Abstract
The pigmentation of skin and hair in mammals is driven by the creation within melanocytes of melanosomes, a specialized pigment-producing organelle, and the subsequent intercellular transfer of this organelle to keratinocytes. This latter process is absolutely required for visible pigmentation and effective photo-protection because it serves to disperse the pigment in skin and hair. Therefore, the transfer of melanosomes from the melanocyte to the keratinocyte is as important for the biological endpoint of mammalian pigmentation as the biogenesis of this fascinating organelle. Here we review new findings that shed light on, and raise additional questions about, the mechanism of this enigmatic process.
Introduction
Skin and hair are comprised almost entirely of keratinocytes, which are created continuously from stem cells present at the base of the epidermis and hair follicle to drive skin replenishment and hair growth [1–5]. Along the way, keratinocytes inherit pigment from a relatively small cohort of pigment-producing cells called melanocytes. The principal role of the melanocyte is to produce the pigment within melanosomes and to transfer these organelles to keratinocytes. For the relatively small number of melanocytes to accomplish this feat, they use long dendrites to reach out and contact many passing keratinocytes (one epidermal melanocyte feeds pigment to upwards of 40 keratinoctyes). Effective transfer also requires that the melanocyte transport its melanosomes from their site of formation in the cell center to their principal site of transfer at dendritic tips. Melanocytes accomplish this feat by coupling long-range, bidirectional microtubule-dependent transport of melanosomes along the length of dendrites with the capture and local movement of the organelles in actin-rich dendritic tips by the actin-based motor protein myosin Va [6]. Finally, the melanosomes accumulated at dendritic tips by this cooperative: capture mechanism are transferred out of the melanocyte and into the keratinocyte. This serves to distribute pigment in the hair and skin so that the animal appears pigmented. Once inside the keratinocyte, the organelles are moved to a position over top of the nucleus to prevent harmful portions of sunlight from inducing mutations in nuclear DNA, which can lead to skin cancer. Melanosome transfer is essential, therefore, not only for visible pigmentation, but for photo protection as well.
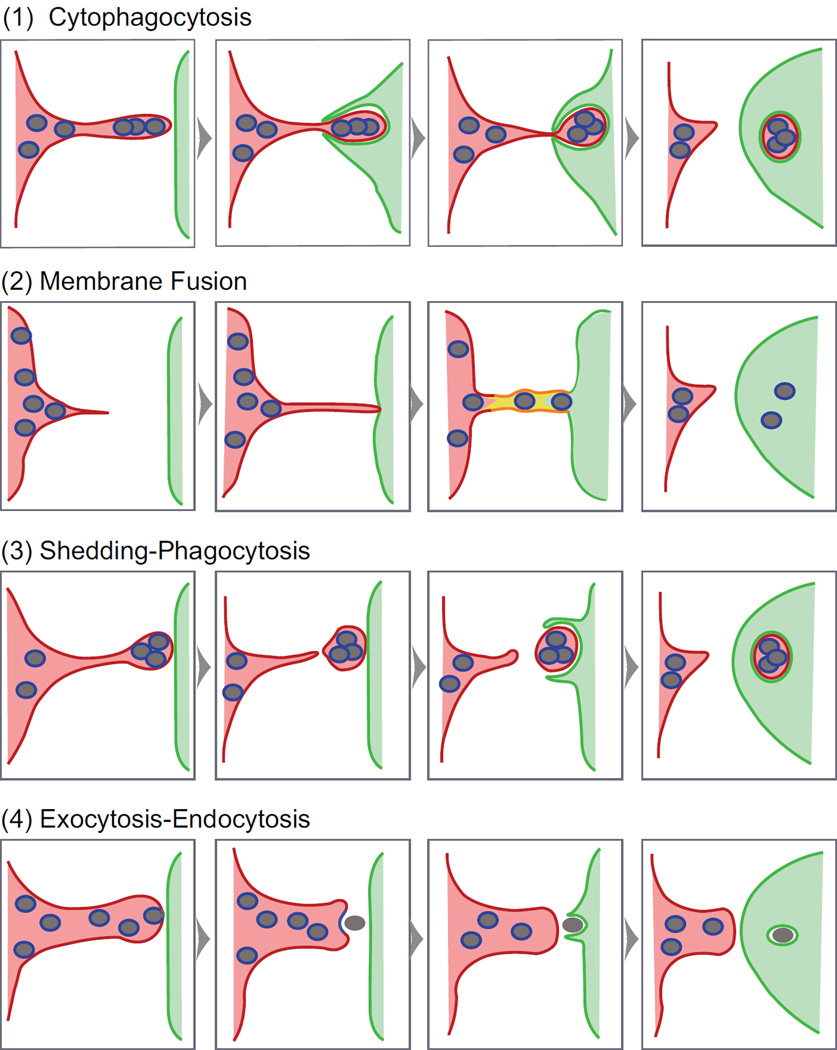
The last comprehensive review on melanosome transfer [7] described four possible transfer mechanisms (Mechanisms 1–4 in Figure 1), which were supported to various degrees by prior experimental work (please see that review for older supporting references). In Mechanism 1 (the Cytophagocytosis model), the keratinocyte phagocytizes the melanocyte’s melanosome-rich dendritic tip. In Mechanism 2 (the Membrane Fusion model), the melanosome moves through a transient membrane conduit connecting the cytoplasm’s of the melanocyte and keratinocyte. In Mechanism 3 (the Shedding-Phagocytosis model), the melanocyte sheds plasma membrane-enclosed, melanosome-rich packages that are subsequently internalized by the keratinocyte via phagocytosis. Finally, in Mechanism 4 (the Exocytosis-Endocytosis model), the melanocyte releases the melanosome’s melanin core into the extracellular space via exocytosis, and the keratinocyte then internalizes this “melanocore” via phagocytosis.
