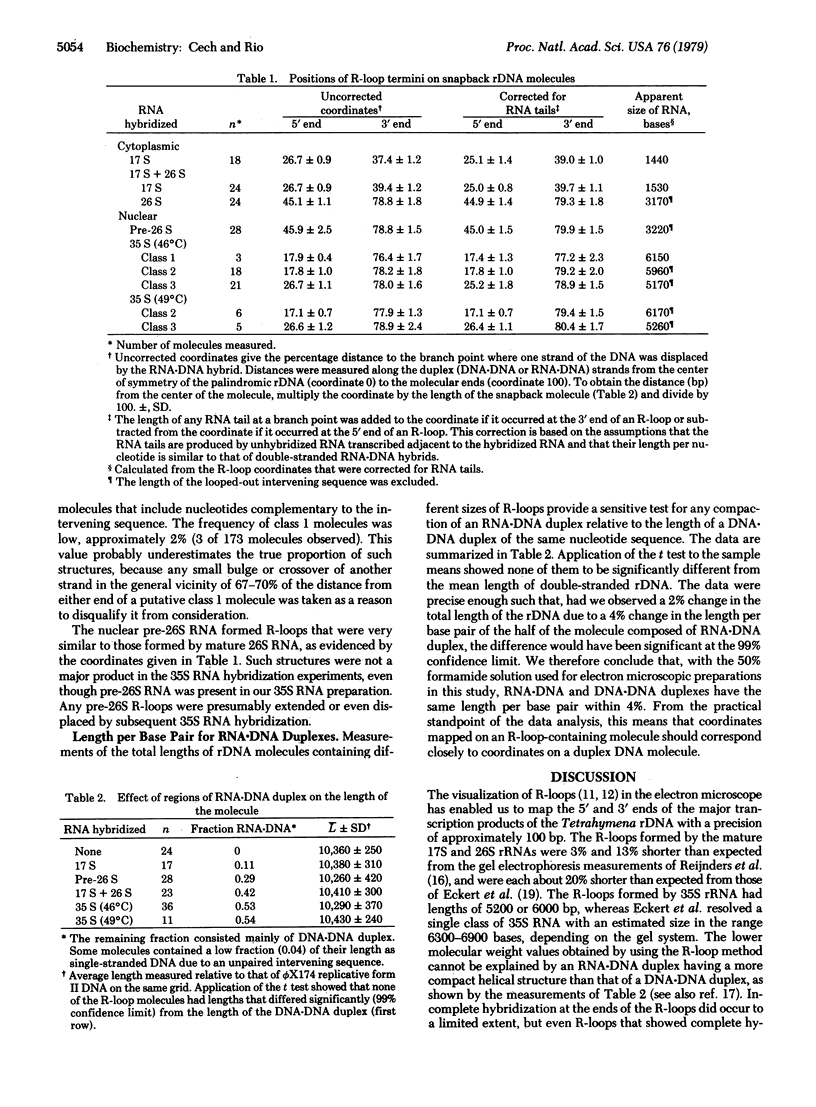
Abstract
R-loop hybridization and electron microscopy were used to map the RNA transcription products of the extrachromosomal rRNA genes of Tetrahymena thermophila. The mature 17S and 26S rRNAs and the nuclear 35S pre-rRNA and pre-26S rRNA were located with a precision of approximately 100 base pairs. A 370-base pair intervening sequence was found in the 26S coding region. It has the same size and relative location as that found in Tetrahymena pigmentosa [Wild, M.A. & Gall, J.G. (1979) Cell 16, 565-573]. One class of R-loop structures formed by nuclear pre-rRNA provided preliminary evidence for a primary transcript that contains the intervening sequence. The results suggested a processing scheme in which splicing of the intervening sequences is followed by a series of strand cleavages to give the mature 17S and 26S rRNAs. Analysis of the data also showed that RNA . DNA and DNA . DNA duplexes, when mounted for electron microscopy by the R-loop procedure, have the same length per base pair within 4%.
