Abstract
Sonic hedgehog (Shh) is a pleiotropic factor in the developing central nervous system (CNS), driving proliferation, specification, and axonal targeting in multiple sites within the forebrain, hindbrain, and spinal cord. Studies in embryonic CNS have shown how gradients of this morphogen are translated by neuroepithelial precursors to determine the types of neurons and glial cells they produce [1, 2]. Shh also has a well-characterized role as a mitogen for specific progenitor cell types in neural development [3, 4]. As we begin to appreciate that Shh continues to act in the adult brain, a central question is what functional role this ligand plays when major morphogenetic and proliferative processes are no longer in operation. A second fundamental question is whether similar signaling mechanisms operate in embryonic and adult CNS. In the two major germinal zones of the adult brain, Shh signaling modulates the self-renewal and specification of astrocyte-like primary progenitors, frequently referred to as neural stem cells (NSCs). It also may regulate the response of the mature brain to injury, as Shh signaling has been variously proposed to enhance or inhibit the development of a reactive astrocyte phenotype. The identity of cells producing the Shh ligand, and the conditions that trigger its release, are also areas of growing interest; both germinal zones in the adult brain contain Shh-responsive cells but do not autonomously produce this ligand. Here, we review recent findings revealing the function of this fascinating pathway in the postnatal and adult brain, and highlight ongoing areas of investigation into its actions long past the time when it shapes the developing brain.
Keywords: Sonic Hedgehog, neural stem cells, astrocytes, reactive astrocytes, ventricular-subventricular zone, subgranular zone, brain tumor stem cells, stem cell microdomains, primary cilium
1.1 Introduction
The Hedgehog (Hh) signaling pathway, of which Shh is the major activating ligand in the brain, is central to the development and patterning of the CNS and other organs [4, 5]. Signaling is initiated when secreted Shh binds Patched (Ptc) at the cell surface, relieving inhibition of the transmembrane protein Smoothened (Smo) [6, 7] and ultimately triggering the activation of the Gli transcription factors. In the absence of Hh signal, Gli3 is proteolytically processed and acts as a transcriptional repressor. Gli2 functions primarily as a transcriptional activator upon Hh stimulation and can initiate transcription of Gli1, a constitutive transcriptional activator that indicates high levels of pathway activity [8–11]. However, in some contexts, Gli3 may act as a weak activator, and Gli2 may act as a weak repressor [12, 13]. The relative levels of the Gli transcription factors, and the balance between repression and activation of Hh pathway target genes, are a major mechanism by which cells in the developing neural tube, one of the best-characterized Shh-responsive tissues, translate a gradient of Hh ligand into a pattern of distinct neuronal fates [6, 14–22]. Expression of Shh and modulation of downstream target genes is also thought to be critical for establishing the early patterning of the ganglionic eminences, a major source of inhibitory interneurons for the telencephalon [18, 23]. Shh also functions in regulating the patterning and proliferation of precursor cells in the postnatal cerebellum, with a concomitant role (when mutated) in cerebellar tumor development [24–28]. More recently, Gli3, and transduction of the Shh signal via the primary cilium, have also been implicated in development of the cortex [29, 30]. Finally, Shh appears to act through non-canonical mechanisms, which are likely Gli-independent, to regulate axon guidance and cortical microcircuit formation in the developing brain [31, 32].
1.2 Establishing Neurogenic Niches - Shh and the Primary Cilium in Maturing Brain
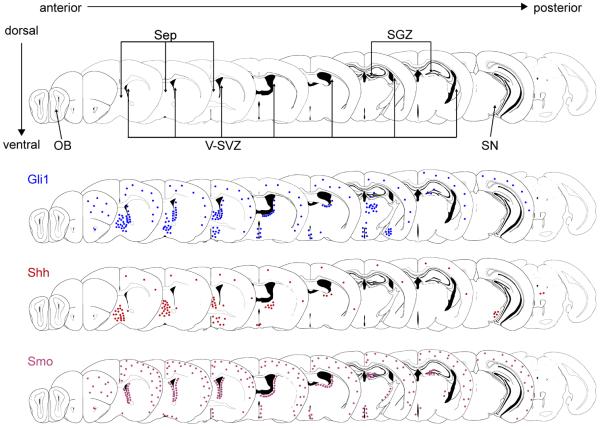
In addition to the many functions Hh signaling has in development, this pathway is key for the formation and patterning of brain germinal niches that continue to produce neurons and glial cells throughout the life of most mammals [33–36]. These niches are the ventricular-subventricular zone (V-SVZ), which is found along the walls of the lateral ventricles, and the subgranular zone (SGZ), within the dentate gyrus in the hippocampus (highlighted in Figure 1) [37, 38]. The V-SVZ primarily generates neuroblasts, which migrate anteriorly to the olfactory bulb and differentiate into several types of neurons [39–42]. The V-SVZ also generates oligodendrocytes in vivo, and stem cells isolated from each of these regions generate astrocytes, oligodendrocytes, and neurons in vitro. The adult V-SVZ NSCs have many characteristics of astrocytes, including expression of glial fibrillary acidic protein (GFAP), and are known as B1 cells. These cells arise from radial glia, the embryonic stem cells that persist in the periventricular region of neonatal mice [43, 44].
Figure 1.
