Abstract
Transforming growth factor-β (TGF-β) signaling plays an important and complex role in renal fibrogenesis. The seemingly simple TGF-β/Smad cascade is intensively regulated at several levels, including crosstalk with other signaling pathways. Epidermal growth factor (EGF) is a potent mitogen for epithelial cells and is elevated in diseased kidneys. In this study, we examined its effect on TGF-β-induced fibrotic changes in human proximal tubular epithelial cells. Simultaneous treatment with EGF specifically inhibited basal and yTGF-β-induced type-I collagen and α-smooth muscle actin (αSMA) expression at both mRNA and protein levels. These effects were prevented by inhibition of either the EGF receptor kinase or its downstream MEK kinase but not by blockade of either the JNK or PI3K pathway. Overexpression of a constitutively active MEK1 construct mimicked the inhibitory effect of EGF. Further, EGF suppressed Smad transcriptional activities, as shown by reduced activation of ARE-luc and SBE-luc. Both reductions were prevented by MEK inhibition. However, EGF did not block Smad2 or Smad3 phosphorylation by TGF-β, or Smad2/3 nuclear import. Finally EGF induced the phosphorylation and expression of TGIF, a known TGF-β/Smad repressor. Both the phosphorylation and the induction were blocked by a MEK inhibitor. Overexpression of TGIF abolished TGF-β-induced αSMA promoter activity. Together these results suggest that EGF inhibits two TGF-β-stimulated markers of EMT through EGF receptor tyrosine kinase and downstream ERK activation, but not through PI3K or JNK. The inhibition results from effector mechanisms downstream of Smads, and most likely involves the transcriptional repressor, TGIF.
Keywords: TGF-β, EGF, EMT, fibrosis, ERK, Smad2/3
1. Introduction
Kidney fibrosis is a primary process by which chronic kidney damage progresses to end-stage renal disease, resulting in significant morbidity and mortality. It is characterized by the excessive deposition of extracellular matrix (ECM) proteins and the replacement of normal tissue with non-functional scar. Type II epithelial-mesenchymal transition (EMT) has been suggested to contribute to kidney tubulointerstitial fibrosis [1], wherein tubular epithelial cells differentiate into mesenchymal-like cells upon fibrotic stimuli, expressing myofibroblast markers such as type I collagen (COL1) and α-smooth muscle actin (αSMA).
Transforming growth factor-β (TGF-β is a well-studied fibrotic cytokine that plays a key role in renal fibrogenesis and it has been shown to stimulate the differentiation of epithelial cells to myofibroblasts by inducing the synthesis of collagen and αSMA. Intracellular signaling by TGF-β is initiated by TGF-β ligand-binding and activation of TGF-β receptors (TβR). Activated TβR kinase phosphorylates downstream regulatory Smad proteins (Smad2 and Smad3), which then oligomerize with the co-Smad (Smad4) and translocate into the nucleus. Once in the nucleus, Smad proteins regulate target gene transcription by binding to the promoter region and interacting with various co-activators or repressors. Both COL1 and αSMA have a Smad-binding sequence in their promoter regions and TGF-β has been shown to induce both in a Smad3-dependent manner [2, 3].
Epidermal growth factor (EGF) plays an important role in regulating cell growth, proliferation and differentiation. It has been implicated in multiple pathological conditions, such as wound healing [4] and cancer cell metastasis and invasion [5, 6]. EGF stimulates multiple biological responses through activation of EGF receptor (EGFR), which is expressed in a variety of mammalian tissues. Activated EGFR phosphorylates and triggers a number of important signaling pathways, including phospholipase C-γ (PLC-α), Ras-Raf-MAPK, phosphatidylinositol-3 kinase (PI3K)-AKT and STATs, which together are considered as the traditional downstream effectors of EGF/EGFR [7]. Crosstalk between EGF and TGF-β signaling has been discussed in multiple publications and the outcome varies depending on the cell type and experiment conditions. EGF synergizes with TGF-β to decrease cell-junction protein expression [8]. On the other hand, EGF has been shown to inhibit BMP/TGF-β signaling through blockade of the nuclear translocation of Smad1 [9] or Smad2/3 [10] via EGFR/Ras/MEK/ERK-mediated phosphorylation of the linker region of Smad2/3. EGF may also antagonize the TGF-β pathway by stabilizing the Smad co-repressor TGIF [11, 12].
In human kidney tissue, both EGF mRNA and protein have been detected in the collecting duct [13] and EGF has been shown to be a potent mitogen for renal tubular epithelial cells [14]. Previous reports have shown that EGF attenuates obstructive nephropathy in neonatal rats [15]. However, the mechanism of the effect of EGF on TGF-β-induced fibrotic changes in human kidney cells is still unclear. In this study, we investigated the effect of EGF on TGF-β-induced fibrogenic markers (COL1 and αSMA) in human tubular epithelial cells. We report that EGF abrogates the expression of these markers in an ERK-dependent manner.
2. Material and Methods
2.1 Cell Culture
A transformed human proximal tubular epithelial cell line (HKC) was kindly provided by Dr. L. Racusen [16] and cultured in Dulbecco's modified eagle medium/F12 supplemented with 10% heat-inactivated fetal bovine serum, L-glutamine, penicillin-streptomycin, amphotericin B and HEPES buffer.
2.2 Materials and Reagents
TGF-β (R&D systems, Minneapolis, MN) was reconstituted in 4mM HCl with 1 mg/ml bovine serum albumin to make a 4 μg/ml stock. Kinase inhibitors AG1478 and wortmannin were purchased from Sigma (St. Louis, MO). SP600125 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and PD0325901 from Selleck Chemicals (Houston, TX). The following antibodies were purchased from the indicated vendors: αSMA, β-actin from Sigma (St. Louis, MO), type I collagen from Southern Biotech (Birmingham, AL), E-Cadherin from BD Biosciences (San Jose, CA), phospho-EGFR (Y1172) and EGFR from Abgent (San Diego, CA), Smad3, phosphor-Smad3 (S423/425), phospho-Smad2 (S465/467) (Ser245/250/255), AKT, phospho-AKT(S473), phospho-p44/42 (T202/Y204), Histone H3 from Cell Signaling technology (Danvers, MA), ERK1, ERK2, phospho-c-Jun, c-Jun, Smad1/2/3, β-tubulin, TGIF, goat anti-mouse IgG-horseradish peroxidase (HRP), mouse anti-goat IgG-HRP antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) and goat anti-rabbit IgG-HRP from Promega (Madison, WI).
