Abstract
The possibility that menthol cigarettes add to the deleterious cardiovascular effects of smoking has been barely discussed. Although cardiovascular diseases (CVD) are at the forefront of medical concerns of people living with HIV (PLWH), an important, yet unknown, issue for clinicians and public health authorities is whether use of menthol-flavored cigarettes heightens CVD risk factors. Our study aims to assess traditional (10-year risk using the Framingham Risk Model) and nontraditional CVD risk factors and to contrast the effects of menthol-flavored versus non-menthol flavored cigarettes on these risk factors. Compared to controls, menthol smokers were twice as likely to have hypertension. Users of menthol-flavored cigarettes had higher body mass index values, and increased risk of abdominal obesity. Multivariate analyses indicated that menthol smokers doubled the odds of having moderate to high CVD risk. This finding is highly significant given the widespread use of menthol-flavored cigarettes, particularly among women, minorities, and PLWH.
Keywords: blood pressure, cardiovascular risks, HIV, mentholated cigarettes, obesity
Despite notable improvements in prevention, cardiovascular diseases (CVD) continue to cause, on average, one death every 39 seconds in the United States (Roger et al., 2012). Each year, an estimated 1,000,000 Americans will experience new coronary attacks or silent myocardial infarctions (Roger et al., 2012). For people living with HIV (PLWH), the situation is even worse, as rates of CVD are approximately two-fold higher than age-matched people without HIV infection (Das, 2010; Kearney, Moore, Donegan, & Lambert, 2010). Increased rates have been attributed, at least in part, to metabolic problems associated with antiretrovirals, including hyperlipidemia and glucose-related abnormalities (Das, 2010; Kearney et al., 2010).
Obesity is increasing rapidly as a health complication for PLWH. Along with aging, obesity has contributed to major increases in the rates of hypertension, hypercholesterolemia, and metabolic disorders in PLWH. Of concern, the increased incidence of obesity will overlap with the smoking epidemic (Miguez, 2012). Recent studies have estimated that, in the era of antiretroviral therapy (ART), PLWH lose more life-years to smoking than to HIV (Petrosillo & Cicalini, 2013). The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study confirmed a two-fold increased odds of myocardial infarctions in current and former smokers compared to those without these risk factors (Friis-Møller et al., 2010). While these studies corroborated a link between smoking and CVD in PLWH, none assessed whether the type of cigarette smoked had any differential effects.
Data emerging from animal studies and a few human studies have provided evidence that menthol-flavored cigarettes may directly affect cardiovascular parameters. Ciftçi et al. (2009) assessed the plausible effects of menthol and non-menthol cigarettes on cardiovascular function and found that menthol-flavored cigarettes exerted detrimental effects on systolic velocity, isovolumic contraction time, and cardiac performance index values. These findings are of great concern given that the market share of menthol-flavored cigarettes has significantly increased in recent years (Giovino et al., 2004). Despite this evidence, no study has attempted to validate this information for PLWH (Das, 2010; Kearney et al., 2010).
Our study aims to address this important issue by examining the relationships between use of menthol-flavored cigarettes and well-defined measures of cardiovascular risk, using baseline measures obtained in our ongoing Florida International Liaison for Transdisciplinary and Educational Research on Smoking (FILTERS) cohort. This information is vitally needed to identify high-risk groups and correlates of risk, and to enable planning of effective prevention programs.
Methods
Study Design and Sample
FILTERS is a 3-year longitudinal study based in South Florida. Subjects completed the consent process and were assigned into one of four socio-demographically-matched groups based on HIV (positive/uninfected) and smoking (smoker/non-smoker) status. The main goal of FILTERS is to evaluate biological (cytokines) and behavioral (i.e., use of menthol cigarettes) mechanisms whereby tobacco use may influence the development of tobacco-related diseases (TRD, CVD), particularly in certain racial ethnic groups. The recruitment process started in June 2011 and ended in June 2013 because the Department of Health closed all studies funded in 2011 for political reasons (Lamendola, 2013). At that time, a total of 393 subjects had enrolled in the study. Individuals were recruited primarily by posting study pamphlets with contact information in local clinics. If phone contact suggested that the candidates knew their HIV status and identified themselves as not using injection drugs and as not dependent on alcohol or any drugs other than tobacco, they were eligible for personal interviews. Individuals with baseline histories of liver cirrhosis, myopathies, malignancies, and immunosuppressive conditions other than HIV were excluded, as were pregnant women. Once enrolled, computerized assessments were conducted by skilled interviewers, brief physical examinations were completed, and blood samples were collected.