Figure 1. Melanosome transfer mechanisms.
Shown are four possible mechanisms of intercellular melanosome transfer. The cartoon, which is based off of a simpler cartoon in [7], uses color coding to mark the identity of the three membranes that are relevant to the characterization of transfer mechanisms: the limiting membrane of the melanosome (blue), the plasma membrane of the melanocyte (red) and the plasma membrane of the keratinocyte (green) (the yellow in Mechanism 2 indicates a mixture of these latter two membranes). Filopodia on the surface of the melanocyte could give rise to the intercellular bridge connecting the cells in Mechanism 2 [7, 10]. Importantly, three of the four mechanisms (Mechanisms 1, 3 and 4) employ phagocytosis by the keratinocyte to complete the transfer process. This step requires the expression on the surface of the keratinoctye of the protease activated receptor Par2 (see [27] for review). We note, however, that phagocytic extensions typically cannot “bite through” the object being engulfed [39], arguing against Mechanism 1 (see the text for other reservations regarding this mechanism).
While little has been published over the last ten years to strengthen support for Mechanisms 1 and 2, several recent studies have provided very strong support for Mechanisms 3 and 4. Here we describe the experiments supporting these two distinct pathways, discuss the underlying molecular mechanisms thought to drive and regulate them, highlight the strengths and limitations of each study, and suggest future experiments that might resolve remaining questions regarding the mechanism of intercellular melanosome transfer.
The shedding mechanism
Two important papers published in 2012 provided support for Mechanism 3. In the first paper, Ando and colleagues [8] •• used scanning and transmission electron microscopy (EM) of human melanocyte/keratinocyte co-cultures to demonstrate the presence of melanosome-rich protrusions or “globules” on the surface of melanocyte dendrites and filopodia. Similar globules were also seen attached to microvilli on adjacent keratinocytes, and what appeared to be remnants of these globules were observed inside keratinocytes. Based on these EM snapshots, the authors argued that transfer is driven by a multi-step process in which the melanocyte packages melanosomes into protrusions or “globules” on its surface and then sheds these globules. The released globules are subsequently captured by keratinocytes and phagocytized, completing the transfer process (for review, see [9]). While the still images presented by Ando et al [8]•• are certainly consistent with Mechanism 3, as well as with previous studies linking filopodia to melanosome transfer [10] [11] • [12], the complete lack of dynamic imaging significantly limits the strength of their argument.