Expression of Shh Pathway Components in the Mature Brain. Coronal sections of adult brain are shown from anterior (left) to posterior (right). The ventricles, which contain cerebrospinal fluid, are shown in black. Specific anatomical regions discussed in the text are indicated in the top row of sections, including the olfactory bulb (OB), ventricular-subventricular zone (V-SVZ), and subgranular zone (SGZ) in the dentate gyrus. Locations where Shh, Smo, and Gli1 (downstream transcriptional activator) expression have been reported are indicated by colored dots. Both the V-SVZ and SGZ contain Smo-expressing astrocyte-like stem cells, some fraction of which also express Gli1. The cortex (in the dorsal portion of each slice) and parts of the ventral forebrain also contain Smo / Gli1-expressing astrocytes. By contrast, Shh-producing cells are often located in distinct subregions from Shh-responsive cells, with Shh-producing neurons found in the septum (Sep), multiple areas in the ventral forebrain, and the substantia nigra (SN).
The SGZ, in contrast, is not located next to the ventricles, but is found deep in the brain parenchyma at the interface of the granule cell layer and hilus in the dentate gyrus of the hippocampus [45]. The NSCs in the SGZ also correspond to cells with astroglial properties: they are radially oriented astrocytes with a cell body in the SGZ and a prominent GFAP-positive process that traverses radially through the granule cell layer to profusely branch in the inner molecular layer [38, 45–47]. These cells are known by various names, including radial astrocytes, radial glial-like cells or radial progenitors; since these cells do not retain radial glia morphology and are not located in a VZ or SVZ, we will refer to them here as radial astrocytes (RA), the original name used when they were first identified as the SGZ primary progenitors in the adult brain [46]. Like the B1 cells in the V-SVZ, RA in the SGZ were thought to be derived from embryonic radial glia [48, 49]. However, recent work has also shown that some RA precursors undergo a curious and extensive tangential migration, taking them from a unique ventral Hh-responsive location at the temporal pole of the developing hippocampus to ultimately disperse throughout the SGZ [50]. Once established in the SGZ, RAs persist throughout life, generating new granule neurons through intermediate precursors. A number of studies focusing on prenatal removal of Hh pathway components have revealed a stringent requirement for canonical Hh signaling, mediated through the primary cilium, in the patterning and maintenance of both the V-SVZ and SGZ.
Shh knockout mice lack ventral structures in the CNS and die after birth, precluding analyses of postnatal brain development [9, 51]. As a consequence, many of the clues indicating that the Shh pathway is central to the establishment of adult germinal zones came from studies using cell type-specific or inducible Cre recombinases to ablate specific pathway members in the mouse. Using the Nestin-Cre transgenic mouse, which drives recombination throughout the developing telencephalon, Machold and colleagues ablated either Shh itself or Smo, which is required for cells to transduce the Shh signal [9]. Although early Shh-dependent dorsoventral patterning, such as the establishment of the ganglionic eminences, is largely normal in these animals, striking defects were present in both postnatal neurogenic niches at two weeks after birth, suggesting a requirement for Shh in their establishment or maintenance. Smo-deficient animals exhibit an overall reduction in brain mass, enlarged ventricles, and reduced numbers of progenitor cells in the germinal regions. Specifically, both the V-SVZ and SGZ are thinner, and have decreased BrdU incorporation and increased apoptotic markers at early postnatal timepoints. These data suggest an ongoing requirement for Smo in both neurogenic niches. Following this initial observation, subsequent studies used tamoxifen-inducible Cre recombinase, again driven by the Nestin promoter, to specifically examine the postnatal requirement for Smoothened in the V-SVZ [52, 53]. Similar to the effects observed following ablation during embryonic development, deletion of Smo during the immediate postnatal period results in a marked decrease in neurogenesis. However, no increase in apoptosis was observed. These data suggest that Smo, and Shh signaling that occurs in the juvenile brain after embryonic and fetal development, have a specific effect in the proliferation, and possibly on the self-renewal, of NSCs.
The function of Shh in developing stem cell niches is also dependent on the presence of a functional primary cilium. Ablation of the motor protein KIF3A, intraflagellar transport protein IFT88, or the ciliary protein Stumpy, and therefore the removal of functional primary cilia in neural precursors, results in decreased Shh target gene expression and a phenotype similar to that observed in Smo-deficient animals [54, 55]. hGFAP-Cre; Kif3afl/fl and hGFAP-Cre; Smofl/fl animals, like Nestin-Cre; Smofl/fl animals, have a hypocellular and disorganized dentate gyrus at birth, accompanied by decreased proliferation and neurogenesis. Removal of primary cilia also blocks the effects of heightened pathway activation via expression of a hypermorphic Smo, SmoM2. Similarly, ablation of primary cilia has significant effects in the postnatal V-SVZ, but here the interpretation is complicated as the promoters used for genetic ablation of primary cilia also affect the function of motile cilia in ependymal cells and therefore cerebrospinal fluid (CSF) flow (unpublished observation). Ependymal cells and CSF are integral components of the adult V-SVZ niche [56–58] and disruption of motile cilia in ependymal cells is likely to indirectly affect V-SVZ progenitors. New approaches to selectively ablate cilia in V-SVZ progenitors, but not ependymal cells, are required to understand the role of primary cilia in these periventricular NSCs.