Cardiovascular Outcomes
Each FILTERS participant received a brief medical examination that included vital signs and anthropometric measurements. After the participant had rested at least 5 minutes, systolic and diastolic measurements were registered as the first and fifth Korotkoff sounds. Subjects were considered to be hypertensive if they were taking antihypertensive medications, self-reported to being diagnosed with hypertension (yes/no), and/or registered systolic pressures 140 mmHg or higher or diastolic pressures equal to 90 mmHg or higher. They were then stratified in the Framingham Risk Model and had a Framingham Risk Score (FRS) assessed. The Framingham Heart risk assessment tool predicts a person’s chance of having a heart attack in the next 10 years and higher scores imply greater risk (main study outcome) (National Heart, Lung, and Blood Institute, 2004). The risk was calculated based on smoking status (yes/no), current drug prescription to treat high blood pressure (yes/no), blood pressure, and lipids obtained at baseline.
Since the FRS does not consider obesity, which has been associated with an enhanced risk for CVD, we decided to collect anthropometric measures along with elements of the metabolic profile. Basic body composition indices using standardized instruments were obtained, including weight and height to calculate body mass index (BMI; weight [lbs]/height [inches]2 × 703). Participants were classified as thin if BMI was less than18.5 kg/m2, eutrophic if BMI was 18.5 to 24.9 kg/m2, overweight if BMI was 25 to 29.9 kg/m2, and obese if BMI was 30 kg/m2 or higher. In accordance with national guidelines, abdominal obesity was defined as a waist circumference greater than 102 centimeters (40 inches) in males and 88 centimeters (35 inches) in females.
The lipid profile encompassed direct measurements of triglycerides and total, high density (HDL), and low density (LDL) cholesterol levels. Subjects were considered to have dyslipidemia if they reported current use of lipid-lowering medications or if they had hypercholesterolemia (> 200 mg/dL), and/or HDL-cholesterol less than 40 mg/dL in men and less than 50 mg/dL in women, and/or triglycerides 150 mg/dL or higher, and/or LDL-cholesterol 160 mg/dL or higher (Roger et al., 2012).
The FRS was calculated based on vital signs, lipid profiles, and medical history. The FRS was selected for use in our study because it is accepted as a reference method and has been validated in the U.S. population (Grundy et al., 2004). While more precise, HIV-specific risk algorithms were based on European cohorts and, therefore, not reflective of our study sample.
Smoking Behaviors
The Fagerström Test for Nicotine Dependence (FTND) was used to obtain operational measures of smoking (Heatherton, Kozlowski, Frecker, & Fagerström, 1991). In addition to the FTND, we used a smoking history that included core questions related to tobacco prevalence and consumption, cessation, second-hand smoke, media and advertising exposure, and economics. In the first section, participants were asked questions regarding age of initiation, number of years smoking, type of tobacco used, and total number of cigarettes smoked every day. Following national guidelines, a participant was classified as a current smoker if s/he reported smoking at a minimum of 100 cigarettes in his or her lifetime and currently smoked every day or some days (Centers for Disease Control and Prevention, 1994).
Use of Menthol-Flavored Cigarettes
This variable was based on self-reports (yes/no) and the participant’s preferred brands. Smokers were dichotomized as menthol users or not menthol users.
Covariates
Computerized questionnaires were used to obtain socio-demographic information and drug use and medical histories, including history of ART. Based on self-reports, subjects were categorized as African American, White non-Hispanic (Caucasian), or Hispanic. Age was stratified as 18 to 29, 30 to 40, or 41 or more years. Annual income was categorized as $0 to $11,000, $11,001 to $20,000, $20,001 to $49,999, or more than $50,000. Education level was assigned a code between 1 and 16, representing each year of schooling through college or vocational training.