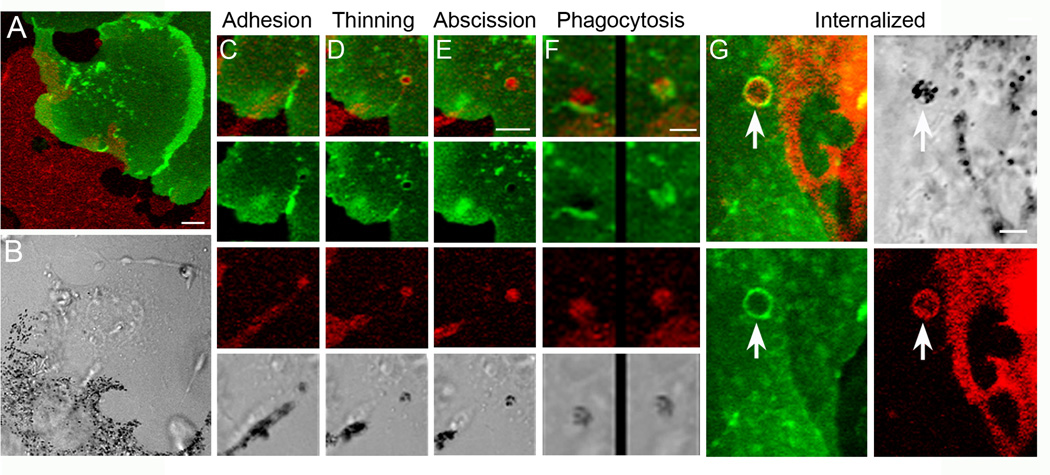
In the second paper, Wu et al [13] •• used primary co cultures prepared from a transgenic mouse (the “Holly” mouse) that yields melanocytes and keratinocytes with red and green plasma membranes, respectively (Figure 2). This allowed a clear understanding of exactly where the black melanosomes were in the transfer process. Using time lapse imaging, they then observed a multi-step transfer process that involved adhesion between the melanosome-rich tip of the melanocyte’s dendrite and the keratinocyte, the thinning of the dendrite behind the attached tip, and the shedding from the tip of a plasma membrane-enclosed package containing multiple melanosomes. The shed package then resides on the surface of the keratinocyte for a variable period of time, at which point it is internalized by phagocytosis, completing the transfer process. Importantly, nascent internalized packages exhibit multiple membrane profiles consistent with Mechanism 3 (Figures 1 and 2). The main strength of this study is that the transfer process was visualized in real time and using techniques that allowed unequivocal interpretation of the images. The main limitation of this study is that the transfer process was not examined in living tissue, leaving open the possibility that Mechanism 3 is not physiologically relevant.
Figure 2. Time lapse imaging of “Holly” mice co cultures reveals the Shedding-Phagocytosis mechanism.
Panel A shows an image of a red melanocyte and a green keratinocyte in a primary skin culture prepared from a Holly mouse. Black melanosomes are easily visible in the transmitted light image in Panel B. Panels C–F show selected video frames that correspond to the four phases of the Shedding-Phagocytosis mechanism: adhesion of the tip of the melanocyte’s dendrite to the surface of the keratinocyte (Panel C), thinning of the dendrite behind the adhered tip (Panel D), package formation by apparent self-abscission (Panel E), and phagocytosis of the package by the keratinocyte (Panel F: two sequential video frames are shown) (in each case, the overlay of the green and red images is shown at the top, and the separate green, red and transmitted light images are shown underneath; note that Panel F is a higher magnification than Panels C–E). Panel G shows the membrane profiles of a shed package that has been internalized by a keratinocyte. An overlay of the green and red images is shown in the upper left, and separate green, red and transmitted light images are shown in the remainder of the panel. Mag bars are 5 μm (A and B), 3.8 μm (C–E), 2 μm (F), and 2.9 μm (G). Reproduced with permission from the Proceedings of the National Academy of Sciences [13] ••.
In terms of the contributions made by the melanocyte and the keratinocyte to shedding-based transfer, Wu and colleagues [13] •• argued from various aspects of their dynamic imaging that the package is generated by a self-abscission event, i.e. the melanocyte cleaves itself to create the package. That said, they did not observe shedding in the absence of keratinocytes, indicating an important role for the receiving cell. This later observation disagrees with Ando and colleagues, who found packages/globules in the media of pure human melanocyte cultures [14]. Further complicating matters, Wu et al [13] •• did not observe shedding in time lapse movies of similarly-prepared pure human melanocyte cultures. Future studies are clearly needed to clarify the roles played by the melanocyte and the keratinocyte in shedding-based transfer.
With regard to the underlying mechanism driving shedding, Wu et al [13] •• suggest that the apparent self-abscission event could be driven by the ESCRT complex, which drives similar membrane rearrangements during MVB formation, HIV budding, and abscission at the end of cell division [15]. While previous studies have identified a link between the ESCRT complex and melanosome biogenesis [16, 17], it should be noted that humans with mutations in the ESCRT III isoforms CHMP1A [18] or CHMP2B [19] do not exhibit pigment dilution.