2.3 Quantitative PCR
Total RNA was extracted from HKCs using the PrepEase RNA spin kit (Affymetrix, Santa Clara, CA) according to the manufacturer's direction. RNA concentration was measured with Quant-it RiboGreen assay (Invitrogen). 1 μg of RNA was reverse-transcribed to cDNA using iScript cDNA synthesis kit (Bio-Rad). Real-time PCR was then performed using the iQ SYBR Green Supermix (Bio-Rad) with the iCycler iQ real-time PCR detection system (Bio-Rad). Real-time data were collected for 40 cycles of 95 °C, 10 s; 57 °C, 45 s; and 75 °C, 30s. Primers are designed with software provided by Invitrogen and synthesized by Integrated DNA Technology (Coralville, CA). Relative expression of the gene of interest was estimated by the ΔΔCt method using β2-microglobulin as a reference gene. Samples were analyzed in triplicate, and experiments were repeated at least three times. Primers used were as follows: αSMA, 5’-AGCAGGCCAAGGGGCTATATAA-3’ (forward) and 5′-CGTAGCTGTCTTTTTGTCCCATT-3’ (reverse); COL1A1, 5’-CAATGCTGCCCTTTCTGCTCCTTT-3’ (forward) and 5’-CACTTGGGTGTTTGAGCATTGCCT-3’ (reverse); TGIF, 5’-TAGAGGAGACCCCATTTCATTCC-3’ (forward) and 5’-GGGGATGACGGCTTAGGAGA-3’ (reverse); β2-microglobulin, 5′-TGTCTGGGTTTCATCCATCCGACA-3′ (forward) and 5′-TCACACGGCAGGCATACTCATCTT-3′ (reverse).
2.4 Whole Cell lysate preparation
Cell fractionation and Western blot Cells were washed twice with ice-cold PBS and whole cell lysate was made in RIPA buffer (50 mM Tris·HCL, pH 7.5; 150 mM NaCl; 1% Nonidet P-40; 0.5% deoxycholate; 0.1% SDS; 1 mM EDTA) containing protease and phosphatase inhibitors (Sigma). For the cellular fractionation studies, cells were first lysed in a hypotonic buffer (20 mM Tris·HCL, pH 7.5; 0.5 mM EDTA; 0.5 mM EGTA; 10 mM β-mercaptoethanol) and a dounce homogenizer was used to disrupt the cell membrane. After low-speed centrifugation, supernatant was saved as cytosolic lysate, and nuclei pellet was lysed in RIPA buffer for nuclear lysate. Protein samples were subjected to immunoblotting for target proteins, and immunoreactive bands were visualized using a chemiluminescence reagent according to the manufacturer's protocol (Santa Cruz Biotechnology, Santa Cruz, CA). Each experiment has been repeated at least three times. Quantitative densitometry was analyzed with Image J. Representative figures were shown.
2.5 Transient transfection and luciferase assay
Cells were cultured on 6-well plates at 2.0 × 105 cells/well 18-24h before transfection. Indicated plasmids were co-transfected with a β-galactosidase expression vector as a control for transfection efficiency. 0.5 μg/well of each DNA was transfected in serum-free medium using FuGENE 6 (2 μ1/1 μg of DNA; Roche Applied Science) according to the manufacturer's instructions. TGF-β or vehicle (BSA) and/or EGF were added to cultures 5 hours after the transfection. In selected experiments, inhibitors were added 1 h prior to TGF-β/EGF treatment. After a 24-hour incubation, the cells were harvested in reporter lysis buffer (Promega, Madison, WI). Luciferase and β-galactosidase activities were measured as described previously [17]. Each condition was tested in triplicate, and experiments were repeated at least three times for statistical analyses. The αSMA promoter-luciferase reporter construct was a generous gift from Dr. Robert Schwartz (Baylor College of Medicine) [18]. The SBE-luc reporter was obtained from B. Vogelstein (Howard Hughes Medical Institute/Johns Hopkins University) [19]. Elk-Gal, Gal-Luc and CA-MEK1 plasmids were part of PathDetect in vivo pathway reporting system purchased from Stratagene (La Jolla, CA). Wild type TGIF plasmid was kindly provided by Dr. David Wotton (University of Virginia) [11].
2.6 Statistics
Each experiment was repeated three times. The representative figures are shown as means ± SE. Fold induction by TGF-β (labeled on top of bars) or percentage inhibition of the fold induction by EGF between groups from three independent experiments was compared using student's t-test. Statistical significance was considered at p values of less than 0.05.
3. Results
3.1 EGF inhibits TGF-β-induced collagen I and α SMA expression in human tubular epithelial cells
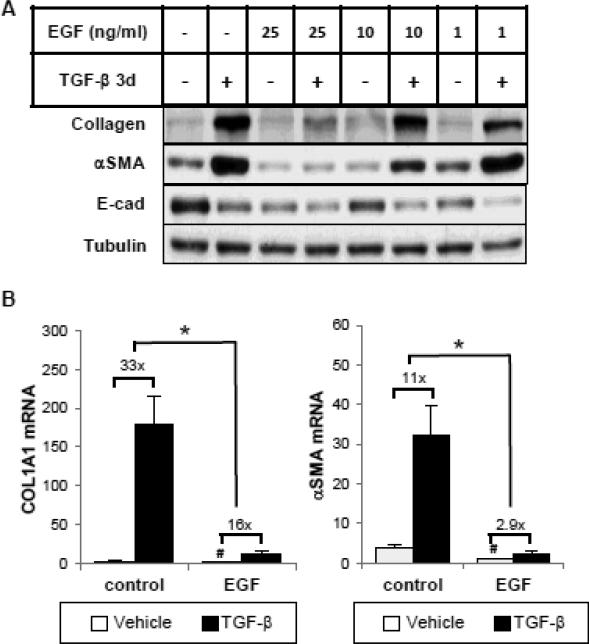
We first examined the effect of increasing doses of EGF (1-25 ng/ml) on TGF-β induced type I collagen (COL1) and αSMA expression in human tubular epithelial cells (HKCs). When treated with 1 ng/ml TGF-β, COL1 and αSMA protein expression in HKCs was markedly increased after 3 days of incubation (Figure 1A). Simultaneous addition of EGF dose-dependently decreased TGF-β-induced COL1 and αSMA protein expression, with their inductions largely abolished at 25 ng/ml. Similar results were observed with COL1 and αSMA mRNA expression as the fold induction by TGF-β was significantly reduced by EGF. These results indicate that the inhibitory effect is, at least partly, at the mRNA level (Figure 1B). Furthermore, treatment with EGF (25 ng/ml) alone inhibited basal COL1 and αSMA expression. Interestingly, under the same experimental condition, EGF did not prevent TGF-β-induced down-regulation of E-cadherin (E-Cad) but did decrease basal E-cad expression at all doses (Figure 1A), which is consistent with previous reports [8, 20].
Figure 1.
