PubMed] [Google Scholar]
- 190.Duprez D, Fournier-Thibault C, Le Douarin N. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development. 1998;125:495–505. doi: 10.1242/dev.125.3.495. [DOI] [PubMed] [Google Scholar]
- 191.Elia D, Madhala D, Ardon E, Reshef R, Halevy O. Sonic Hedgehog promotes proliferation and differentiation of adult muscle cells: involvement of MAPK/ERK and PI3K/Akt pathways. Biochim Biophys Acta. 2007;1773:1438–1446. doi: 10.1016/j.bbamcr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 192.Hirsinger E, Stellabotte F, Devoto SH, Westerfield M. Hedgehog signaling is required for commitment but not initial induction of slow muscle precursors. Dev Biol. 2004;275:143–157. doi: 10.1016/j.ydbio.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 193.Hu JK, McGlinn E, Harfe BD, Kardon G, Tabin CJ. Autonomous and nonau-tonomous roles of Hedgehog signaling in regulating limb muscle formation. Genes Dev. 2012;26:2088–2102. doi: 10.1101/gad.187385.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wolff C, Roy S, Ingham PW. Multiple muscle cell identities induced by distinct levels and timing of Hedgehog activity in the zebrafish embryo. Curr Biol. 2003;13:1169–1181. doi: 10.1016/s0960-9822(03)00461-5. [DOI] [PubMed] [Google Scholar]
- 195.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, Yamamoto H, Miyagoe-Suzuki Y, Takeda S. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 197.Piccioni A, Gaetani E, Neri V, Gatto I, Palladino M, Silver M, Smith RC, Giarretta I, Pola E, Hlatky L, Pola R. Sonic Hedgehog therapy in a mouse model of age-associated impairment of skeletal muscle regeneration. J Gerontol A Biol Sci Med Sci. 2013 doi: 10.1093/gerona/glt076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Straface G, Aprahamian T, Flex A, Gaetani E, Biscetti F, Smith RC, Pecorini G, Pola E, Angelini F, Stigliano E, Castellot JJ, Losordo DW, Pola R. Sonic Hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2009;13:2424–2435. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rubin BP, Nishijo K, Chen HI, Yi X, Schuetze DP, Pal R, Prajapati SI, Abraham J, Arenkiel BR, Chen QR, Davis S, McCleish AT, Capecchi MR, Michalek JE, Zarzabal LA, Khan J, Yu Z, Parham DM, Barr FG, Meltzer PS, Chen Y, Keller C. Evidence for an unanticipated relationship between undifferentiated pleomorphic sarcoma and embryonal rhabdomyosarcoma. Cancer Cell. 2011;19:177–191. doi: 10.1016/j.ccr.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 204.Hitosugi T, Fan J, Chung TW, Lythgoe K, Wang X, Xie J, Ge Q, Gu TL, Polakiewicz RD, Roesel JL, Chen GZ, Boggon TJ, Lonial S, Fu H, Khuri FR, Kang S, Chen J. Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Mol Cell. 2011;44:864–877. doi: 10.1016/j.molcel.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McFate T, Mohyeldin A, Lu H, Thakar J, Henriques J, Halim ND, Wu H, Schell MJ, Tsang TM, Teahan O, Zhou S, Califano JA, Jeoung NH, Harris RA, Verma A. Pyruvate dehydrogenase complex activity controls metabolic and malignant phenotype in cancer cells. J Biol Chem. 2008;283:22700–22708. doi: 10.1074/jbc.M801765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Christofk HR, VanderHeiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 207.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 208.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Israelsen WJ, Dayton TL, Davidson SM, Fiske BP, Hosios AM, Bellinger G, Li J, Yu Y, Sasaki M, Horner JW, Burga LN, Xie J, Jurczak MJ, DePinho RA, Clish CB, Jacks T, Kibbey RG, Wulf GM, Di Vizio D, Mills GB, Cantley LC, Vander Heiden MG. PKM2 isoform-specific deletion reveals a differential requirement for pyruvate kinase in tumor cells. Cell. 2013;155:397–409. doi: 10.1016/j.cell.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tang JY, Mackay-Wiggan JM, Aszterbaum M, Yauch RL, Lindgren J, Chang K, Coppola C, Chanana AM, Marji J, Bickers DR, Epstein EH. Inhibiting the Hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sekulic A, Migden MR, Oro AE, Dirix L, Lewis KD, Hainsworth JD, Solomon JA, Yoo S, Arron ST, Friedlander PA, Marmur E, Rudin CM, Chang AL, Low JA, Mackey HM, Yauch RL, Graham RA, Reddy JC, Hauschild A. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Scott MP. Basal cell carcinomas in mice overexpressing sonic Hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 213.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 214.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. J Cell Sci. 2010;123:499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and cil-iopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 216.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 217.Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Gödel M, Müller K, Herbst M, Hornung M, Doerken M, Köttgen M, Nitschke R, Igarashi P, Walz G, Kuehn EW. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol. 2010;12:1115–1122. doi: 10.1038/ncb2117. [DOI] [PMC free article] [PubMed] [Google Scholar]