Wu and colleagues [13] ••also provided insight into the regulation of shedding by studying dilute suppressor (dsu) [20] [21]. On a black background, dilute (myosin Va null) mice appear grey. This defect results from a reduction in the amount of pigment transferred to keratinocytes, which in turn results from a defect in the distribution of melanosomes inside dilute melanocytes-rather than being concentrated at dendritic tips, the preferred site of intercellular transfer, the organelles are concentrated in the cell center [6]. Importantly, the coat of the dilute mouse can be restored to black by making the mouse homozygous for a functional null allele at the dsu locus, which encodes the protein melanoregulin. Surprisingly, while the color of the dilute/dsu mouse’s coat is rescued, the defect in melanosome distribution within its melanocytes is not. To solve this mystery, Wu and colleagues [13] •• examined cell boundaries and pigment distributions in the ear skin of dilute and dilute/dsu mice. What the images suggested was that the melanosomes concentrated in the center of dilute/dsu melanocytes, but not those concentrated in the center of dilute melanocytes, are undergoing robust transfer to surrounding keratinocytes. Given this result and the fact that it is the loss of expression of melanoregulin that rescues the dilute mouse, Wu and colleagues [13] •• hypothesized that melanoregulin serves as a negative regulator of shedding-based melanosome transfer. This hypothesis was then confirmed by time lapse imaging of primary cultures from dilute and dilute/dsu mice. Specifically, while melanocytes in both cultures shed melanosome-rich packages from their central cytoplasm, the rate was six-fold higher in dilute/dsu cultures, completely consistent with the in situ data. Finally, additional data showed that melanoregulin also negatively regulates melanosome transfer in otherwise wild type mice. How melanoregulin, which is concentrated in the limiting membrane of the melanosome [22, 23], actually regulates shedding is currently unknown.
Coupled exocytosis/endocytosis of melanocores
The recent paper from the Seabra lab [24] •• supporting Mechanism 4 was predicated on two considerations. First, the different transfer mechanisms predict different membrane profiles surrounding melanosome pigment cores immediately following their transfer to the keratinocyte (see color coding in Figure 1). Specifically, Mechanisms 1 (Cytophagocytosis) and 3 (Shedding-Phagocytosis) should both yield pigment cores that are surrounded by multiple membranes: the melanosome membrane, the remnants of the melanocyte’s plasma membrane, and the phagosomal membrane derived from the keratinocyte’s plasma membrane. In contrast, Mechanism 2 (Membrane Fusion) should yield pigment cores surrounded by just one membrane, the melansome’s membrane. Finally, Mechanism 4 (Exocytosis-Endocytosis) should also yield pigment cores surrounded by just one membrane, but in this case it would be the phagosomal membrane. The second consideration is that only serial sectioning allows firm conclusions to be drawn from ultrastructural data regarding membrane profiles surrounding pigment cores following transfer [7]. Indeed, the single-section EMs used as the primary support for Mechanism 1 (the Cytophagocytosis model) are quite problematic for this reason (see [7] for references and further discussion).
Guided by these considerations, Seabra and colleagues [24] •• used serial section transmission EM to obtain three results in human skin that support Mechanism 4 (Figures 1 and 3). First, they observed naked melanin cores in the extracellular space between melanocytes and keratinocytes. Second, the large majority of melanin cores inside keratinocytes were surrounded by a single membrane. Third, both confocal microscopy and cryo-immuno-EM showed that TYRP1, which is highly enriched in the limiting membrane of melanosomes inside melanocytes, is difficult to detect on melanin cores inside keratinocytes (although see [25]). Similarly, immuno-EM showed that LAMP1, another melanosome membrane marker, is “left behind” following transfer from mouse melanocytes to human keratinocytes in co culture. These later results are only consistent with Mechanism 4 (Figure 1).
Figure 3. Serial section and immuno-electron microscopy of human skin support the Exocytosis-Endocytosis mechanism.