Epidermal growth factor (EGF) inhibits basal and TGF-β-induced collagen I and α-smooth muscle actin (αSMA) mRNA and protein expression in human tubular epithelial cells (HKCs). (A) Subconfluent HKCs were serum starved for 24 hours before incubation with or without TGF-β (1 ng/ml) and/or EGF (1, 10 or 25 ng/ml) for 3 days. Whole cell lysates were immunoblotted with antibodies against E-cad, αSMA, type-I collagen and β-actin, respectively. β-actin was used as a loading control. (B) Cells were treated with TGF-β (1 ng/ml) and/or EGF (25 ng/ml) for 24 hours. Quantitative PCR was performed to assess the mRNA abundance of COL1A1 and αSMA. #p<0.05 comparing baseline activities (no TGF-β) with or without EGF, *p<0.05 fold induction by TGF-β comparing with and without EGF treatment (n=3).
3.2 EGF inhibits EMT marker expression through EGFR-ERK activation
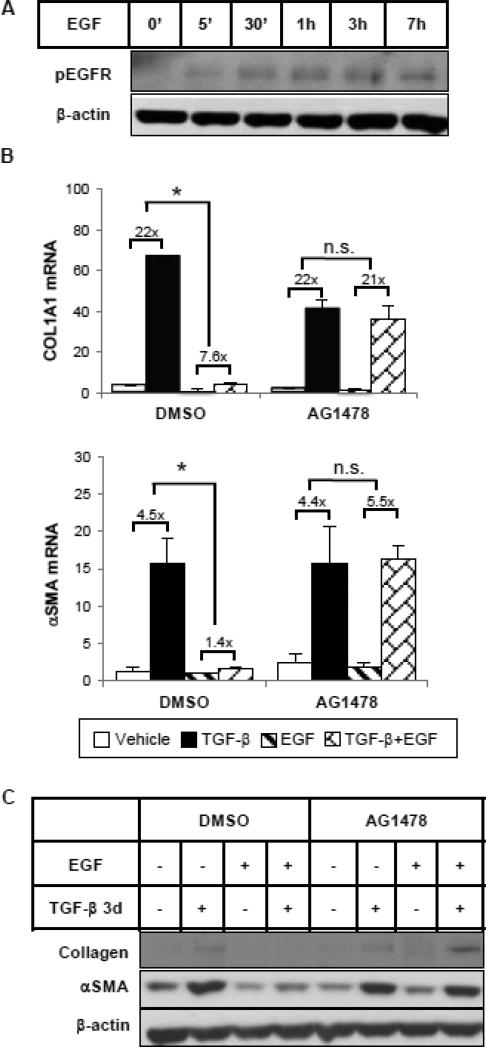
Using the EGFR tyrosine kinase-specific inhibitor, AG1478, we then tested whether EGF exerts its inhibitory effects on EMT marker expression by activating EGFR. As shown in Figure 2A, EGF activated and phosphorylated EGFR in HKCs as early as 5 minutes after treatment and the phosphorylation was sustained for at least 7 hours. Pre-treatment with AG1478 (5 μM) for 1 hour completely inhibited EGFR phosphorylation by EGF (Supplemental Figure 1A). Blocking EGFR activation did not alter TGF-β induction of COL1 and αSMA expression, but it prevented their downregulation by EGF at both mRNA (Figure 2B) and protein (Figure 2C) levels, suggesting that the inhibitory effect of EGF is EGFR dependent.
Figure 2.
EGF represses TGF-β-induced collagen and αSMA expression through EGFR. (A) HKCs were treated with EGF (25 ng/ml) for various times as indicated. Phospho-EGFR was examined by western blot. β-actin was used as a loading control. (B) Cells were pretreated with DMSO or EGFR tyrosine kinase inhibitor AG1478 (5 μM) for 1 hour, followed by incubation with TGF-β (1 ng/ml) and/or EGF (25 ng/ml) for 24 hours. COL1A1 and αSMA mRNA levels were examined by quantitative PCR. *p<0.05 fold induction by TGF-β comparing with and without EGF treatment (n=3). (C) Cells were treated as above but for 72 hours. COL1A1 and αSMA protein levels were examined by western blot. β-actin was used as a loading control.
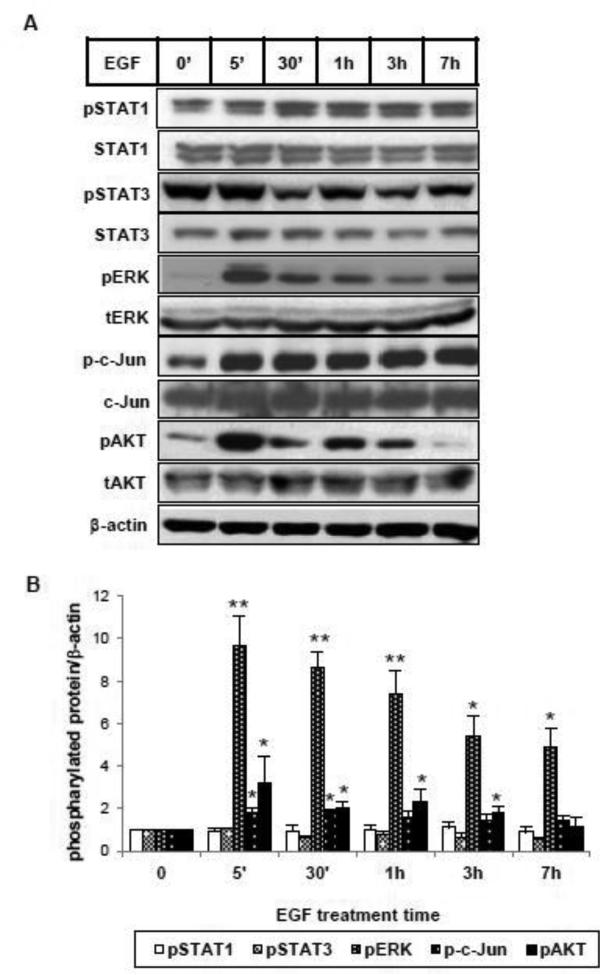
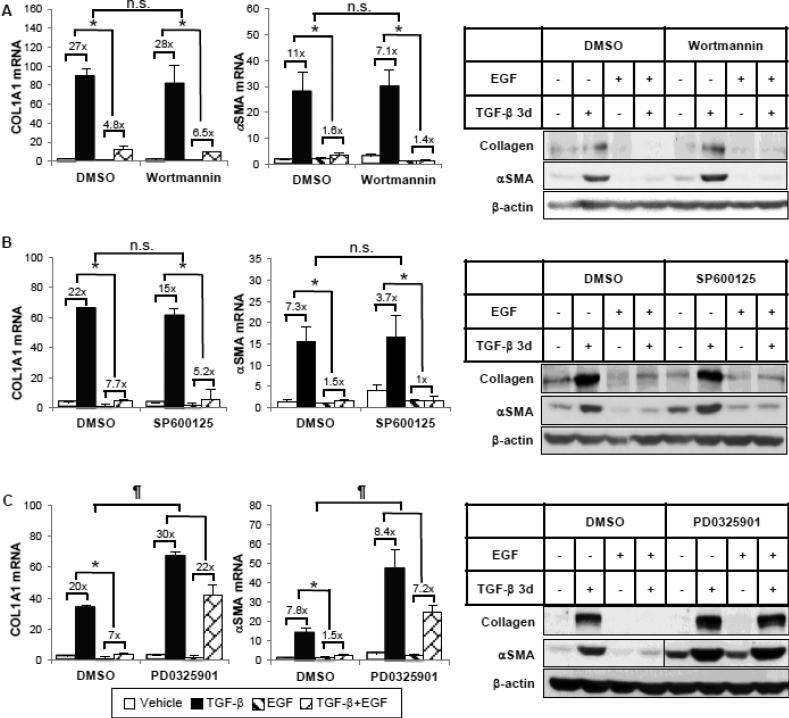
To identify the downstream signaling pathways from EGFR that might mediate the inhibitory effect of EGF, we first examined the signaling cascades that are specifically activated by EGF in HKCs. As shown in Figure 3, ERK, AKT and c-Jun phosphorylation was rapidly induced by EGF as early as 5 minutes. We did not detect any significant increase of phosphorylated STAT1 or STAT3, which have been shown to be part of the EGF signaling network [21], suggesting that EGF activates varying signaling pathways in a cell type-specific manner. Next, we utilized small molecule inhibitors to further examine which of the activated signaling pathways is responsible for inhibiting TGF-β-induced COL1 and αSMA expression. Experiments were performed with specific