Full text
PDF
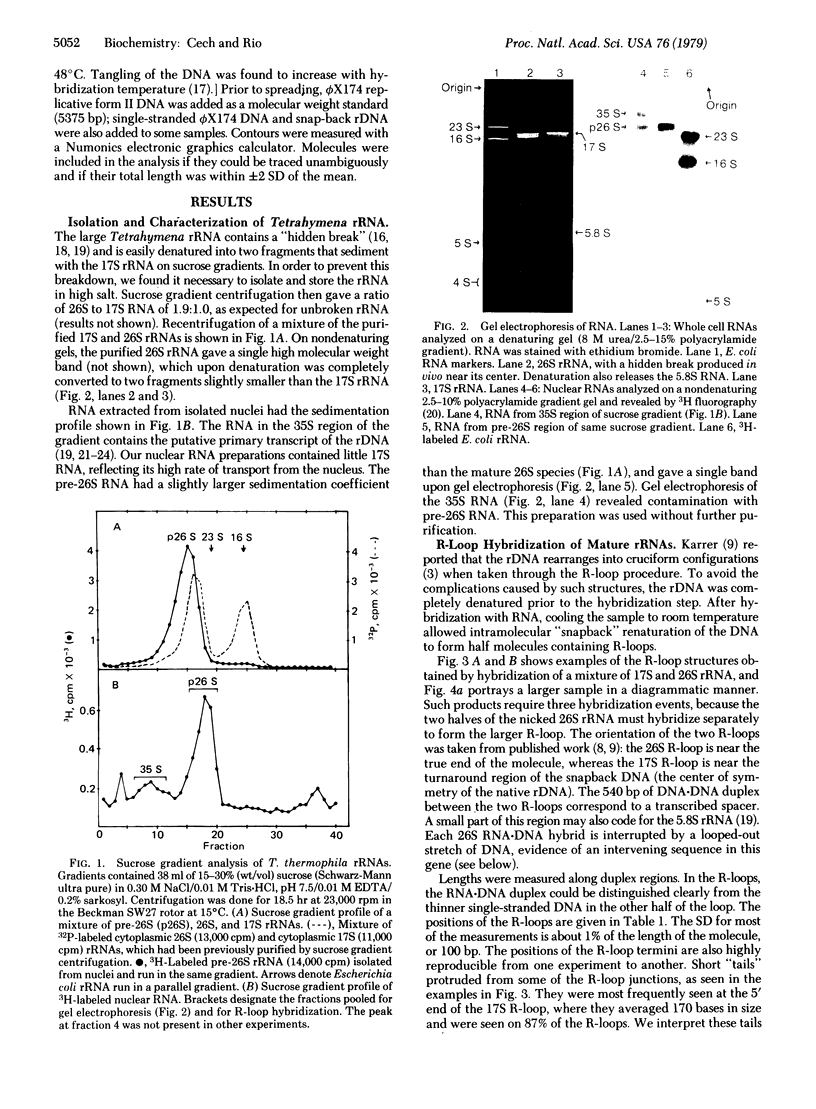
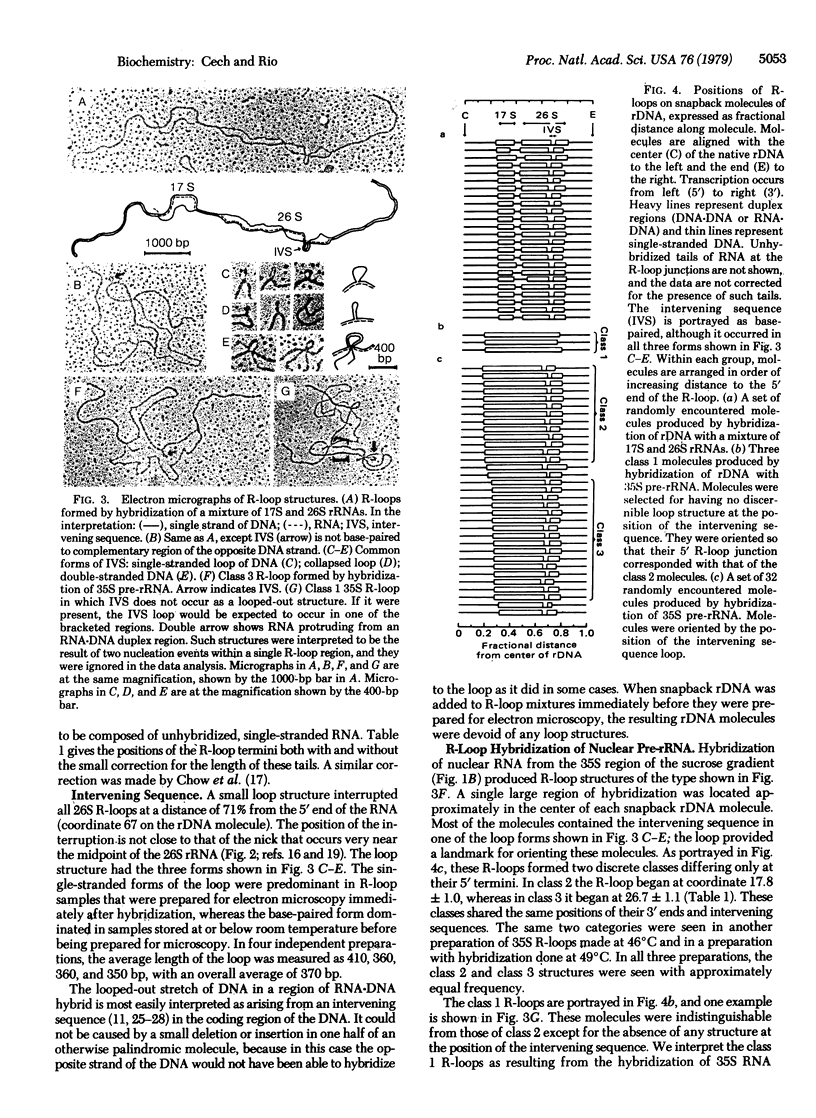

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett T., Rae P. M. A 9.6 kb intervening sequence in D. virilis rDNA, and sequence homology in rDNA interruptions of diverse species of Drosophila and other diptera. Cell. 1979 Apr;16(4):763–775. doi: 10.1016/0092-8674(79)90092-8. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Heyting C., Borst P., Arnberg A. C., Van Bruggen E. F. An insert in the single gene for the large ribosomal RNA in yeast mitochondrial DNA. Nature. 1978 Sep 28;275(5678):336–338. doi: 10.1038/275336a0. [DOI] [PubMed] [Google Scholar]
- Bostock C. J., Prescott D. M., Lauth M. Lability of 26 S ribosomal RNA in Tetrahymena pyriformis. Exp Cell Res. 1971 May;66(1):260–262. doi: 10.1016/s0014-4827(71)80038-1. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Eckert W. A., Kaffenberger W., Krohne G., Franke W. W. Introduction of hidden breaks during rRNA maturation and ageing in Tetrahymena pyriformis. Eur J Biochem. 1978 Jul 3;87(3):607–616. doi: 10.1111/j.1432-1033.1978.tb12413.x. [DOI] [PubMed] [Google Scholar]
- Engberg J., Andersson P., Leick V., Collins J. Free ribosomal DNA molecules from Tetrahymena pyriformis GL are giant palindromes. J Mol Biol. 1976 Jun 25;104(2):455–470. doi: 10.1016/0022-2836(76)90281-3. [DOI] [PubMed] [Google Scholar]
- Engberg J., Christiansen G., Leick V. Autonomous rDNA molecules containing single copies of the ribosomal RNA genes in the macronucleus of Tetrahymena pyriformis. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1356–1365. doi: 10.1016/0006-291x(74)90463-x. [DOI] [PubMed] [Google Scholar]
- Gall J. G. Free ribosomal RNA genes in the macronucleus of Tetrahymena. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3078–3081. doi: 10.1073/pnas.71.8.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., Hogness D. S. A novel arrangement of the 18S and 28S sequences in a repeating unit of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):167–176. doi: 10.1016/0092-8674(77)90212-4. [DOI] [PubMed] [Google Scholar]