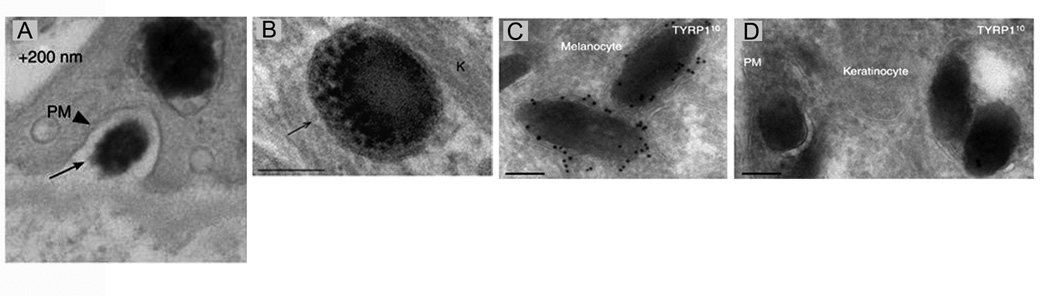
Panel A shows an image of a melanocore in the extracellular space between a melanocyte and a keratinocyte (this image is from a series of consecutive sections that together prove this melanocore is extracellular). Panel B shows the single membrane surrounding a melanocore inside a keratinocyte. Panel C shows the extensive immunogold labeling of a two melanosomes inside a melanocyte using an antibody to TYRP1, a glycoprotein present in the limiting membrane of the melanosome. Panel D shows the minimal immunogold labeling for TYRP1 on several melanocores inside a keratinocyte. Together, Panels C and D argue that the limiting membrane of the melanosome is “left behind” during transfer, consistent with Mechanism 4. Reproduced with permission from the Journal of Investigative Dermatology [24] ••.
While the EM results presented by Seabra and colleagues [24] •• are compelling, especially given that they were obtained from real tissue, they do suffer from the limitations inherent in using static images to understand a dynamic process. For example, any compartment that survives for only a short period of time immediately following transfer may be largely missing from static EM images, especially given that melanosome transfer is relatively infrequent, while the aftermath (pigment inside the keratinocyte) is long-lived. One such missing compartment could be nascent phagosomes inside the keratinocyte, where contents (e.g. melanosome membrane markers and multiple melanocyte-derived membranes) might be subject to rapid degradation following fusion of the nascent phagosome with a lysosome soon after endocytosis. If such a short-lived compartment exists, it would be largely invisible for kinetic reasons in static EMs and would undermine support for Mechanism 4.
With regard to the underlying machinery supporting Mechanism 4, the coupled exocytosis and endocytosis of melanocores would presumably be driven by conventional pathways of secretion (i.e. employing Rab GTPases, tethering factors, Snares, etc.) [26] and phagocytosis [27]. Rather than focus on these components, Seabra and colleagues explored the roles of Rab27a and Rab11b in the transfer process using their co culture system. The first result was that Rab27a does not play an obvious role in the keratinocyte-induced exocytosis of melanin from melanocytes, as gauged by measuring the amount of pigment accumulated in the media over seven days in control and Rab27a-knockdown cultures. Similarly, melanosome exocytosis in co cultures containing melan-ash melanocytes, an immortal melanocyte cell line derived from ashen (Rab27a null) mice, was normal. We agree with the authors (see the Discussion in their paper) that these results should be interpreted cautiously given the extensive cell biological, biochemical, and genetic data from ashen mice indicating that this Rab is required for proper melanosome distribution and effective animal pigmentation [28–32] [33] •.
The second result was that Rab11b is required for robust melanosome transfer in co culture, as a ~50% reduction in Rab11b mRNA levels reduced both keratinocyte-induced melanin exocytosis and subsequent melanin up take by the keratinocyte by ~50%. As a segway to eventually understanding why this is the case, the authors showed that Rab11b-positive vesicles are often in close proximity to end-stage melanosomes. These results are interesting, especially given previous reports linking Rab11-positive compartments to exocytosis in melanocytes [34] • and T cells [35]. That said, Rab11b plays an important role in cargo transport to melanosomes [17], clouding the issue to some extent. Moreover, Rab11b plays important roles in regulating the recycling endosome [36], an essential membrane trafficking pathway present in all cell types. Interference in such pathways can have pleiotropic effects on cells.