inhibitors of PI3K (wortmannin), JNK (SP600125) or MEK (PD0325901) (Supplemental Figure 1B-1D). As shown in Figure 4A, when EGF-activated PI3K/AKT pathway activity was blocked by wortmannin, EGF was still able to inhibit TGF-β-stimulated COL1 and αSMA mRNA and protein expression. Similar results were observed where the JNK pathway was blocked by SP600125 (Figure 4B). However, in the presence of a MEK inhibitor, PD0325901, that blocks EGF-activated ERK phosphorylation, the percentage inhibition of TGF-β-induced COL1 and αSMA expression by EGF was significantly abrogated, indicating a necessary role of MEK/ERK activation (Figure 4C). To be noted, the inhibitor PD0325901 also had an enhancing effect on basal and TGF-β-induced αSMA expression. Altogether, the data suggest that EGF inhibits TGF-β-induced collagen and αSMA expression through the EGFR/MEK/ERK signaling cascade.
Figure 3.
EGF activates multiple signaling pathways in HKC. (A) Cells were incubated with EGF (25 ng/ml) for the indicated intervals. Total cell lysates were analyzed by western blot using specific antibodies against phosphorylated forms of STAT1, STAT3, ERK, c-Jun, AKT (pSTAT1, pSTAT3, pERK, p-c-Jun, pAKT) and their corresponding non-phosphorylated forms. β-actin was used as a loading control. (B) Densitometric analysis of pSTAT1, pSTAT3, pERK, p-c-Jun and pAKT was normalized to β-actin and expressed as fold change over vehicle alone. *p<0.05, **p<0.005 comparing with vehicle control (n=3).
Figure 4.
EGF requires the ERK pathway, but not PI3K or JNK, for its inhibitory effects. HKCs were pretreated with DMSO or (A) PI3K inhibitor wortmannin (50 nM), (B) JNK inhibitor SP600125 (20 μM) or (C) MEK inhibitor PD0325901 (100 nM) for 1 hour, then incubated with TGF-β (1 ng/ml) and/or EGF (25 ng/ml) for 24 hours or 72 hours. COL1A1 and αSMA mRNA levels were examined by quantitative PCR after 24-hour treatment. COL1A1 and αSMA protein expression after 72-hour treatment was examined by western blot. β-actin was used as a loading control. The vertical line in the middle of the blot in (C) indicates the removal of irrelevant lanes within the same blot. *p<0.05 fold induction by TGF-β comparing with and without EGF treatment. n.s. or ¶p<0.05 comparing percentage inhibition of TGF-β fold induction by EGF between DMSO and the inhibitor group (n=3).
3.3 EGF inhibits TGF-β-induced Smad2/3 transcriptional activity through ERK activation
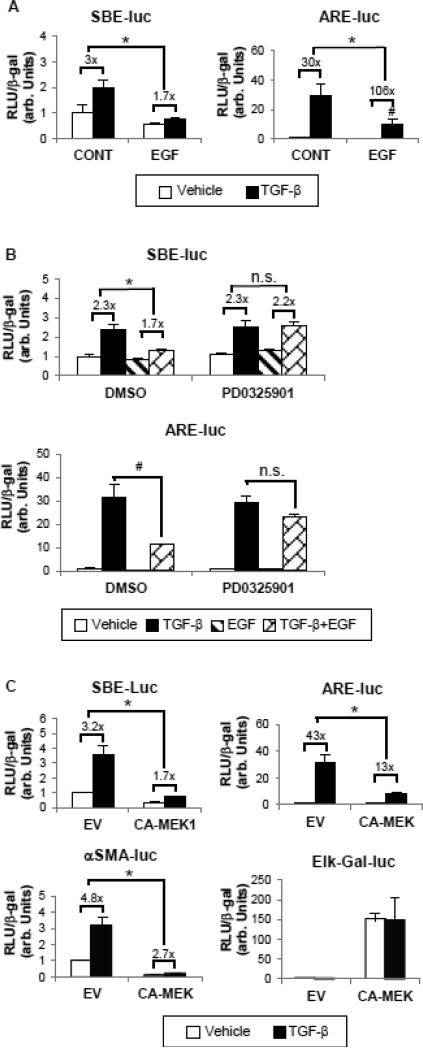
It has been shown that TGF-β induces collagen and αSMA expression through its canonical Smad pathway [22]. To determine whether EGF represses EMT marker induction by inhibiting the TGF-β/Smad pathway, we first tested the effect of EGF on the transcriptional activity of TGF-β/Smad-responsive promoters. As shown in Figure 5A, EGF treatment suppressed the Smad3/4-specific transcriptional response to TGF-β of the SBE-Luc promoter-reporter construct. EGF also reduced the absolute transcriptional activity of a Smad2-dependent promoter, ARE-Luc, although the TGF-β fold induction was increased due to extremely low baseline activity after EGF treatment.
Figure 5.
