Abstract
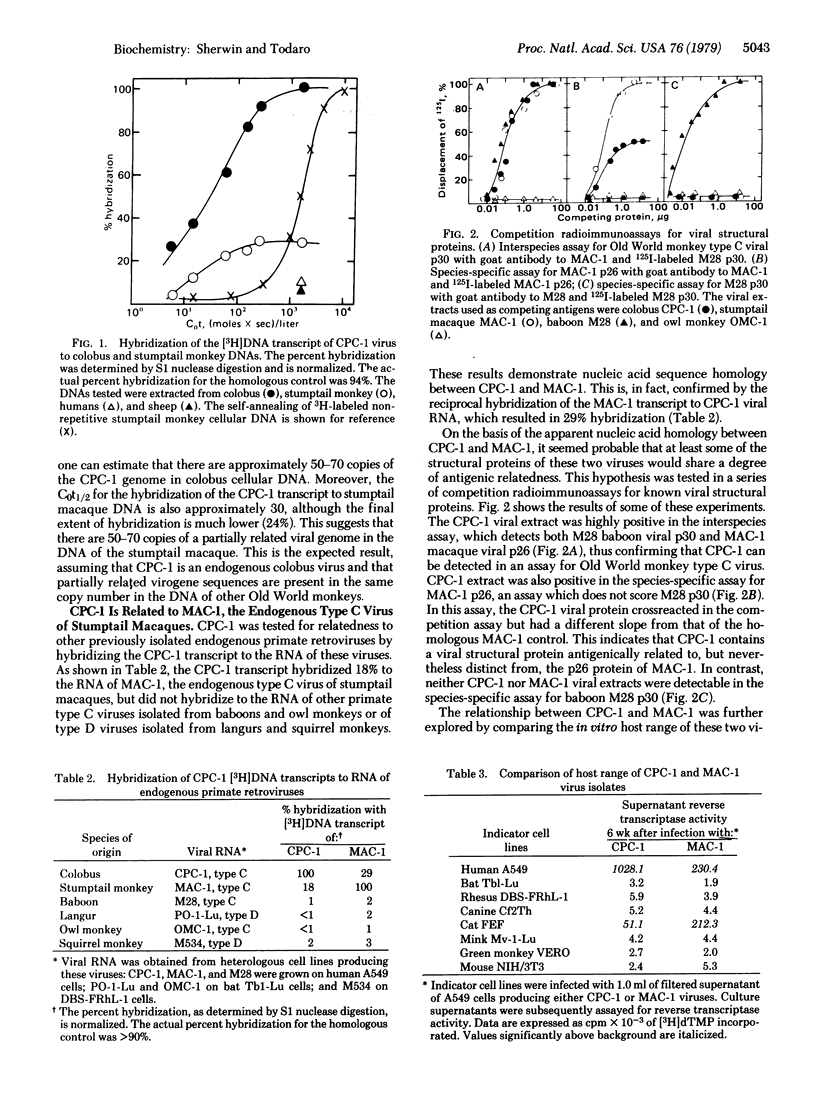
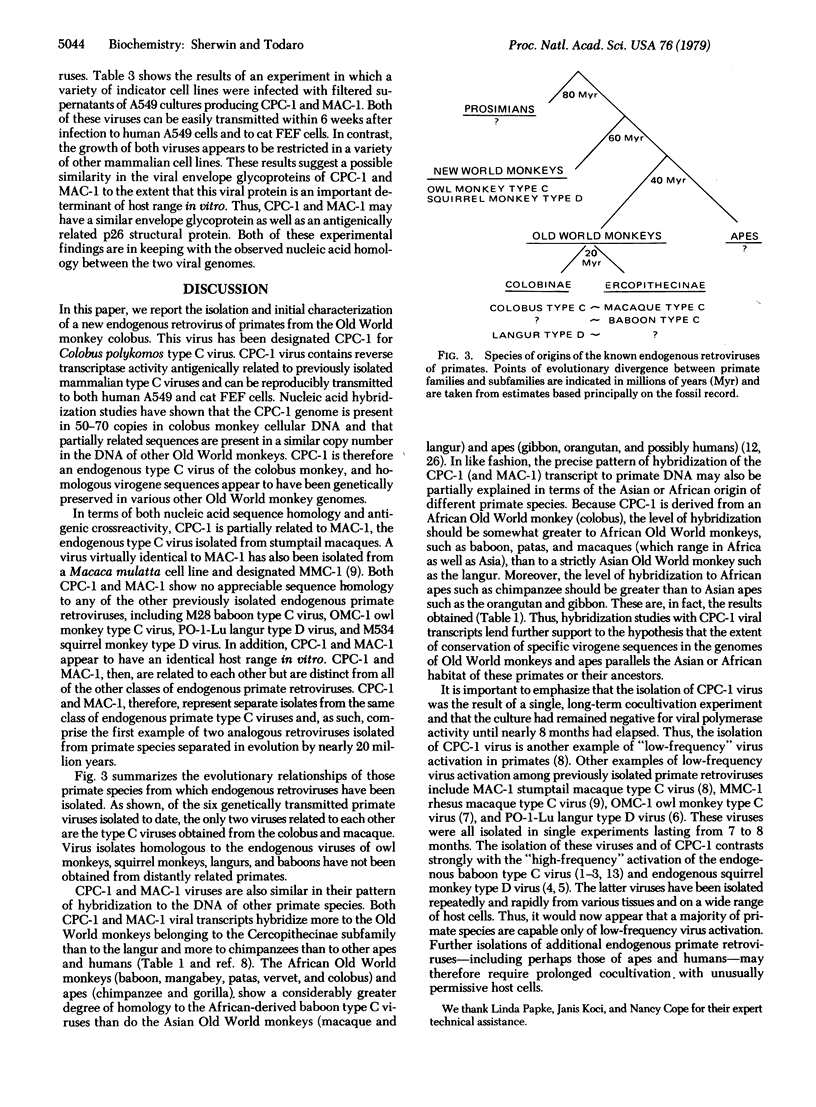
A new, genetically transmitted retrovirus has been isolated from the Old World monkey Colobus polykomos. This virus, designated CPC-1, is readily transmitted to both feline and human cells in culture. Nucleic acid hybridization studies reveal that there are 50-70 copies of the CPC-1 genome in colobus cellular DNA. Related virogene sequences can be detected in the DNA of all other Old World monkeys, as well as in the DNA of at least one ape species, the chimpanzee, indicating that this virus has been genetically transmitted in primates for 30-40 million years. CPC-1 is partially related to the type C virus previously isolated from stumptail monkeys (MAC-1). These two viruses have nucleic acid sequence homology, antigenic crossreactivity in their major viral structural protein, and a very similar host range in vitro. CPC-1 and MAC-1 therefore belong to the same class of genetically transmitted primate type C viruses and, as such, represent the first example in primates of analogous endogenous retroviruses isolated from two distantly related species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R. E., Heinemann R., Wilson G. L., Callahan R., Todaro G. J. Detection of baboon type C viral sequences in various primate tissues by molecular hybridization. J Virol. 1974 Jul;14(1):56–67. doi: 10.1128/jvi.14.1.56-67.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Lieber M. M., Livingston D. M., Sherr C. J., Todaro G. J., Kalter S. S. Infectious C-type virus isolated from a baboon placenta. Nature. 1974 Mar 1;248(5443):17–20. doi: 10.1038/248017a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of primate oncornaviruses: An endogenous virus from langurs (Presbytis spp.) with related virogene sequences in other Old World monkeys. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4557–4561. doi: 10.1073/pnas.74.10.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Evolution of type C viral genes: evidence for an Asian origin of man. Nature. 1976 May 13;261(5556):101–108. doi: 10.1038/261101a0. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Todaro G. J. Multiple divergent copies of endogenous C-type virogenes in mammalian cells. Nature. 1974 Nov 8;252(5479):170–173. doi: 10.1038/252170a0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Bryant M. L., Sherr C. J., Sen A., Todaro G. J. Molecular diversity among five different endogenous primate retroviruses. J Virol. 1978 Oct;28(1):300–313. doi: 10.1128/jvi.28.1.300-313.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcher D., Heberling R. L., Kalter S. S., Schlom J. Squirrel monkey retrovirus: an endogenous virus of a new world primate. J Virol. 1977 Aug;23(2):294–301. doi: 10.1128/jvi.23.2.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R. J., Scolnick E. M., Parks W. P., Yakovleva L. A., Lapin B. A. Isolation of a primate type-C virus from a lymphomatous baboon. Int J Cancer. 1974 Dec 15;14(6):722–730. doi: 10.1002/ijc.2910140605. [DOI] [PubMed] [Google Scholar]
- Heberling R. L., Barker S. T., Kalter S. S., Smith G. C., Helmke R. J. Oncornavirus: isolation from a squirrel monkey (Saimiri sciureus) lung culture. Science. 1977 Jan 21;195(4275):289–292. doi: 10.1126/science.63993. [DOI] [PubMed] [Google Scholar]
- Jainchill J. L., Aaronson S. A., Todaro G. J. Murine sarcoma and leukemia viruses: assay using clonal lines of contact-inhibited mouse cells. J Virol. 1969 Nov;4(5):549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohne D. E. Evolution of higher-organism DNA. Q Rev Biophys. 1970 Aug;3(3):327–375. doi: 10.1017/s0033583500004765. [DOI] [PubMed] [Google Scholar]
- Lieber M. M., Sherr C. J., Todaro G. J. S-tropic murine type-C viruses: frequency of isolation from continuous cell lines, leukemia virus preparations and normal spleens. Int J Cancer. 1974 May 15;13(5):587–598. doi: 10.1002/ijc.2910130503. [DOI] [PubMed] [Google Scholar]
- Lieber M., Smith B., Szakal A., Nelson-Rees W., Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976 Jan 15;17(1):62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Rabin H., Benton C. V., Tainsky M. A., Rice N. R., Gilden R. V. Isolation and characterization of an endogenous type C virus of rhesus monkeys. Science. 1979 May 25;204(4395):841–842. doi: 10.1126/science.87013. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Baltimore D. Increased length of DNA made by virions of murine leukemia virus at limiting magnesium ion concentration. J Virol. 1977 Jan;21(1):168–178. doi: 10.1128/jvi.21.1.168-178.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Benveniste R. E., Todaro G. J. Interspecies antigenic determinants of the reverse transcriptases and p30 proteins of mammalian type C viruses. J Virol. 1975 Jun;15(6):1440–1448. doi: 10.1128/jvi.15.6.1440-1448.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherr C. J., Schlom J., Schidlovsky G., Stephenson J. R. Isolation and characterization of a new type D retrovirus from the asian primate, Presbytis obscurus (spectacled langur). Virology. 1978 Jan;84(1):189–194. doi: 10.1016/0042-6822(78)90231-3. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Benveniste R. E., Sherwin S. A., Sherr C. J. MAC-1, a new genetically transmitted type C virus of primates: "low frequency" activation from stumptail monkey cell cultures. Cell. 1978 Apr;13(4):775–782. doi: 10.1016/0092-8674(78)90227-1. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Benveniste R. E. Baboons and their close relatives are unusual among primates in their ability to release nondefective endogenous type C viruses. Virology. 1976 Jul 1;72(1):278–282. doi: 10.1016/0042-6822(76)90331-7. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Sherr C. J., Sen A., King N., Daniel M. D., Fleckenstein B. Endogenous New World primate type C viruses isolated from owl monkey (Aotus trivirgatus) kidney cell line. Proc Natl Acad Sci U S A. 1978 Feb;75(2):1004–1008. doi: 10.1073/pnas.75.2.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Tevethia S. S., Melnick J. L. Isolation of an RD-114 related type-C virus from feline sarcoma virus-transformed baboon cells. Intervirology. 1973;1(5):399–404. doi: 10.1159/000148868. [DOI] [PubMed] [Google Scholar]
- Wallace R. E., Vasington P. J., Petricciani J. C., Hopps H. E., Lorenz D. E., Kadanka Z. Development and characterization of cell lines from subhuman primates. In Vitro. 1973 Mar-Apr;8(5):333–341. doi: 10.1007/BF02619057. [DOI] [PubMed] [Google Scholar]