- Gocke E., Leer J. C., Nielsen O. F., Westergaard O. Transcriptional properties of nucleoli isolated from Tetrahymena. Nucleic Acids Res. 1978 Nov;5(11):3993–4006. doi: 10.1093/nar/5.11.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallick R. B., Chelm B. K., Gray P. W., Orozco E. M., Jr Use of aurintricarboxylic acid as an inhibitor of nucleases during nucleic acid isolation. Nucleic Acids Res. 1977 Sep;4(9):3055–3064. doi: 10.1093/nar/4.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karrer K. M., Gall J. G. The macronuclear ribosomal DNA of Tetrahymena pyriformis is a palindrome. J Mol Biol. 1976 Jun 25;104(2):421–453. doi: 10.1016/0022-2836(76)90280-1. [DOI] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- Kumar A. Ribosome synthesis in Tetrahymena pyriformis. J Cell Biol. 1970 Jun;45(3):623–634. doi: 10.1083/jcb.45.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Leick V. Formation of subribosomal particles in the macronuclei of Tetrahymena pyriformis. Eur J Biochem. 1969 Mar;8(2):221–228. doi: 10.1111/j.1432-1033.1969.tb00518.x. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Gorovsky M. A. Subunit structure of rDNA-containing chromatin. Biochemistry. 1976 Feb 24;15(4):750–755. doi: 10.1021/bi00649a005. [DOI] [PubMed] [Google Scholar]
- Niles E. G. Isolation of a high specific activity 35S ribosomal RNA precursor from Tetrahymena pyriformis and identification of its 5' terminus, pppAp. Biochemistry. 1978 Oct 31;17(22):4839–4844. doi: 10.1021/bi00615a035. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Cordell B., Valenzuela P., Rutter W. J., Goodman H. M. Structure and processing of yeast precursor tRNAs containing intervening sequences. Nature. 1978 Aug 3;274(5670):438–445. doi: 10.1038/274438a0. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Manning J., Davidson N. Sequence arrangement of the rDNA of Drosophila melanogaster. Cell. 1977 Feb;10(2):213–214. doi: 10.1016/0092-8674(77)90215-x. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Bostock C., Gamow E., Lauth M. Characterization of rapidly labeled RNA in Tetrahymena pyriformis. Exp Cell Res. 1971 Jul;67(1):124–128. doi: 10.1016/0014-4827(71)90627-6. [DOI] [PubMed] [Google Scholar]