EGF decreases Smad2 and Smad3 transcriptional activities via ERK activation. (A) Cells were transfected with either ARE-Luc plus FAST-1 or SBELuc reporter constructs to detect the transcriptional activity of Smad2 and Smad3, respectively. 24 hours after transfection, cells were exposed to 1 ng/ml TGF-β with or without EGF (25 ng/ml) for another 24 hours. *p<0.05 fold induction by TGF-β comparing with and without EGF treatment. #p<0.05 comparing absolute activities induced by TGF-β in the presence or absence of EGF treatment (n=3). (B) Transfected cells were pretreated with DMSO or PD0325901 (100 nM) for 1 hour before incubation with TGF-β and/or EGF for 24 hours. *p<0.05 fold induction by TGF-β comparing with and without EGF treatment. #p<0.05 comparing absolute activities induced by TGFβ in the presence or absence of EGF treatment (n=3). (C) A constitutively active MEK1 construct (CA-MEK1, 0.5 μg) or its empty vector (EV) was overexpressed in HKCs along with ARE/SBE-Luc or αSMA promoter reporter construct (SMA-Luc). Elk-Gal-Luc was used to determine the ERK functional activity. *p<0.05 fold induction by TGF-β compared with EV group. In all experiments, the luciferase activity assayed in triplicates was normalized to β-galactosidase (β-gal) to control transfection efficiency.
To confirm that the MEK/ERK pathway plays an essential role in mediating the inhibitory effect of EGF, we tested the effect of EGF on SBE-Luc/ARE-Luc in presence of PD0325901 (MEK1 inhibitor). Consistent with our previous results, blocking ERK signaling abolished EGF action, thus leading to the restoration of ARE-Luc and TGF-β-induced SBE-Luc activities (Figure 5B). To further investigate whether the ERK pathway is sufficient for mediating the inhibitory effect of EGF, we sought to determine whether overexpressing constitutively active MEK1 (CA-MEK1) has the same effect as treatment with EGF. Figure 5C shows that CA-MEK1 overexpression robustly activated ERK as measured by an Elk-Gal-Luc reporter, which is a specific read-out for ERK functional activity. Moreover constitutive activation of ERK mimicked EGF action by repressing TGF-β-induced transcriptional activity of SBE-Luc, ARE-Luc and the αSMA promoter-Luc (αSMA-Luc). These results indicate that ERK activity is not only necessary but also sufficient for mediating the inhibitory effects by EGF.
3.4 EGF treatment does not affect Smad phosphorylation or trafficking
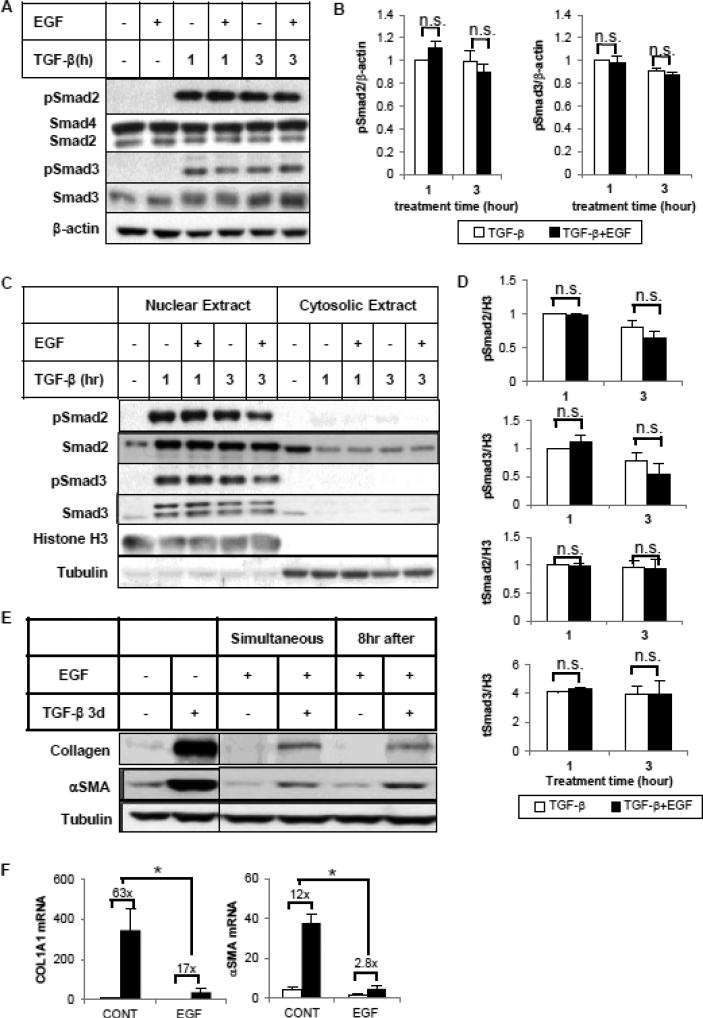
To investigate the mechanism by which EGF modulates Smad transcriptional activity, we first examined whether EGF affects the phosphorylation status of Smad 2/3. Smad activity is initiated by its phosphorylation at C-terminal serine residues. As shown in Figure 6A and 6B, simultaneous incubation with EGF had no effect on the C-terminal phosphorylation of Smad2 or Smad3 in HKCs stimulated by TGF-β for 1 hour or 3 hours. Similar results were observed with longer TGF-β treatment (Supplemental Figure S2). In some cell types, ERK modifies cell function by phosphorylating serine/threonine residues in the Smad2/3 linker regions [23]. However, EGF did not change the phosphorylation of specific linker-region sites in HKC (data not shown). It has been suggested that EGF can inhibit TGF-β signaling by blocking the nuclear accumulation of R Smads [10]. Therefore we tested whether EGF treatment of HKCs interferes with TGF-β-induced Smad2/3 nuclear translocation. As shown in Figure 6C, TGF-β treatment increased Smad2/3 nuclear concentrations and decreased their cytosolic levels after 1-hour or 3-hour incubation. The nuclear level of total and phosphorylated Smad2/3 remained unchanged with EGF treatment (Figure 6D). Together these results demonstrate that even though EGF attenuates Smad2/3-dependent transcriptional response, it does not affect the extent and kinetics of TGF-β-activated phosphorylation of Smad2/3, nor does it affect the TGF-β-induced nuclear translocation of Smad2/3 protein.
Figure 6.
EGF does not block TGF-β-induced Smad2/3 C-terminal phosphorylation or nuclear translocation. Cells were treated with TGF-β (1 ng/ml) in the absence or presence of EGF (25 ng/ml) for 1 or 3 hours. Whole cell lysates (A, B) or subcellular fractionated extracts (C, D) were analyzed by western blot with antibodies against phospho-Smad2 (Ser465/467), total Smad2, Smad4, phospho-Smad3 (Ser423/425) or total Smad3. β-actin was used as loading control of whole cell lysate. Histone H3 and tubulin served as markers for nuclear and cytosol separation. (B, D) Densitometric analysis of phospho-/total Smad2/Smad3 was normalized to β-actin or Histone H3 and expressed as fold change over TGF-β alone. (E) EGF inhibits TGF-β-induced collagen I and αSMA expression when it is added 8 hour after TGF-β. HKCs were treated with TGFβ (1 ng/ml) for 3 days. EGF (25 ng/ml) was added simultaneously or 8 hours after TGF-β treatment. Whole cell lysates were examined for collagen I and αSMA expression by western blot. Tubulin was used as a loading control. The vertical line in the middle of the blot indicates the removal of irrelevant lanes within the same blot. (F) HKCs were treated as above for 24 hours. COL1A1 and αSMA mRNA levels were examined by quantitative PCR. *p<0.05 fold induction by TGF-β comparing with and without EGF treatment (n=3).