Statistical Analyses
During the study design, a Monte Carlo simulation was used to determine the power to detect a difference between study groups. We had 90% power to detect differences between menthol flavored and non-flavored groups and 80% power to detect differences between people living with and without HIV. Analyses were performed using SPSS 19 (IBM Inc., Chicago, IL) for Windows. Correlations between the main variables of interest (i.e., FRS, CD4+ T cell counts, lipid levels, ART, smoking, and menthol use) were examined with Pearson correlation coefficients. Chi-square, student's t-test, and ANOVA were used to evaluate differences in risk factors between groups (smokers of menthols = 1, non-menthol smokers = 2 and nonsmokers = 3 or 0 in the quantitative variables). Regression models were employed to examine potential predictors of Framingham risks (nominal below or above 10 years). Covariates also included cigarettes per day (continuous), a squared term for cigarettes per day, menthol, age, sex, race, anthropometrics, and HIV status. The criterion for statistical significance was α = .05.
Results
Sociodemographics
Table 1 shows the descriptive characteristics of the total sample by smoking status. No significant differences were found between the groups for income or general health (albumin, liver enzymes, CD4+ T cell counts). Groups differed only in race and gender, as menthol users were more likely to be African American. Although no significant gender differences in smoking rates were observed (61% of males and 57% of females smoked), of the 91 female smokers, 22% used non-mentholated cigarettes while the remaining 78% were menthol users. On the other hand, of the 149 males smokers, 34% smoked non-mentholated cigarettes versus 66% menthol users (Odds Ratio [OR] = 1.8, 95%; Confidence Interval [CI]: 1–2.9, p = .03).
Table 1.
Sociodemographic Information by Smoking Group (n = 393)
| Variables | Non- Smokers (n = 153) |
Smokers of Non-mentholated Cigarettes (n = 70) |
Smokers of Mentholated Cigarettes (n = 170) |
p value |
|---|---|---|---|---|
| Age | 39.3 ± 9 | 40.4 ± 8.5 | 40.8 ± 8.5 | 0.7 |
| Gender | ||||
| • Male | 49% (75) | 72% (50) | 58% (99) | .03 |
| • Female | 51% (78) | 28% (20) | 42% (71) | |
| Race/Ethnicity | ||||
| • Black | 57% (87) | 17% (12) | 68% (116) | .006 |
| • Hispanic | 40% (61) | 71% (50) | 25% (42) | |
| • White | 3% (5) | 12% (8) | 7% (12) | |
| Income | ||||
| • Less than $11,000 | 15% (23) | 6% (4) | 6% (10) | .2 |
| • $11,001–$20,000 | 80% (122) | 91% (64) | 92% (157) | |
| • $20,001–$49,000 | 3% (5) | 0% (0) | 2% (3) | |
| • > $50,000 | 2% (3) | 3% (2) | 0% | |
| HIV | ||||
| • Yes | 65% (99) | 52% (36) | 57% (97) | .1 |
| • No | 35% (54) | 48% (34) | 43% (73) | |
| Albumin | 4.2 ± 0.5 | 4.2 ± 0.4 | 4.1 ± 0.6 | .9 |
| Liver Enzymes | ||||
| • AST | 38 ± 22 | 45 ± 22 | 36 ± 18 | 0.6 |
| • ALT | 35 ± 25 | 41 ± 39 | 35 ± 19 | .8 |
| CD4+ T cell counts | 508.9 ± 311 | 462.6 ± 299 | 470 ± 322 | .4 |
| Viral Load Log | 2.4 ± 1.4 | 2.6 ± 1.3 | 2.5 ± 1.2 | .1 |
Note. Values are means ± SD or percentages with exact n in the parenthesis.
Note. Significant racial and gender differences were found between groups.