1.3 Shh Signaling in Adult Germinal Niches
In the adult rodent brain, multiple roles have been attributed to Shh signaling – both fate specification and regulation of proliferative activity. Early indicators that Shh signaling might continue in adult germinal regions came from transcriptional studies cataloging the locations of Shh transcript as well as transcripts of other canonical pathway members, Ptc and Smo [59–62]. These data, as well as localization patterns indicated by subsequent experiments using mouse reporter alleles, are summarized in Figure 1. Although Ptc and Shh are not prevalent in the V-SVZ, Smo is expressed throughout this region. In addition, injection of myristoylated Shh in the lateral ventricle in rodents results in elevated BrdU incorporation and Ptc transcription, suggesting that Shh-responsive cells are indeed present in this region. Both Ptc and Smo are also present at high levels in the dentate gyrus [61, 62]. Shh-expressing regions are largely distinct from these areas, with high Shh transcript found in several locations in the basal forebrain, brainstem, and cranial nerve nuclei [9, 61, 62]. Sparse expression of Shh is also found in the juvenile (P15) hilus, near the SGZ, but it is not clear if this expression persists into adulthood [9, 61]. Exogenous Shh protein has also been shown to increase the production of multipotent, self-renewing neurospheres cultured from adult stem cells of the V-SVZ or SGZ, suggesting that signaling through this pathway affects stem cell proliferation, the balance between self-renewal and differentiation, or both [11, 63]. In contrast to the localization of Shh transcript and subsequent analyses using knock-in mouse alleles to label cell bodies, Shh protein has been detected in the dentate gyrus, cerebrospinal fluid, and the neuropil surrounding the ventral V-SVZ [63, 64]. However, the mechanisms through which Shh protein is secreted and reaches these regions remain to be elucidated. In the case of the V-SVZ, although many stem cells extend a primary cilium that contacts the cerebrospinal fluid in the lateral ventricles, the Shh-producing cells under normal conditions all appear to be neurons, and the soma of these neurons are frequently located far from the ventricles [64]. However, a small subpopulation of these neurons exists very close to the ventral V-SVZ [64]. Radiolabeling data in developing and adult optic nerve in rodent brain indicates that Shh can be anterogradely transported in neurons, suggesting that cells distant from the ventricular surface may still form contacts with the stem cells of the V-SVZ to generate locally elevated Shh levels [65–67]. Intriguingly, a recent study compromising the production of Shh by dopaminergic neurons in the ventral midbrain also implicated this ligand in regulating the survival of specific neuronal subtypes [68]. Ablating Shh in adult dopaminergic neurons in the substantia nigra and associated areas (using a dopamine transporter locus-driven Cre) resulted in a progressive loss of fast-spiking and cholinergic neurons in the striatum, where long-range projections from dopaminergic neurons are found. These results suggest a further, possibly Gli-independent, role in regulation of neural activity and survival that depends on proper transport of Shh ligand [68].
Recently, multiple groups have mapped the cell types and microdomains within the V-SVZ that are responsive to Shh signal, using expression of gli1 transcript and Gli1-driven reporters as indicators of high pathway activation. Quantification of Shh and other pathway members in individual cells isolated from the V-SVZ, or subpopulations enriched by flow cytometric sorting, indicates that type B1 cells (stem cells) are likely the major Shh-responsive populations in this region, although transcript can also be detected in putative transit-amplifying cells [11]. Studies of Gli1-nlacZ, Gli2-nlacZ, and Gli3-nlacZ mice have demonstrated persistent β–galactosidase activity in V-SVZ and SGZ, and the generation of Gli1-CreERT2 mice allowed temporally precise lineage tracing of strongly Shh-responsive cells [8, 64, 69]. Co-localization analyses of brain sections from these reporter mice again indicate that in the V-SVZ type B1 astrocyte-like neural stem cells are the primary Gli-expressing population. Immunostaining for Smo protein shows substantial coexpression of Smo with GFAP, which marks the astrocyte-like stem cell population and mature astrocytes, but is not expressed in transit-amplifying progenitors or immature neuroblasts [64]. Further, prospective labeling of Shh-responsive cells prior to antimitotic (cytosine-β-D-arabinofuranoside [Ara-C]) administration, a procedure to eliminate actively dividing intermediate progenitors, demonstrates that the Shh-responsive population in both the V-SVZ and SGZ includes slow-cycling neural stem cells (as opposed to “activated” neural stem cells, which are initiating division) [8]. These cells persist during antimitotic treatment and subsequently repopulate the germinal zones. In the case of antimitotic treatment, as Shh-responsive cells were prospectively labeled prior to infusion, it is not possible to tell how the pattern of pathway activation might change during or after Ara-C administration. As we now appreciate (see below) that dorsal and ventral microdomains of the normal V-SVZ have distinct levels of Shh activity, it will be of interest to trace pathway activation during recovery from Ara-C.
It is now known that the V-SVZ is an organized mosaic of NSCs which can be subdivided into several microdomains, distinguished by their ability to generate specific neuronal subtypes in the olfactory bulb [64, 70–76]. Recent data focusing on stem cell regional heterogeneity have indicated different roles for the various Gli family members in distinct parts of the V-SVZ. While upstream components of the Hh pathway, such as Smo, are expressed in GFAP-positive cells throughout the germinal zone, gli1 expression is predominantly localized to the ventral SVZ, and is sufficient to drive the production of the most common ventrally-derived neuron types – deep granule interneurons (deep GCs, shown in Figure 2) and calbindin-positive periglomerular cells (PGCs) [64]. Intriguingly, recent lineage tracing data indicate that the Gli1-expressing domain of the ventral V-SVZ also contributes to the generation of four recently identified novel interneuron subtypes in the olfactory bulb [70]. However, ectopic activation of Hh signaling via expression of the SmoM2 allele, while sufficient to drive dorsal stem cells to produce deep GCs and calbindin-positive PGCs, does not result in the production of these novel interneuron subtypes. Additional lineage tracing analyses indicate that the ventral domain, defined by Gli1 expression, contains distinct subregions defined by expression of the Nkx2.1, Nkx6.2, and Zic transcription factors, all of which have been suggested to be Shh target genes in the developing CNS [16, 77]. While the Nkx2.1-expressing microdomain does not contribute to the generation of novel interneuron subtypes, both Nkx6.2- and Zic-expressing cells do, suggesting that additional subtle gradations in the interpretation of the Shh signal may be present within the adult germinal niche. Alternatively, the mechanisms regulating these graded responses may be established early in development and inherited in the adult. Identification of microdomain-specific transcription factors within the V-SVZ, where distinct groups of progenitors can be distinguished by their anatomical location, will also be useful in future work to determine if the Gli1-positive and – negative progenitors of the SGZ are similarly heterogeneous.