To further investigate the mechanisms regulating Smad activity, we varied the time points of adding EGF relative to TGF-β treatment. As shown in Figure 6E and 6F, EGF inhibited TGF-β-induced COL1 and αSMA protein and mRNA expression even when it was added 8 hours after TGF-β treatment. This observation is consistent with the previous results (Figure 6A, 6B) suggesting EGF does not inhibit TGF-β responses during the initial activation of TGF-β/Smad signaling.
3.5 EGF induces the expression and phosphorylation of TGIF, a Smad corepressor
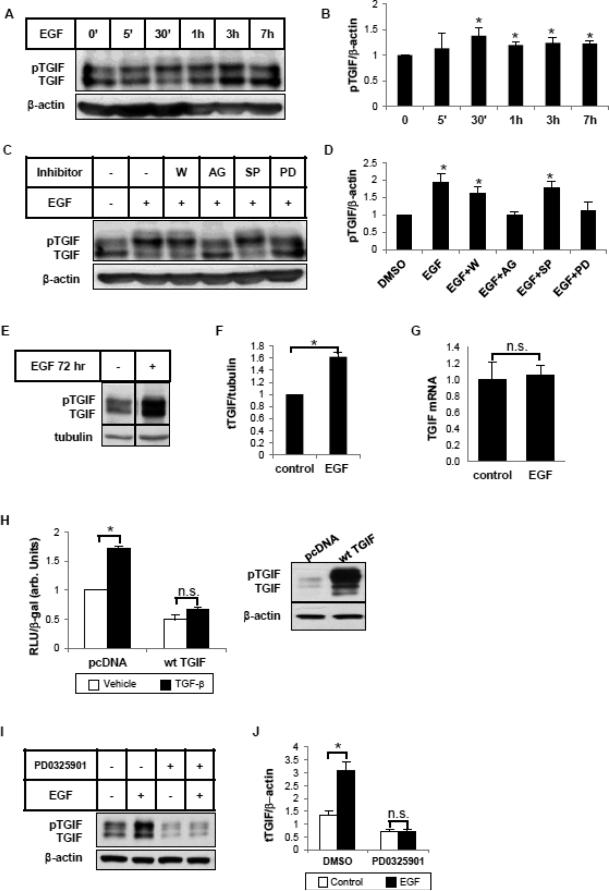
In addition to phosphorylation and nuclear translocation, Smad protein activity can also be regulated by co-activators or co-repressors with which it interacts at the transcriptional complex. EGF previously has been shown to stabilize TGIF, a short-lived Smad repressor, via ERK-mediated phosphorylation at its C-terminus [11]. We therefore assessed whether EGF could influence TGIF expression or phosphorylation in HKCs. Figure 7A shows a double-band, with the upper one corresponding to the phosphorylated form of TGIF [12]. EGF induced TGIF phosphorylation after 30 minutes of incubation (Figure 7B). The induction was blocked by the EGFR inhibitor AG1478, or by the MEK inhibitor PD0325901, but not by PI3K inhibitor wortmannin or JNK inhibitor SP600125 (Figure 7C, 7D). We then examined if EGF also induces TGIF expression. After 72 hours of exposure to EGF, total TGIF protein expression level was markedly increased (Figure 7E, 7F), whereas TGIF mRNA was unchanged (Figure 7G). These data are consistent with a previous report that showed that EGF increases TGIF expression at the post-translational level [11]. To confirm the role of TGIF as a TGF-β signaling antagonist, we first overexpressed TGIF in HKCs. As seen in Figure 7H, TGIF overexpression blocked TGF-β-induced transcriptional activity of SMA-Luc. We then reasoned that, if EGF-induced TGIF expression contributes to the antagonism against TGF-β, the induction of TGIF should be absent when EGF action is blocked. As shown in Figure 7I and 7J, this is indeed the case. Pretreatment with MEK inhibitor PD0325901 abolished EGF-induced TGIF under the same conditions that blocked EGF repression of TGF-β-stimulated collagen 1 and αSMA (Figure 4C). Together these data support the notion that TGIF plays a role in mediating the inhibitory effects of EGF.
Figure 7.
EGF induces the phosphorylation and expression of TGIF. (A) HKCs were treated with EGF (25 ng/ml) for indicated periods of time. Whole cell lysates were examined for TGIF using an antibody that recognizes total TGIF. β-actin was used as a loading control. (B) Densitometric analysis of pTGIF was normalized to β-actin and expressed as fold change over control. *p<0.05 compared with control (n=3). (C) Cells were pretreated with the inhibitors, wortmannin (W, 50 nM), AG1478 (AG, 5 μM), SP600125 (SP, 20 μM) and PD0325901 (PD, 100 nM) for 1 hour before 30 min treatment with EGF (25 ng/ml). Whole cell lysates were examined for TGIF by western blot. β-actin was used as a loading control. (D) Densitometric analysis of pTGIF was normalized to β-actin and expressed as fold change over DMSO control. *p<0.05 compared with DMSO control (n=3). (E) Cells were treated with EGF (25 ng/ml) for 3 days. Whole cell lysates were examined for total TGIF. Tubulin was used as a loading control. The vertical line in the middle of the blot indicates the removal of irrelevant lanes within the same blot. (F) Densitometric analysis of total TGIF was normalized to tubulin and expressed as fold change over control. *p<0.05 compared with control (n=3). (G) Cells were treated with TGF-β (1 ng/ml) and/or EGF (25 ng/ml) for 24 hours. Quantitative PCR was performed to assess the mRNA level of TGIF. (H) A wild type TGIF construct (wt TGIF) was overexpressed in HKCs along with αSMA promoter reporter construct (SMALuc). 3 hours after transfection, cells were exposed to 1 ng/ml TGF-β for another 24 hours. The luciferase activity assayed in triplicates was normalized to β-galactosidase (β-gal) to control transfection efficiency. * p<0.05 compared with vehicle control (n=3). Western blots confirmed the overexpression of TGIF. (I) HKCs were pretreated with DMSO or PD0325901 (100 nM) for 1 hour, then incubated with EGF (25 ng/ml) for 72 hours. TGIF protein expression was examined by western blot. β-actin was used as a loading control. (J) Densitometric analysis of total TGIF was normalized to β-actin and expressed as fold change over DMSO control. *p<0.05 compared with non-EGF-treated control (n=3).