Note: AST = Aspartate transaminase also called glutamic oxaloacetic transaminase (SGOT); ALT= alanine aminotransferase also called glutamic pyruvic transaminase (SGPT).
Smoking by HIV Status
Most participants had been diagnosed with HIV for more than a decade (13.9 ± 8 years). Although all enrolled participants were prescribed ART (mostly Truvada® [44%] or Atripla® [22%], alone or in combination with ritonavir [32%] or lopinavir/ritonavir [13%]), 5% of the subjects decided not to start a prescribed regimen or had discontinued ART because of side effects. CD4+ T cell counts were indicative of reasonably good immunologic status as a result of ART. Overall, the mean CD4+ T cell count was higher, and the mean log viral load lower, in non-smokers.
In the sample, PLWH had smoked for more years than people living without HIV (PLWOH; 25 ± 9 versus 19.8 ± 10 years, p = .001). However, PLWH smoked, on average, a similar number of cigarettes per day (13 ± 9 vs. 12 ± 8) and had comparable FTND scores (4.4 ± 2.4 vs. 4 ± 2.6) to PLWOH. PLWH preferred menthol-flavored brands (70%) more than PLWOH did (60%).
Smoking and Lipid Profile
Across most domains, CVD risk profiles for PLWH (both smokers and nonsmokers) were significantly different than PLWOH, including both traditional and nontraditional CVD risk factors. PLWH exhibited higher rates of hypertriglyceridemia (33% vs. 1%), hypercholesterolemia (30% vs. 8%), low high-density lipoprotein (HDL) cholesterol (35 % vs. 9%), and high blood pressure (28% vs. 25%). Compared to controls (non-smoker, uninfected), PLWH who smoked menthol cigarettes had higher rates of hypertension (54% vs. 38%) and hypertriglyceridemia (33% vs. 1%). As depicted in Table 2, PLWH who were menthol cigarette smokers exhibited higher total cholesterol, low-density lipoproteins (LDL), and glucose levels, but decreased levels of HDL cholesterol. Overall, the menthol cigarette smokers had higher FRS values (5.2 ± 4.5 vs. 3.2 ± 4.3). While the differences in HDL, triglycerides, and glucose did not reach statistical significance, the consistency and direction of these CVD risk measures suggest that menthol has a global deleterious effect.
Table 2.
Cardiovascular Risk Factors by Type of Cigarettes (n = 240)
| Variables | Mean | Standard Deviation |
p value | |
|---|---|---|---|---|
| Framingham | ||||
| Menthol Flavored | 5.2 | 4.5 | ||
| Non-Menthol | 3.2 | 4.3 | .002 | |
| Total Cholesterol mg/dL | ||||
| Menthol Flavored | 195.69 | 46.43 | ||
| Non-Menthol | 177.81 | 38.92 | .02 | |
| High Density Cholesterol mg/dL | ||||
| Menthol Flavored | 48.92 | 46.43 | ||
| Non-Menthol | 52.70 | 38.92 | .2 | |
| Low Density Cholesterol mg/dL | ||||
| Menthol Flavored | 115.40 | 41.75 | ||
| Non-Menthol | 99.79 | 36.85 | .003 | |
| Triglycerides mg/dL | ||||
| Menthol Flavored | 162.70 | 108.43 | ||
| Non-Menthol | 191.8 | 177.14 | .4 | |
| Glucose | ||||
| Menthol Flavored | 99.80 | 61.37 | ||
| Non-Menthol | 90.67 | 24.68 | .16 | |
Note. Significant differences were found between menthol and non-menthol groups on mean Framingham risk scores, total cholesterol, and LDL.