Figure 2.
Forced Activation of Shh Signaling Respecifies Neuronal Progeny. Localized dorsal injection of Ad:GFAPpCre virus (shown in green), which drives recombination in GFAP-expressing stem cells and astrocytes, was used to induce expression of a GFP reporter in combination with a conditional SmoM2 allele. Dorsal stem cells expressing only GFP primarily generate superficial granule cells (shown in blue). In contrast, expression of GFP and SmoM2 re-specifies these dorsal neural stem cells and results in most of these progenitors producing deep granule cells (shown in red), mimicking the behavior of ventral neural stem cells. This forced activation of Hh signaling in dorsal neural stem cells also results in the up-regulation of gli1 transcript in labeled neural stem cells (not shown).
Interestingly, ablation of Shh in adult ShhCreER; Shhfl mice, and loss of gli1 transcript expression in the V-SVZ, does not phenocopy postnatal loss of the Smo protein in this niche – while a shift in the types of neurons produced by the V-SVZ is observed, following Shh reduction neurogenesis is not broadly compromised. Recent studies ablating Gli family members throughout the neurogenic niche have begun to illuminate why Shh reduction and Smo loss may result in distinct phenotypes [69]. Using the mGFAP-Cre transgene, which drives recombination in astrocytes and GFAP-positive neural stem cells in the postnatal brain, Petrova and colleagues conditionally removed Smo, Gli2, Gli3, or Gli2 and Gli3 together. These experiments revealed that Gli-mediated repression is broadly required for the maintenance of normal V-SVZ neurogenesis. Similar to the results obtained with postnatal ablation of Smo in other mouse strains, mGFAP-Cre; Smofl/fl animals exhibit a loss of NSCs and a gradual reduction in V-SVZ proliferation and neurogenesis. Although loss of Gli2 or Gli3 alone does not affect V-SVZ neurogenesis, the concomitant removal of Smo and Gli3 largely rescues the phenotype of Smo loss, suggesting that Gli-mediated repression has a major role in regulating V-SVZ neurogenic activity. Further, the expression of a constitutive Gli3R, in the absence of both normal Gli2 and Gli3, phenocopies the Smo knockout phenotype, arguing that observed differences in neurogenesis between Smo loss and Gli2/3 loss are largely due to the absence of Gli3R in the latter models. A similar requirement for Gli3R was also observed in astrocytes that depend on Shh, as discussed in more detail below. Some questions remain about the potential function of each pathway member in the specification of neuronal subtypes within the niche. After ablation of Smo using the mGFAP-Cre driver, lineage tracing revealed a shift in the proportion of deep and superficial GCs present in the olfactory bulb, with fewer deep GCs and more superficial GCs produced when Smo is lost. This shift is not observed when Gli3 is ablated (either alone or together with Smo), and ablation of both Gli2 and Gli3 results in an increase in the fraction of deep GCs produced. The use of BrdU in the above studies allows labeling of a cohort of SVZ-derived neurons – however, with use of this method alone, changes in the absolute number of specific subtype of interneurons produced may be difficult to determine. Shifts in the proportions of neurons produced may occur because of respecification of new neurons derived from a particular microdomain or could be due to relative changes in proliferation of different stem cell subgroups. Specific ablation of the Gli genes using either viral microinjection or microdomain-specific Cre drivers would distinguish between these possibilities. Collectively, these results argue that a major function of Shh in the adult brain is to maintain the neurogenic niche by attenuating Gli-mediated repression.
1.4 Shh Signaling In Differentiated Astrocytes
In the juvenile and adult brain, Hh signaling also appears to have prominent functions outside the germinal niches. Shh signaling has been suggested to regulate mature astrocytes in both the normal and injured brain [69, 78–80]. In the case of the uninjured adult brain, Shh-responsive astrocytes, revealed by Gli1-nlacZ, Gli2-nlacZ, and Gli3-nlacZ reporter alleles, are present in the ventral forebrain, but also in other parts of the telencephalon including the cerebral cortex [69, 78]. The primary sources of Shh ligand in these brain regions also appear to be neuronal, as labeling using the Shh-CreER transgene identifies subgroups of labeled cells with neuronal morphology [78]. Ablation of Smo using the mGFAP-Cre driver, which targets both astrocyte-like neural stem cells and a subpopulation of mature astrocytes, results in reactive gliosis; interestingly, this effect is most prominently observed among cortical astrocytes. These cortical reactive Gli1-expressing astrocytes, but not those in more ventral structures, are present in greater numbers, have increased expression of GFAP, and exhibit cellular hypertrophy when Smo is removed [78]. As in the case of the V-SVZ, much of this phenotype appears to be dependent on attenuation of Gli3-mediated repression: mGFAP-Cre; Smofl/fl; Gli3fl/fl animals do not exhibit elevated expression of GFAP in the cortex, while adding back a repressor form of Gli3 results in the reappearance of a reactive gliosis phenotype [69]. These phenotypes suggest an ongoing dependence on a Shh signal in the adult brain to restrain astrocytes from becoming reactive. In this case, it remains unclear whether the very small number of Shh-producing cortical neurons (labeled by the Shh-CreER reporter allele) are the sole source of ligand, or whether more distant sources are also required.