4. Discussion
The phenotypic transition of tubular epithelial cell to mesenchymal cells contributes to the generation of matrix-producing myofibroblasts, which are the key pathogenic cells in all fibrotic diseases including renal fibrosis. Given the central role of TGF-β in promoting this transition by inducing the expression of collagen and αSMA, interrupting the TGF-β pathway has become a key target for the current therapeutic intervention in renal fibrosis. In this study we have demonstrated that in human tubular epithelial cells EGF inhibits both basal and TGF-β-induced collagen and αSMA expression.
Our results show that EGF activates the MEK/ERK, JNK and PI3K/AKT signaling pathways through EGFR activation in HKCs. We further demonstrate that the EGFR/MEK/ERK signaling axis is required and sufficient to repress TGF-β-induced collagen and αSMA expression in HKC. MEK/ERK activation has been shown previously to attenuate TGF-β signaling through multiple mechanisms [24, 25]. It has been reported that ERK MAP kinase activated by Ras causes cytoplasmic retention of Smad protein via linker region phosphorylation [10]. Although we ([23, 26]), and others ([27, 28]) previously have implicated Smad linker region phosphorylation in regulating Smad function, we did not observe any change in linker region phosphorylation with EGF treatment in HKC with current, commercially available antibodies. Furthermore in our studies EGF did not block Smad C-terminal phosphorylation or nuclear translocation. Although the nuclear content of Smad was not altered, Smad transcriptional activity was significantly decreased by EGF as measured by ARE-Luc or SBE-Luc. This finding suggests that the suppression does not occur at the level of Smad protein phosphorylation or nuclear translocation, but rather further downstream in the nucleus where the Smad complex transactivates target genes after associating with numerous other cofactors. These cofactors, including histone modifiers, and chromatin remodelers, interact with activated Smad proteins and regulate Smad transcriptional outcomes positively or negatively depending on cellular context [29, 30]. For example, in a different kidney epithelial cell line (HK-2), a Smad transcriptional co-repressor, SnoN, is upregulated by HGF to suppress TGF-βsignaling by interacting with activated Smad2 and sequestering it from transactivation of its target genes [31]. However, in HKC, SnoN protein expression did not change after EGF treatment (Supplemental Figure S3). Instead, another Smad transcriptional co-repressor, TGIF, is phosphorylated and stabilized by EGF via EGFR/ERK activation. TGIF has been shown to interact with Smad4 and overexpression of TGIF suppresses TGF-β-induced αSMA gene expression in kidney mesangial cells [32]. Our results suggest a role for TGIF in mediating the inhibitory effect of EGF via EGFR/MEK/ERK pathway.
In contrast to the inhibitory role of EGF in TGF-β/Smad signaling in HKC, we found that the same EGF treatment does not prevent, and may enhance, TGF-β-induced collagen and αSMA expression in human mesangial cells, in spite of ERK activation (Supplemental Figure S4). This observation is consistent with our previous work which has shown that ERK MAP kinase enhances TGF-β- dependent collagen induction in human mesangial cells [26], suggesting a different, perhaps opposite effect of ERK signaling in epithelial cells versus mesenchymal cells. It indicates that the crosstalk between the MEK/ERK and TGF-β/Smad pathways is cell-type specific and the interaction between ERK and Smad could alter the signaling outcomes either positively or negatively.
Extensive research in cancer cells has strongly suggested a role of EGF/EGFR in promoting type III EMT, mostly through down-regulating cell-junction proteins such as the cadherins. Synergistic activities between TGF-β and EGF have been identified in several studies [8, 33]. Consistently we have seen a similar additive effect of TGF-β and EGF on E-cadherin disappearance in HKC. However, under the same experimental conditions, we also have observed that EGF blocks TGF-β-induced collagen I and αSMA expression. Thus, it is likely that the mechanism regulating E-cadherin expression is distinct from that regulating collagen I and αSMA. Others have shown in tubular epithelial cells that BMP7 opposes TGF-β-induced αSMA expression yet simultaneously synergizes with TGF-β in downregulating E-cadherin [34]. Given that E-cadherin disappearance and αSMA activation are both widely accepted EMT markers, it suggests that loss of E-cadherin may not always lead to a complete phenotypic transition to mesenchymal cells. Thus, the events involved in EMT are complex and likely to be regulated through multiple, independent mechanisms.
5. Conclusion
In summary, we have shown that EGF suppresses basal and TGF-β-induced collagen and αSMA expression through EGFR/MEK/ERK activation in human tubular epithelial cells. The antagonistic effect of EGF is independent of Smad phosphorylation and nucleocytoplasmic dynamics, but most likely results from the induction of a Smad co-repressor, TGIF. The cell type-specific, inhibitory role of ERK activation may provide additional therapeutic strategies for renal tubular interstitial fibrosis.
Supplementary Material
Highlights.
EGF inhibits TGF-β-induced EMT marker expression.
EGF-induced ERK activity is required for the inhibition.
ERK acts in this system by inhibiting TGF-β-induced Smad transcriptional activity.
No changes in TGF-β-induced Smad phosphorylation are observed.
EGF induces the phosphorylation and expression of the Smad repressor, TGIF.
Acknowledgements
We thank Dr. R.Schwartz, Dr. B. Vogelstein and Dr. D. Wotton for providing us with the plasmids, and Dr. L. Racusen for sharing the human proximal tubular epithelial cell line. We also appreciate the discussions and inputs from Dr. T. Hayashida (Northwestern University, Chicago, IL). This work was supported by the grant RO1 DK049362 from the National Institute of Diabetes, Digestive and Kidney Diseases to H.W.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
X.L., S.C.H., J.A.B. performed experiments; X.L. prepared figures; X.L and H.W.S., drafted manuscript; X.L., S.C.H, J.A.B. and H.W.S., approved final version of manuscript.