Smoking and Obesity
Of concern, more than two thirds of the sample was overweight (35%) or obese (38%), and BMI scores were similar between PLWH and PLWOH (30 ± 7 vs. 29 ± 7). However, the waist/hip ratio was larger for PLWH (PLWH: 0.94 ± 0.07 vs. PLWOH: 0.92 ± 0.07, p = .014). Despite similar albumin levels, dietary intake, and exercise patterns, PLWH who smoked menthol-flavored cigarettes had significantly higher BMI scores than non-menthol smokers (31 ± 7.5 vs. 27.4 ± 5.3, p = .02). A similar trend was also observed in PLWOH (29 ± 7 vs. 27.3 ± 5, p = .05). Indeed, compared to smokers of non-menthol-flavored cigarettes, menthol cigarette smokers exhibited a 40% increased risk of abdominal obesity (OR = 1.4, 95%; CI: 1–1.6, p = .05), and a 20% increased risk when compared to the nonsmoking controls (OR = 1.2, 95%; CI: 1–1.5, p = .07).
Smoking and Hypertension
We also examined whether smoking, particularly of menthol cigarettes, had detrimental effects on systolic and diastolic pressure. Among PLWH, data demonstrated a progressive increase in systolic blood pressure from nonsmokers to smokers of regular cigarettes to smokers of menthol-flavored cigarettes (118 ± 7 vs. 120 ± 11 vs. 124 ± 13 mmHg, p = .05). Diastolic pressure measurements for PLWH were lower among nonsmokers (73 ± 8), with progressive increases observed in non-menthol smokers (76 ± 6) and menthol smokers (77 ± 9 mmHg; p = .04). A similar trend was observed for PLWOH, but differences did not reach statistical significance (mean non-menthol smokers: 119/74 vs. menthol smokers: 121/76 mmHg). Additional analyses for PLWH indicated that users of menthol-flavored cigarettes were twice as likely to have hypertension as non-menthol smokers (OR = 1.7, 95%; CI: 9-3.3, p = .05). Smokers of menthol-flavored cigarettes more commonly used antihypertensive medications (p = .05).
Cardiovascular Risk Factors
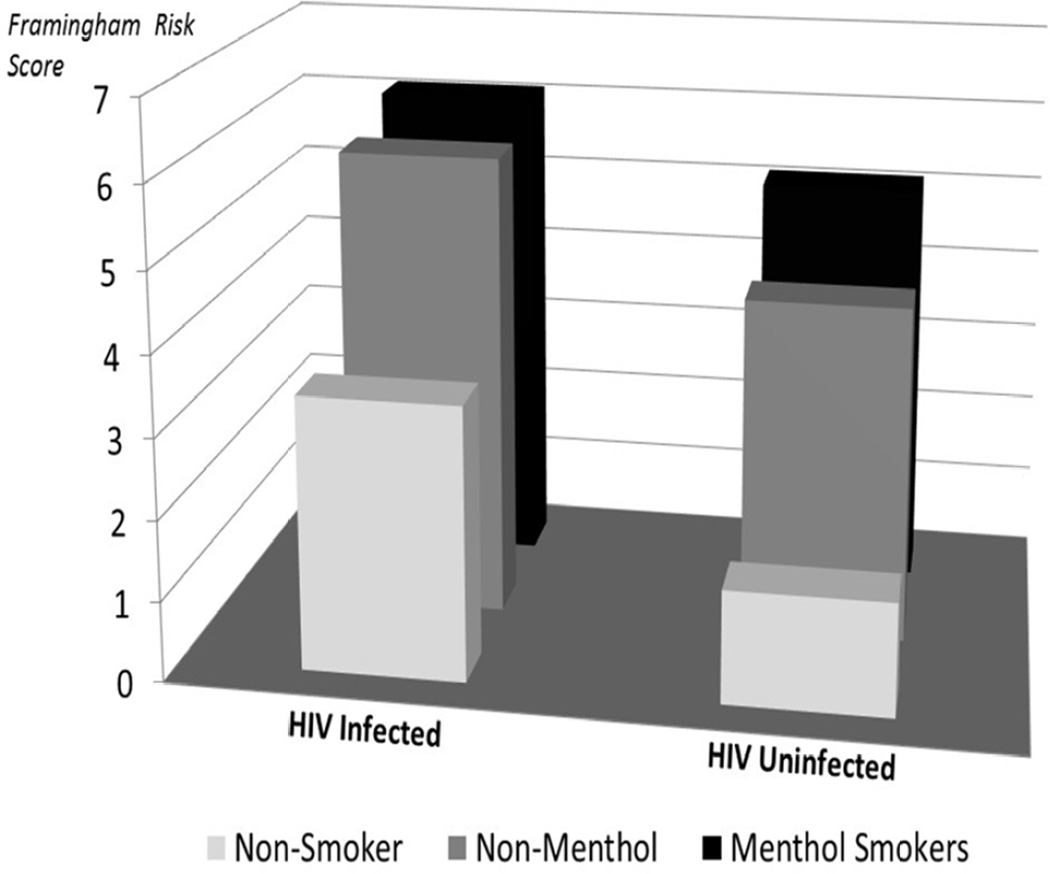
Overall, the mean 10-year risk for coronary events according to FRS was 6.4 ± 0.5. In total, 62% of PLWH and 77% of PLWOH were categorized as being at low risk for CVD (< 10% see Figure 1). The remaining 25% of PLWH and 19% of PLWOH had either moderate (10–20%) or high (> 20%) 10-year risk for CVD, respectively (13% of PLWH vs. 4% of PLWOH). We could not confirm prior observations of a direct relationship between higher CD4+ T cell counts with higher cardiovascular risk estimates. Additional analyses indicated that PLWH who smoked menthol-flavored cigarettes were twice as likely to have moderate to high CVD risk (> 10%) when compared to non-menthol users (OR = 2.5; 95% CI: 1–6, p = .02). Among uninfected people, only 1% had a high CVD risk, and all were menthol users.
Figure 1.
Cardiovascular risks by smoking status
Final Analyses