1.5 Shh Signaling in the Injured Brain
In contrast to the uninjured brain, reactive astrocytes themselves have been suggested to produce Shh ligand after different types of damage, including cortical stab or freeze injuries and tumor development [80, 81]. In these cases, the ligand has been postulated to enhance, rather than inhibit the reactive glial phenotype – inflammatory factors that enter the brain after injury appear to result in an upregulation of Shh expression in reactive astrocytes [80]. In a cortical stab injury model, elevated Shh signaling has been proposed to increase the capacity of reactive astrocytes to form neurospheres in culture, although in this case the precise source of ligand after injury (and before culture) is unclear [79]. Reactive astrogliosis can be either beneficial or detrimental depending on the duration and severity of an injury and resulting inflammation [82]. It will be important in future experiments to determine the precise roles of Shh in response to injury and determine if it is beneficial, or whether it perpetuates and potentiates an inflammatory response.
Shh is also associated with oligodendrocyte production in neural development, and has recently been shown to be upregulated after focal demyelinating lesions in the adult mouse [83]. Introduction of exogenous Shh in this system, via overexpression of human Shh in V-SVZ cells, enhanced remyelination after injury. This effect might be attributable to the combination of multiple alterations observed in this system, as transduction of V-SVZ cells with Shh increases the number of proliferating oligodendrocyte precursors but also results in a reduction of reactive glia and macrophages in these lesions [83]. Interestingly, in this case, as in the normal cortex, Shh appears to act as a factor that inhibits the reactive astrocyte phenotype.
High activation of the Shh pathway can also have deleterious consequences for the normal proliferation of progenitor cells, especially in the brain, where most cells are normally quiescent. Shh signaling may abnormally increase proliferation or promote tumor cell growth. Active Shh signaling occurs in stem-like brain tumor cells and molecular subclasses of brain tumors, including pediatric brain tumors derived from cerebellar precursors and juvenile and adult gliomas [10, 25, 84–87]. Similar to its role in the normal adult neural stem cell niche, Shh may be important for maintaining the tumor-propagating “brain tumor stem cell” population. In some cases, a Shh-responsive cell may also be a brain tumor cell of origin. For example, a population of Gli1-expressing cells is present in the juvenile pons, correlating with the time in human development when pontine gliomas occur [86]. In both pontine gliomas and glioblastoma multiforme, the most common adult brain tumor, inhibition of Shh signaling via the small molecule cyclopamine inhibits the propagation of glioma cells in sphere culture and/or intracranial xenograft models [10, 85, 86]. Shh, therefore, is likely an important mitogen or self-renewal factor not only under normal conditions, but also during oncogenesis in the juvenile or postnatal brain.
1.6 Perspectives and Future Directions
Although much progress has been made in understanding the function of the Hh pathway in the adult brain, many questions still remain. In particular, while most, if not all, Shh producing cells in the adult brain under normal conditions correspond to neurons, the conditions that trigger Shh ligand release have not been identified. The finding that specific groups of neurons produce Shh raises the question of whether activation of specific neural circuits is responsible for transiently elevating levels of this ligand, and thereby influencing neuronal production in the V-SVZ or SGZ or neuronal and glial survival in other brain regions. It will be fascinating to address this unique aspect of pathway regulation using the many novel molecular and optogenetic tools that are now available to selectively ablate or activate neuronal populations of interest. Similarly, the mechanism for transport of Shh to the relevant responsive cells has not yet been defined, although evidence supporting anterograde transport within neurons exists [65–67]. Alternatively, another type of specialized contact may be at play. Recent live-imaging data in embryonic limb bud suggests that Shh-producing and –responsive cells may also contact each other through specialized filopodia termed cytonemes [88–90]. As these extensions cannot be readily detected in fixed tissue, imaging of explanted V-SVZ or SGZ tissue will be necessary to determine if these structures are also present among adult NSCs. Finally, whether the potential role of Shh pathway activation in pathological conditions is dependent on autocrine signaling, neuronally produced Shh, locally produced Shh from reactive astrocytes or other cells, or systemic sources of this ligand remains to be fully elucidated.
Shh may function either as a classical morphogen, dictating cell identity through the expression of lineage-specific transcription factors, or as a mitogen, regulating the cell cycle kinetics of progenitor cells [1, 2, 4, 26, 27, 77, 91–93]. In the adult brain, a case may be made for both of these roles: existing data implicate both Gli2/3-mediated regulation of quiescence and cell division as well as Gli1/2-mediated identity specification as active processes in the stem cell niche. In future experiments, it will be interesting to determine whether the primary function of the Shh protein is in regulation of proliferation, specification, or both in adult stem cells. A rigorous identification of the transcriptional targets of this pathway in the neural stem cell niches will likely be central to answering this question. Although Hedgehog signaling is a major driver for the development of many organs, the many ways this pathway is repurposed in the adult brain are only now being revealed, and represent a fascinating puzzle for future investigation.
Highlights.