Disclosure
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Burns WC, Thomas MC. The molecular mediators of type 2 epithelial to mesenchymal transition (EMT) and their role in renal pathophysiology. Expert Rev Mol Med. 2010;12:e17. doi: 10.1017/S1462399410001481. [DOI] [PubMed] [Google Scholar]
- 2.Poncelet AC, Schnaper HW. Sp1 and Smad proteins cooperate to mediate transforming growth factor-beta 1-induced alpha 2(I) collagen expression in human glomerular mesangial cells. The Journal of biological chemistry. 2001;276(10):6983–92. doi: 10.1074/jbc.M006442200. [DOI] [PubMed] [Google Scholar]
- 3.Hu B, Wu Z, Phan SH. Smad3 mediates transforming growth factor-beta-induced alpha-smooth muscle actin expression. Am J Respir Cell Mol Biol. 2003;29(3 Pt 1):397–404. doi: 10.1165/rcmb.2003-0063OC. [DOI] [PubMed] [Google Scholar]
- 4.Barrientos S, et al. Growth factors and cytokines in wound healing. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 5.Lu X, Kang Y. Epidermal growth factor signalling and bone metastasis. British journal of cancer. 2010;102(3):457–61. doi: 10.1038/sj.bjc.6605490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Z, et al. Epidermal growth factor-induced tumor cell invasion and metastasis initiated by dephosphorylation and downregulation of focal adhesion kinase. Molecular and cellular biology. 2001;21(12):4016–31. doi: 10.1128/MCB.21.12.4016-4031.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo HW, Hsu SC, Hung MC. EGFR signaling pathway in breast cancers: from traditional signal transduction to direct nuclear translocalization. Breast cancer research and treatment. 2006;95(3):211–8. doi: 10.1007/s10549-005-9011-0. [DOI] [PubMed] [Google Scholar]
- 8.Uttamsingh S, et al. Synergistic effect between EGF and TGF-beta1 in inducing oncogenic properties of intestinal epithelial cells. Oncogene. 2008;27(18):2626–34. doi: 10.1038/sj.onc.1210915. [DOI] [PubMed] [Google Scholar]
- 9.Kretzschmar M, Doody J, Massague J. Opposing BMP and EGF signalling pathways converge on the TGF-beta family mediator Smad1. Nature. 1997;389(6651):618–22. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 10.Kretzschmar M, et al. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes Dev. 1999;13(7):804–16. doi: 10.1101/gad.13.7.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo RS, Wotton D, Massague J. Epidermal growth factor signaling via Ras controls the Smad transcriptional co-repressor TGIF. The EMBO journal. 2001;20(1-2):128–36. doi: 10.1093/emboj/20.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang S, Nugent MA, Panchenko MP. EGF antagonizes TGF-beta-induced tropoelastin expression in lung fibroblasts via stabilization of Smad corepressor TGIF. American journal of physiology. Lung cellular and molecular physiology. 2008;295(1):L143–51. doi: 10.1152/ajplung.00289.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeng F, Singh AB, Harris RC. The role of the EGF family of ligands and receptors in renal development, physiology and pathophysiology. Experimental cell research. 2009;315(4):602–10. doi: 10.1016/j.yexcr.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norman J, et al. EGF-induced mitogenesis in proximal tubular cells: potentiation by angiotensin II. The American journal of physiology. 1987;253(2 Pt 2):F299–309. doi: 10.1152/ajprenal.1987.253.2.F299. [DOI] [PubMed] [Google Scholar]
- 15.Chevalier RL, et al. Obstructive nephropathy in the neonatal rat is attenuated by epidermal growth factor. Kidney international. 1998;54(1):38–47. doi: 10.1046/j.1523-1755.1998.00966.x. [DOI] [PubMed] [Google Scholar]
- 16.Racusen LC, et al. Cell lines with extended in vitro growth potential from human renal proximal tubule: characterization, response to inducers, and comparison with established cell lines. The Journal of laboratory and clinical medicine. 1997;129(3):318–29. doi: 10.1016/s0022-2143(97)90180-3. [DOI] [PubMed] [Google Scholar]
- 17.Hayashida T, et al. TGF-beta1 activates MAP kinase in human mesangial cells: a possible role in collagen expression. Kidney international. 1999;56(5):1710–20. doi: 10.1046/j.1523-1755.1999.00733.x. [DOI] [PubMed] [Google Scholar]
- 18.Min BH, Foster DN, Strauch AR. The 5′-flanking region of the mouse vascular smooth muscle alpha-actin gene contains evolutionarily conserved sequence motifs within a functional promoter. The Journal of biological chemistry. 1990;265(27):16667–75. [PubMed] [Google Scholar]
- 19.Zawel L, et al. Human Smad3 and Smad4 are sequence-specific transcription activators. Molecular cell. 1998;1(4):611–7. doi: 10.1016/s1097-2765(00)80061-1. [DOI] [PubMed] [Google Scholar]
- 20.Lu Z, et al. Downregulation of caveolin-1 function by EGF leads to the loss of E-cadherin, increased transcriptional activity of beta-catenin, and enhanced tumor cell invasion. Cancer Cell. 2003;4(6):499–515. doi: 10.1016/s1535-6108(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Z, Wen Z, Darnell JE., Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–8. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 22.Poncelet AC, de Caestecker MP, Schnaper HW. The transforming growth factor-beta/SMAD signaling pathway is present and functional in human mesangial cells. Kidney international. 1999;56(4):1354–65. doi: 10.1046/j.1523-1755.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- 23.Browne JA, et al. Serine-204 in the linker region of Smad3 mediates the collagen-I response to TGF-beta in a cell phenotype-specific manner. Experimental cell research. 2013 doi: 10.1016/j.yexcr.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bottinger EP, Bitzer M. TGF-beta signaling in renal disease. Journal of the American Society of Nephrology : JASN. 2002;13(10):2600–10. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Kelm RJ, Jr., Strauch AR. Transforming growth factor beta1-mediated activation of the smooth muscle alpha-actin gene in human pulmonary myofibroblasts is inhibited by tumor necrosis factor-alpha via mitogen-activated protein kinase kinase 1-dependent induction of the Egr-1 transcriptional repressor. Molecular biology of the cell. 2009;20(8):2174–85. doi: 10.1091/mbc.E08-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayashida T, Decaestecker M, Schnaper HW. Cross-talk between ERK MAP kinase and Smad signaling pathways enhances TGF-beta-dependent responses in human mesangial cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(11):1576–8. doi: 10.1096/fj.03-0037fje. [DOI] [PubMed] [Google Scholar]
- 27.Wang G, et al. Transforming growth factor-{beta}-inducible phosphorylation of Smad3. The Journal of biological chemistry. 2009;284(15):9663–73. doi: 10.1074/jbc.M809281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alarcon C, et al. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-beta pathways. Cell. 2009;139(4):757–69. doi: 10.1016/j.cell.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13(10):616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102(3):593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Dai C, Liu Y. A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. Journal of the American Society of Nephrology : JASN. 2005;16(1):68–78. doi: 10.1681/ASN.2003090795. [DOI] [PubMed] [Google Scholar]
- 32.Dai C, Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. Journal of the American Society of Nephrology : JASN. 2004;15(6):1402–12. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- 33.Docherty NG, et al. TGF-beta1-induced EMT can occur independently of its proapoptotic effects and is aided by EGF receptor activation. American journal of physiology. Renal physiology. 2006;290(5):F1202–12. doi: 10.1152/ajprenal.00406.2005. [DOI] [PubMed] [Google Scholar]
- 34.Veerasamy M, et al. Differential regulation of E-cadherin and alpha-smooth muscle actin by BMP 7 in human renal proximal tubule epithelial cells and its implication in renal fibrosis. American journal of physiology. Renal physiology. 2009;297(5):F1238–48. doi: 10.1152/ajprenal.90539.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.