Multivariate analyses indicated that abdominal obesity predicted CVD risks (Framingham 10 year-risk below/above) beyond BMI. Of greatest importance, use of menthol-flavored cigarettes was strongly associated with FRS (see Table 3). Notably, when menthol was included in the model, HIV, race, and gender became insignificant predictors of FRS. Age was the only variable that remained significant among those used in the calculation of FRS.
Table 3.
Multivariate Analyses of Framingham 10-Year Risks
| Model | Unstandardized Coefficients |
||||
|---|---|---|---|---|---|
| B | Standard Error |
Wald | Exp (B) |
p value |
|
| Constant | 7.54 | 2.893 | 6.794 | 1881.4 | 0.009 |
| Abdominal Obesity | −1.001 | 0.476 | 4.422 | 0.368 | 0.035 |
| Menthol Groups | 1.435 | 0.495 | 8.419 | 4.199 | 0.004 |
| Age | 0.144 | 0.059 | 5.831 | 0.866 | 0.016 |
Discussion
Our large study of ambulatory PLWH receiving regular HIV care and PLWOH demonstrated a striking discordance in the prevalence of CVD risks by type of cigarettes. Data indicated that menthol added an incremental adverse effect beyond smoking to the CVD risk profile. Of concern, a sizable portion of our study population (70%) used menthol cigarettes. Particularly remarkable was the strong relationship with obesity, which has emerged as a critical risk factor for CVD in PLWH (Amorosa et al., 2005). The dangers of tobacco products and the excessive rates of menthol use are disquieting when considering that PLWH age at a rapid pace and experience diseases associated with aging 10 to 20 years earlier than in their non-infected peers (El-Sadr et al., 2006; Newman et al., 2009). We provide additional evidence regarding the risks of menthol cigarettes. Collectively, these findings further justify the need for more studies to closely monitor this public health threat.
While tobacco companies have portrayed menthol as causing innocuous cold sensations, our findings indicated that use of menthol-flavored cigarettes was associated with overall higher prevalence of hypertension. Increases in blood pressure readings were likely the result, at least in part, of excess nicotine levels found in menthol users. In addition to its direct effects on the sympathetic system, the identification of transient receptor potential cation channel subfamily M member 8 (TRPM8; also known as the cold and menthol receptor 1) as a regulator of Ca2+-permeable channel expressed in the vasculature has opened a new pathway to explain the vasopressor effects of menthols (Johnson et al., 2009). Neunteufl et al. (2002) further supported our findings that acute exposure to menthol cigarettes was associated with impaired endothelial function. This finding is highly relevant, considering that hypertension can affect a number of other co-morbid conditions, including metabolic syndrome, lipid abnormalities, CVD, and diabetes.