Hedgehog signaling is required to establish adult neural stem cell niches
Modulation of Hedgehog signaling regulates adult stem cell maintenance and identity
Sonic Hedgehog ligand in the adult brain is produced by mature neurons
Some mature astrocytes are also Hedgehog-responsive
Hedgehog signaling is often upregulated after acute brain injury
Acknowledgments
We thank C.C. Harwell and all the members of the Álvarez-Buylla and Ihrie labs for helpful discussions and critical feedback. We apologize to colleagues whose work could not be discussed in detail due to space constraints. A. A-B. is the is the Heather and Melanie Muss Endowed Chair of Neurological Surgery at UCSF. Work in the Álvarez-Buylla laboratory is supported by grants from the US National Institutes of Health, and work in the Ihrie laboratory is supported by grants from an American Cancer Society Institutional Research Grant, Vanderbilt-Ingram Cancer Center Young Ambassadors, and the Tuberous Sclerosis Alliance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ribes V, Briscoe J. Establishing and Interpreting Graded Sonic Hedgehog Signaling during Vertebrate Neural Tube Patterning: The Role of Negative Feedback. Cold Spring Harbor Perspect Biol. 2009;1:a002014. doi: 10.1101/cshperspect.a002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dessaud E, McMahon AP, Briscoe J. Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development. 2008;135:2489–503. doi: 10.1242/dev.009324. [DOI] [PubMed] [Google Scholar]
- [3].Hatton BA, Knoepfler PS, Kenney AM, Rowitch DH, de Alboran IM, Olson JM, et al. N-myc is an essential downstream effector of Shh signaling during both normal and neoplastic cerebellar growth. Cancer Res. 2006;66:8655–61. doi: 10.1158/0008-5472.CAN-06-1621. [DOI] [PubMed] [Google Scholar]
- [4].Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–83. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- [5].McMahon AP, Ingham PW, Tabin CJ. Developmental roles and clinical significance of hedgehog signaling. Curr Top Dev Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- [6].Ho KS, Scott MP. Sonic hedgehog in the nervous system: functions, modifications and mechanisms. Curr Opin Neurobiol. 2002;12:57–63. doi: 10.1016/s0959-4388(02)00290-8. [DOI] [PubMed] [Google Scholar]
- [7].Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–6. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- [8].Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. doi: 10.1038/nature03994. [DOI] [PubMed] [Google Scholar]
- [9].Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39:937–50. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- [10].Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–72. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, et al. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–44. doi: 10.1242/dev.01567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang C, Ruther U, Wang B. The Shh-independent activator function of the full-length Gli3 protein and its role in vertebrate limb digit patterning. Dev Biol. 2007;305:460–9. doi: 10.1016/j.ydbio.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bowers M, Eng L, Lao Z, Turnbull RK, Bao X, Riedel E, et al. Limb anterior-posterior polarity integrates activator and repressor functions of GLI2 as well as GLI3. Dev Biol. 2012;370:110–24. doi: 10.1016/j.ydbio.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oosterveen T, Kurdija S, Enstero M, Uhde CW, Bergsland M, Sandberg M, et al. SoxB1-driven transcriptional network underlies neural-specific interpretation of morphogen signals. Proc Natl Acad Sci U S A. 2013;110:7330–5. doi: 10.1073/pnas.1220010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cohen M, Briscoe J, Blassberg R. Morphogen interpretation: the transcriptional logic of neural tube patterning. Curr Opin Genet Dev. 2013;23:423–8. doi: 10.1016/j.gde.2013.04.003. [DOI] [PubMed] [Google Scholar]
- [16].Peterson KA, Nishi Y, Ma W, Vedenko A, Shokri L, Zhang X, et al. Neural-specific Sox2 input and differential Gli-binding affinity provide context and positional information in Shh-directed neural patterning. Genes Dev. 2012;26:2802–16. doi: 10.1101/gad.207142.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee EY, Ji H, Ouyang Z, Zhou B, Ma W, Vokes SA, et al. Hedgehog pathway-regulated gene networks in cerebellum development and tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:9736–41. doi: 10.1073/pnas.1004602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic hedgehog signaling confers ventral telencephalic progenitors with distinct cortical interneuron fates. Neuron. 2010;65:328–40. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Himmelstein DS, Bi C, Clark BS, Bai B, Kohtz JD. Balanced Shh signaling is required for proper formation and maintenance of dorsal telencephalic midline structures. BMC Dev Biol. 2010;10:118. doi: 10.1186/1471-213X-10-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vokes SA, Ji H, Wong WH, McMahon AP. A genome-scale analysis of the cis-regulatory circuitry underlying sonic hedgehog-mediated patterning of the mammalian limb. Genes Dev. 2008;22:2651–63. doi: 10.1101/gad.1693008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Alvarez-Medina R, Cayuso J, Okubo T, Takada S, Marti E. Wnt canonical pathway restricts graded Shh/Gli patterning activity through the regulation of Gli3 expression. Development. 2008;135:237–47. doi: 10.1242/dev.012054. [DOI] [PubMed] [Google Scholar]
- [22].Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132:4987–98. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- [23].Gulacsi A, Anderson SA. Shh maintains Nkx2.1 in the MGE by a Gli3-independent mechanism. Cereb Cortex. 2006;16(Suppl 1):i89–95. doi: 10.1093/cercor/bhk018. [DOI] [PubMed] [Google Scholar]
- [24].Fleming JT, He W, Hao C, Ketova T, Pan FC, Wright CC, et al. The Purkinje neuron acts as a central regulator of spatially and functionally distinct cerebellar precursors. Developmental cell. 2013;27:278–92. doi: 10.1016/j.devcel.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Schuller U, Heine VM, Mao J, Kho AT, Dillon AK, Han YG, et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 2008;14:123–34. doi: 10.1016/j.ccr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kenney AM, Cole MD, Rowitch DH. Nmyc upregulation by sonic hedgehog signaling promotes proliferation in developing cerebellar granule neuron precursors. Development. 2003;130:15–28. doi: 10.1242/dev.00182. [DOI] [PubMed] [Google Scholar]