Conclusion
Given the strong support for Mechanism 3 provided by Ando et al [8] •• and Wu et al [13] ••, and the strong support for Mechanism 4 provided by Tarafder et al [24] ••, the question now is who is right! One possibility is that melanosome transfer is driven by different mechanisms in mice (Mechanism 3) and humans (Mechanism 4), although the data in Ando el al [8] •• using human melanocytes does not support this idea. It is certainly possible that melanosome transfer is accomplished by multiple mechanisms in both mice and humans, and that the choice of mechanism may depend on skin type (e.g. dark versus light skin) [37] and/or melanocyte location (epidermal versus follicular) [38]. One tool that would aid in resolving the mechanism of melanosome transfer would be the availability of one or more mouse coat color mutants exhibiting specific defects in melanosome transfer. Indeed, the numerous mouse coat color mutants affecting pigment synthesis, melanosome biogenesis, or melanosome transport/distribution have been extremely valuable in unraveling these complex processes. Surprisingly, coat color mutants defective specifically in the transfer process have not been identified, perhaps because such mutants are not viable. The second tool would be the visualization of the transfer process in real time in living skin, as this should identify in unequivocal fashion the physiologically relevant mechanism(s) of melanosome transfer. This approach should be feasible in the mouse using the transgenic animal described by Wu et al [13] ••. Stay tuned.
Highlights.
The transfer of melanosomes from melanocytes to keratinocytes drives visible pigmentation.
Two recent studies support a shedding-phagocytosis mechanism of transfer.
Another study links coupled exocytosis-endocytosis of the melanosome core to transfer.
Multiple mechanisms may support intercellular melanosome transfer.
Real-time imaging of transfer in living tissue should clarify the mechanism(s) involved.
Acknowledgements
We thank Mickey Marks for his comments on the manuscript. We apologize to those whose work we could not cite due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kondo T, Hearing VJ. Update on the regulation of mammalian melanocyte function and skin pigmentation. Expert Rev Dermatol. 2011;6(1):97–108. doi: 10.1586/edm.10.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sitaram A, Marks MS. Mechanisms of protein delivery to melanosomes in pigment cells. Physiology (Bethesda) 2012;27(2):85–99. doi: 10.1152/physiol.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marks MS, Heijnen HF, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25(4):495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hume AN, Seabra MC. Melanosomes on the move: a model to understand organelle dynamics. Biochem Soc Trans. 2011;39(5):1191–1196. doi: 10.1042/BST0391191. [DOI] [PubMed] [Google Scholar]
- 5.Ohbayashi N, Fukuda M. Role of Rab family GTPases and their effectors in melanosomal logistics. J Biochem. 2012;151(4):343–351. doi: 10.1093/jb/mvs009. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, et al. Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143(7):1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Den Bossche K, Naeyaert JM, Lambert J. The quest for the mechanism of melanin transfer. Traffic. 2006;7(7):769–778. doi: 10.1111/j.1600-0854.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 8. Ando H, et al. Melanosomes are transferred from melanocytes to keratinocytes through the processes of packaging, release, uptake, and dispersion. J Invest Dermatol. 2012;132(4):1222–1229. doi: 10.1038/jid.2011.413. This paper used scanning and electron microcopy of human melanocyte/keratinocyte co cultures to identify melanosome-rich globules on the surface of melanocyte dendrites and filopodia. Other images are consistent with these globules being shed by the melanocyte and then taken up by keratinocytes via phagocytosis. Together, the images support the Shedding-Phagocytosis pathway of melanosome transfer.
- 9.Scott G. Demonstration of melanosome transfer by a shedding microvesicle mechanism. J Invest Dermatol. 2012;132(4):1073–1074. doi: 10.1038/jid.2012.20. [DOI] [PubMed] [Google Scholar]
- 10.Scott G, et al. Filopodia are conduits for melanosome transfer to keratinocytes. J Cell Sci. 2002;115(Pt 7):1441–1451. doi: 10.1242/jcs.115.7.1441. [DOI] [PubMed] [Google Scholar]
- 11. Singh SK, et al. Melanin transfer in human skin cells is mediated by filopodia--a model for homotypic and heterotypic lysosome-related organelle transfer. FASEB J. 2010;24(10):3756–3769. doi: 10.1096/fj.10-159046. This paper provided nice evidence that melanocyte filopodia are involved in the transfer of melanosomes from melanocytes to keratinocytes, as well as between keratinocytes. Evidence was also presented that myosin X, and unconventional myosin linked to filopodia formation and phagocytosis, is required in melanocytes to support filopodial-based transfer and in keratinocytes to phagocytize filopodial tips.