The most striking difference in CVD risk factors was the much higher frequency of obesity in menthol users compared to non-menthol users, both in HIV-infected and uninfected groups. Differences in weight were probably related to the appetite-enhancing properties of menthols. The rates of obesity are of concern, given that associated hormonal signals lead to endocrine alterations and enhanced production of pro-inflammatory cytokines (Amorosa et al., 2005). In addition, the high frequency of abdominal obesity among menthol users represents a major challenge because this fat distribution pattern may play a significant role in modulating CVD risks beyond obesity (Dervaux, Wubuli, Megnien, Chironi, & Simon, 2008). Although body composition assessments were limited to anthropometric measures, our findings indicate the need for future studies to determine the mechanism mediating these observations. Strategies should be developed to improve overall health of PLWH by reducing both smoking and obesity because these are interlinked epidemics.
The finding that menthol may be associated with alterations of the lipid profile is new to the smoking literature. However, in the pharmacotherapy field, researchers have been using the disruptive properties of menthol over lipids to accelerate the absorption of other medications (Watanabe, Obata, Ishida, & Takayama, 2009). The discovery that PLWH have excessive CVD risk confirms the findings of previous studies that showed that both HIV and antiretrovirals could exacerbate CVD risks (Das, 2010; Kearney et al., 2010). However, we could not confirm reports of a linear association between CD4+ T cell counts and CVD risks (Ho, et al., 2012).
Our findings need to be analyzed in the context of some limitations. Our study design did not allow inference of causation. It was conducted in South Florida and included a limited number of White participants; results may differ by geographic region or race/ethnicity. Despite these cautions, consistency across separate studies in the direction of association, and the existence of a plausible biological mechanism, strengthen the support of our observations.
We have added to the body of scientific evidence that describes the deleterious effects of menthol-flavored cigarettes above and beyond smoking. Public health professionals should develop campaigns aimed at reducing or eradicating the use of menthol-flavored cigarettes, particularly in PLWH. Strategies should be developed to improve overall health by reducing both smoking and obesity because these are interlinked epidemics for PLWH.
Key Considerations.
Smoking is common in PLWH and is one of the biggest preventable risks for morbidity and mortality.
Although concerns have been raised regarding menthol-flavored cigarettes in the general population, no data are currently available for PLWH. Closer examination may be needed to better inform clinical guidelines for the care of HIV-infected individuals.
PLWH need CV screening and prevention information in light of studies indicating that they have enhanced risk. However, messages should include specific warnings about menthol-flavored cigarettes.
Strategies that identify individuals at increased risk, such as menthol users, are likely to facilitate tobacco and cardiovascular disease prevention.
Acknowledgements
The grant was funded by the James and Esther King Florida Health Department Tobacco Grant (KG 10 MJM). The study described used the CRC facilities supported by Grant Number 1UL1TR000460, Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors report no real or perceived vested interests that relate to this article that could be construed as a conflict of interest.
Contributor Information
María José Míguez-Burbano, School of Integrated Science and Humanity Department of Art and Science, Florida International University, Miami, Florida, USA.
Mayra Vargas, School of Integrated Health and Science, Department of Art and Science, Florida International University, Miami, Florida, USA.
Clery Quiros, School of Integrated Health and Science, Department of Art and Science, Florida International University, Miami, Florida, USA.
John E. Lewis, Department of Psychiatry & Behavioral Sciences, University of Miami School of Medicine, Miami, Florida, USA.
Luis Espinoza, Department of Medicine, University of Miami School of Medicine, Miami, Florida, USA.
Deshratan Asthana, Department of Psychiatry & Behavioral Sciences, University of Miami School of Medicine, Miami, Florida, USA.