- [27].Kenney AM, Rowitch DH. Sonic hedgehog promotes G(1) cyclin expression and sustained cell cycle progression in mammalian neuronal precursors. Mol Cell Biol. 2000;20:9055–67. doi: 10.1128/mcb.20.23.9055-9067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goodrich LV, Milenkovic L, Higgins KM, Scott MP. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–13. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- [29].Wilson SL, Wilson JP, Wang C, Wang B, McConnell SK. Primary cilia and Gli3 activity regulate cerebral cortical size. Dev Neurobiol. 2012;72:1196–212. doi: 10.1002/dneu.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang H, Ge G, Uchida Y, Luu B, Ahn S. Gli3 is required for maintenance and fate specification of cortical progenitors. J Neurosci. 2011;31:6440–8. doi: 10.1523/JNEUROSCI.4892-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harwell CC, Parker PR, Gee SM, Okada A, McConnell SK, Kreitzer AC, et al. Sonic hedgehog expression in corticofugal projection neurons directs cortical microcircuit formation. Neuron. 2012;73:1116–26. doi: 10.1016/j.neuron.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;62:349–62. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- [33].Suh H, Deng W, Gage FH. Signaling in adult neurogenesis. Annu Rev Cell Dev Biol. 2009;25:253–75. doi: 10.1146/annurev.cellbio.042308.113256. [DOI] [PubMed] [Google Scholar]
- [34].Ihrie RA, Alvarez-Buylla A. Cells in the astroglial lineage are neural stem cells. Cell Tissue Res. 2008;331:179–91. doi: 10.1007/s00441-007-0461-z. [DOI] [PubMed] [Google Scholar]
- [35].Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- [36].Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–8. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–86. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–89. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- [40].Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–8. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- [41].Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–16. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- [42].Doetsch F, Garcia-Verdugo JM, Alvarez-Buylla A. Regeneration of a germinal layer in the adult mammalian brain. ProcNatlAcadSciUSA. 1999;96:11619–24. doi: 10.1073/pnas.96.20.11619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–8. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Merkle FT, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci U S A. 2004;101:17528–32. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- [46].Seri B, Garcia-Verdugo JM, Collado-Morente L, McEwen BS, Alvarez-Buylla A. Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J Comp Neurol. 2004;478:359–78. doi: 10.1002/cne.20288. [DOI] [PubMed] [Google Scholar]
- [47].Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- [48].Breunig JJ, Haydar TF, Rakic P. Neural stem cells: historical perspective and future prospects. Neuron. 2011;70:614–25. doi: 10.1016/j.neuron.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kriegstein A, Alvarez-Buylla A. The glial nature of embryonic and adult neural stem cells. Annu Rev Neurosci. 2009;32:149–84. doi: 10.1146/annurev.neuro.051508.135600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Li G, Fang L, Fernandez G, Pleasure SJ. The ventral hippocampus is the embryonic origin for adult neural stem cells in the dentate gyrus. Neuron. 2013;78:658–72. doi: 10.1016/j.neuron.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- [52].Balordi F, Fishell G. Hedgehog signaling in the subventricular zone is required for both the maintenance of stem cells and the migration of newborn neurons. J Neurosci. 2007;27:5936–47. doi: 10.1523/JNEUROSCI.1040-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Balordi F, Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007;27:14248–59. doi: 10.1523/JNEUROSCI.4531-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Han YG, Spassky N, Romaguera-Ros M, Garcia-Verdugo JM, Aguilar A, Schneider-Maunoury S, et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci. 2008;11:277–84. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- [55].Breunig JJ, Sarkisian MR, Arellano JI, Morozov YM, Ayoub AE, Sojitra S, et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA. 2008:13127–32. doi: 10.1073/pnas.0804558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Lehtinen MK, Zappaterra MW, Chen X, Yang YJ, Hill AD, Lun M, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lim DA, TA D, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin Antagonizes BMP Signaling to Create a Niche for Adult Neurogenesis. Neuron. 2000;28:713–26. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- [58].Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–32. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- [59].Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A, Ruat M. Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci. 2002;16:2351–7. doi: 10.1046/j.1460-9568.2002.02412.x. [DOI] [PubMed] [Google Scholar]
- [60].Charytoniuk D, Porcel B, Rodriguez Gomez J, Faure H, Ruat M, Traiffort E. Sonic Hedgehog signalling in the developing and adult brain. J Physiol Paris. 2002;96:9–16. doi: 10.1016/s0928-4257(01)00075-4. [DOI] [PubMed] [Google Scholar]
- [61].Traiffort E, Charytoniuk D, Watroba L, Faure H, Sales N, Ruat M. Discrete localizations of hedgehog signalling components in the developing and adult rat nervous system. Eur J Neurosci. 1999;11:3199–214. doi: 10.1046/j.1460-9568.1999.00777.x. [DOI] [PubMed] [Google Scholar]
- [62].Traiffort E, Charytoniuk DA, Faure H, Ruat M. Regional distribution of Sonic Hedgehog, patched, and smoothened mRNA in the adult rat brain. J Neurochem. 1998;70:1327–30. doi: 10.1046/j.1471-4159.1998.70031327.x. [DOI] [PubMed] [Google Scholar]