- Reeder R. H., Higashinakagawa T., Miller O., Jr The 5' leads to 3' polarity of the Xenopus Ribosomal RNA precursor molecule. Cell. 1976 Jul;8(3):449–454. doi: 10.1016/0092-8674(76)90158-6. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Sloof P., Sival J., Borst P. Gel electrophoresis of RNA under denaturing conditions. Biochim Biophys Acta. 1973 Oct 26;324(3):320–333. doi: 10.1016/0005-2787(73)90278-5. [DOI] [PubMed] [Google Scholar]
- Rochaix J. D., Malnoe P. Anatomy of the chloroplast ribosomal DNA of Chlamydomonas reinhardii. Cell. 1978 Oct;15(2):661–670. doi: 10.1016/0092-8674(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Spradling A., Pardue M. L., Penman S. Messenger RNA in heat-shocked Drosophila cells. J Mol Biol. 1977 Feb 5;109(4):559–587. doi: 10.1016/s0022-2836(77)80091-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., White R. L., Davis R. W. Hybridization of RNA to double-stranded DNA: formation of R-loops. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2294–2298. doi: 10.1073/pnas.73.7.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Curtis P. J., Tiemeier D. C., Leder P., Weissmann C. The intervening sequence of a mouse beta-globin gene is transcribed within the 15S beta-globin mRNA precursor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1309–1313. doi: 10.1073/pnas.75.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. The structural organization of ribosomal DNA in Drosophila melanogaster. Cell. 1977 Feb;10(2):193–212. doi: 10.1016/0092-8674(77)90214-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Hogness D. S. R loop mapping of the 18S and 28S sequences in the long and short repeating units of Drosophila melanogaster rDNA. Cell. 1977 Feb;10(2):177–192. doi: 10.1016/0092-8674(77)90213-6. [DOI] [PubMed] [Google Scholar]
- Wild M. A., Gall J. G. An intervening sequence in the gene coding for 25S ribosomal RNA of Tetrahymena pigmentosa. Cell. 1979 Mar;16(3):565–573. doi: 10.1016/0092-8674(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Gall J. G. A single integrated gene for ribosomal RNA in a eucaryote, Tetrahymena pyriformis. Cell. 1977 Sep;12(1):121–132. doi: 10.1016/0092-8674(77)90190-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Sandeen D. The ribonuclease activity of crystallized pancreatic deoxyribonuclease. Anal Biochem. 1966 Feb;14(2):269–277. doi: 10.1016/0003-2697(66)90137-0. [DOI] [PubMed] [Google Scholar]