References
- Amorosa V, Synnestvedt M, Gross R, Friedman H, MacGregor RR, Gudonis D, Tebas P. A tale of 2 epidemics: The intersection between obesity and HIV infection in Philadelphia. Journal of Acquired Immune Deficiency Syndromes. 2005;39:557–561. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults -- United States, 1992, and changes in the definition of current cigarette smoking. Morbidity and Mortality Weekly Report. 1994;43:342–346. [PubMed] [Google Scholar]
- Ciftçi O, Güllü H, Calişkan M, Topçu S, Erdoğan D, Yildirir A, Muderrisoğlu H. Mentholated cigarette smoking and brachial artery, carotid artery, and aortic vascular function. Turk Kardiyoloji Dernegi Arsivi. 2009;37:234–240. [PubMed] [Google Scholar]
- Das S. Risk of cardiovascular disease in HIV-infected patients. Journal of Antimicrobial Chemotherapy. 2010;65:386–389. doi: 10.1093/jac/dkp460. [DOI] [PubMed] [Google Scholar]
- Dervaux N, Wubuli M, Megnien J-L, Chironi G, Simon A. Comparative associations of adiposity measures with cardiometabolic risk burden in asymptomatic subjects. Atherosclerosis. 2008;201(2):413–417. doi: 10.1016/j.atherosclerosis.2007.11.032. [DOI] [PubMed] [Google Scholar]
- El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, Rappaport C. CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- Friis-Møller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, Law MG. Predicting the risk of cardiovascular disease in HIV-infected patients: The Data Collection on Adverse Effects of Anti-HIV Drugs Study. European Journal of Cardiovascular Prevention & Rehabilitation. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- Giovino G, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cumming M. Epidemiology of menthol cigarette use. Nicotine & Tobacco Research. 2004;6(Suppl 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Bairey Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult treatment Panel III guidelines. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(8):149–161. doi: 10.1161/01.ATV.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Ho JE, Scherzer R, Hecht FM, Maka K, Selby V, Martin JN, Hsue PY. The association of CD4+ T-cell counts and cardiovascular risk in treated HIV disease. AIDS. 2012;26(9):1115–1120. doi: 10.1097/QAD.0b013e328352ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. American Journal of Physiology-Heart and Circulatory Physiology. 2009;296(6):H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney F, Moore AR, Donegan CF, Lambert J. The ageing of HIV: Implications for geriatric medicine. Age and Ageing. 2010;39(5):536–541. doi: 10.1093/ageing/afq083. [DOI] [PubMed] [Google Scholar]
- Lamendola B. DOH cancels studies in mid-stream. 2013 Retrieved from http://health.wusf.usf.edu/post/doh-cancels-studies-mid-stream. [Google Scholar]
- Miguez MJ. Current issues in cigarette smoking among persons living with HIV/AIDS: A growing public health problem surrounded by missing information and misconceptions. Journal of AIDS and Clinical Research. 2012;3:e109. [Google Scholar]
- National Heart, Lung, and Blood Institute. National Cholesterol Education Program, third report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) 2004 Retrieved from www.nhlbi.nih.gov/guidelines/cholesterol/index.htm.
- Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, Stefenelli T. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. Journal of the American College of Cardiology. 2002;39:251–256. doi: 10.1016/s0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- Newman AB, Sachs MC, Arnold AM, Fried LP, Kronmal R, Cushman M, Lumley T. Total and cause-specific mortality in the cardiovascular health study. Journals of Gerontology - Series A. 2009;64(12):1251–1261. doi: 10.1093/gerona/glp127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo N, Cicalini S. Smoking and HIV: Time for a change? BMC Medicine. 2013;11:16. doi: 10.1186/1741-7015-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Turner MB. Heart disease and stroke statistics—2012 update: A report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Obata Y, Ishida K, Takayama K. Effect of l-menthol on the thermotropic behavior of ceramide 2/cholesterol mixtures as a model for the intercellular lipids in stratum corneum. Colloids and Surfaces B: Biointerfaces. 2009;73:116–121. doi: 10.1016/j.colsurfb.2009.05.007. [DOI] [PubMed] [Google Scholar]