- [63].Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–7. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- [64].Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–62. doi: 10.1016/j.neuron.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Traiffort E, Moya KL, Faure H, Hassig R, Ruat M. High expression and anterograde axonal transport of aminoterminal sonic hedgehog in the adult hamster brain. Eur J Neurosci. 2001;14:839–50. doi: 10.1046/j.0953-816x.2001.01708.x. [DOI] [PubMed] [Google Scholar]
- [66].Wallace VA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126:2901–9. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- [67].Dakubo GD, Beug ST, Mazerolle CJ, Thurig S, Wang Y, Wallace VA. Control of glial precursor cell development in the mouse optic nerve by sonic hedgehog from retinal ganglion cells. Brain research. 2008;1228:27–42. doi: 10.1016/j.brainres.2008.06.058. [DOI] [PubMed] [Google Scholar]
- [68].Gonzalez-Reyes LE, Verbitsky M, Blesa J, Jackson-Lewis V, Paredes D, Tillack K, et al. Sonic hedgehog maintains cellular and neurochemical homeostasis in the adult nigrostriatal circuit. Neuron. 2012;75:306–19. doi: 10.1016/j.neuron.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Petrova R, Garcia AD, Joyner AL. Titration of GLI3 repressor activity by sonic hedgehog signaling is critical for maintaining multiple adult neural stem cell and astrocyte functions. J Neurosci. 2013;33:17490–505. doi: 10.1523/JNEUROSCI.2042-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Merkle FT, Fuentealba LC, Sanders TA, Magno L, Kessaris N, Alvarez-Buylla A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat Neurosci. 2014;17:207–14. doi: 10.1038/nn.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–4. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- [72].Lopez-Juarez A, Howard J, Ullom K, Howard L, Grande A, Pardo A, et al. Gsx2 controls region-specific activation of neural stem cells and injury-induced neurogenesis in the adult subventricular zone. Genes Dev. 2013;27:1272–87. doi: 10.1101/gad.217539.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Azim K, Fiorelli R, Zweifel S, Hurtado-Chong A, Yoshikawa K, Slomianka L, et al. 3-dimensional examination of the adult mouse subventricular zone reveals lineage-specific microdomains. PLoS One. 2012;7:e49087. doi: 10.1371/journal.pone.0049087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Young KM, Fogarty M, Kessaris N, Richardson WD. Subventricular zone stem cells are heterogeneous with respect to their embryonic origins and neurogenic fates in the adult olfactory bulb. J Neurosci. 2007;27:8286–96. doi: 10.1523/JNEUROSCI.0476-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kohwi M, Osumi N, Rubenstein JL, Alvarez-Buylla A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J Neurosci. 2005;25:6997–7003. doi: 10.1523/JNEUROSCI.1435-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kelsch W, Mosley CP, Lin CW, Lois C. Distinct mammalian precursors are committed to generate neurons with defined dendritic projection patterns. PLoS Biol. 2007;5:e300. doi: 10.1371/journal.pbio.0050300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Vokes SA, Ji H, McCuine S, Tenzen T, Giles S, Zhong S, et al. Genomic characterization of Gli-activator targets in sonic hedgehog-mediated neural patterning. Development. 2007;134:1977–89. doi: 10.1242/dev.001966. [DOI] [PubMed] [Google Scholar]
- [78].Garcia AD, Petrova R, Eng L, Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–608. doi: 10.1523/JNEUROSCI.0830-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, et al. Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog. Cell Stem Cell. 2013;12:426–39. doi: 10.1016/j.stem.2013.01.019. corrected. [DOI] [PubMed] [Google Scholar]
- [80].Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29:10299–308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Becher OJ, Hambardzumyan D, Fomchenko EI, Momota H, Mainwaring L, Bleau AM, et al. Gli activity correlates with tumor grade in platelet-derived growth factor-induced gliomas. Cancer Res. 2008;68:2241–9. doi: 10.1158/0008-5472.CAN-07-6350. [DOI] [PubMed] [Google Scholar]
- [82].Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ferent J, Zimmer C, Durbec P, Ruat M, Traiffort E. Sonic Hedgehog signaling is a positive oligodendrocyte regulator during demyelination. J Neurosci. 2013;33:1759–72. doi: 10.1523/JNEUROSCI.3334-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gruber Filbin M, Dabral SK, Pazyra-Murphy MF, Ramkissoon S, Kung AL, Pak E, et al. Coordinate activation of Shh and PI3K signaling in PTEN-deficient glioblastoma: new therapeutic opportunities. Nature medicine. 2013;19:1518–23. doi: 10.1038/nm.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the Hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009;28:3468–76. doi: 10.1038/onc.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Monje M, Mitra SS, Freret ME, Raveh TB, Kim J, Masek M, et al. Hedgehog-responsive candidate cell of origin for diffuse intrinsic pontine glioma. Proc Natl Acad Sci U S A. 2011;108:4453–8. doi: 10.1073/pnas.1101657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gibson P, Tong Y, Robinson G, Thompson MC, Currle DS, Eden C, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–9. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–32. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development. 2014;141:729–36. doi: 10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kornberg TB. Cytonemes extend their reach. The EMBO journal. 2013;32:1658–9. doi: 10.1038/emboj.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb Symp Quant Biol. 1997;62:451–66. [PubMed] [Google Scholar]
- [92].Cayuso J, Ulloa F, Cox B, Briscoe J, Marti E. The Sonic hedgehog pathway independently controls the patterning, proliferation and survival of neuroepithelial cells by regulating Gli activity. Development. 2006;133:517–28. doi: 10.1242/dev.02228. [DOI] [PubMed] [Google Scholar]
- [93].Persson M, Stamataki D, te Welscher P, Andersson E, Bose J, Ruther U, et al. Dorsal ventral patterning of the spinal cord requires Gli3 transcriptional repressor activity. Genes Dev. 2002;16:2865–78. doi: 10.1101/gad.243402. [DOI] [PMC free article] [PubMed] [Google Scholar]