- 12.Singh SK, et al. The silver locus product (Silv/gp100/Pmel17) as a new tool for the analysis of melanosome transfer in human melanocyte-keratinocyte co-culture. Exp Dermatol. 2008;17(5):418–426. doi: 10.1111/j.1600-0625.2008.00702.x. [DOI] [PubMed] [Google Scholar]
- 13. Wu XS, et al. Melanoregulin regulates a shedding mechanism that drives melanosome transfer from melanocytes to keratinocytes. Proc Natl Acad Sci U S A. 2012;109(31):E2101–E2109. doi: 10.1073/pnas.1209397109. This paper used time lapse imaging of primary co cultures made from Holly mice, which yield red melanocytes and green keratinocytes, to demonstrate in real time the Shedding-Phagocytosis pathway for melanosome transfer. The paper also demonstrated that melanoregulin, the product of the dilute suppressor locus, is a negative regulator of shedding-based melanosome transfer.
- 14.Ando H, et al. Involvement of pigment globules containing multiple melanosomes in the transfer of melanosomes from melanocytes to keratinocytes. Cell Logist. 2011;1(1):12–20. doi: 10.4161/cl.1.1.13638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCullough J, Colf LA, Sundquist WI. Membrane fission reactions of the mammalian ESCRT pathway. Annu Rev Biochem. 2013;82:663–692. doi: 10.1146/annurev-biochem-072909-101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truschel ST, et al. ESCRT-I function is required for Tyrp1 transport from early endosomes to the melanosome limiting membrane. Traffic. 2009;10(9):1318–1336. doi: 10.1111/j.1600-0854.2009.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Niel G, et al. The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell. 2011;21(4):708–721. doi: 10.1016/j.devcel.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mochida GH, et al. CHMP1A encodes an essential regulator of BMI1-INK4A in cerebellar development. Nat Genet. 2012;44(11):1260–1264. doi: 10.1038/ng.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skibinski G, et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat Genet. 2005;37(8):806–808. doi: 10.1038/ng1609. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan TN, et al. dsu functions in a MYO5A-independent pathway to suppress the coat color of dilute mice. Proc Natl Acad Sci U S A. 2004;101(48):16831–16836. doi: 10.1073/pnas.0407339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KJ, et al. The murine dilute suppressor gene encodes a cell autonomous suppressor. Genetics. 1994;138(2):491–497. doi: 10.1093/genetics/138.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu XS, Martina JA, Hammer JA., 3rd Melanoregulin is stably targeted to the melanosome membrane by palmitoylation. Biochem Biophys Res Commun. 2012;426(2):209–214. doi: 10.1016/j.bbrc.2012.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohbayashi N, et al. Melanoregulin regulates retrograde melanosome transport through interaction with the RILP-p150Glued complex in melanocytes. J Cell Sci. 2012;125(Pt 6):1508–1518. doi: 10.1242/jcs.094185. [DOI] [PubMed] [Google Scholar]
- 24. Tarafder AK, et al. Rab11b Mediates Melanin Transfer between Donor Melanocytes and Acceptor Keratinocytes via Coupled Exo/Endocytosis. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.432. This paper used serial section electron microscopy and immuno-electron microscopy of human skin samples to provide support for the Exocytosis-Endocytosis pathway of melanosome transfer. The paper also presents data from melanocyte: keratinocyte co cultures that Rab27a is not involved in the exocytosis of melanin cores, while Rab11b-positive vesicles that surround peripheral melanosomes are.
- 25.Kim HJ, et al. Visualization of the melanosome transfer-inhibition in a mouse epidermal cell co-culture model. Int J Mol Med. 2010;25(2):249–253. [PubMed] [Google Scholar]
- 26.Yatsu A, et al. Syntaxin-3 is required for melanosomal localization of Tyrp1 in melanocytes. J Invest Dermatol. 2013;133(9):2237–2246. doi: 10.1038/jid.2013.156. [DOI] [PubMed] [Google Scholar]
- 27.Seiberg M. Keratinocyte-melanocyte interactions during melanosome transfer. Pigment Cell Res. 2001;14(4):236–242. doi: 10.1034/j.1600-0749.2001.140402.x. [DOI] [PubMed] [Google Scholar]
- 28.Provance DW, James TL, Mercer JA. Melanophilin, the product of the leaden locus, is required for targeting of myosin-Va to melanosomes. Traffic. 2002;3(2):124–132. doi: 10.1034/j.1600-0854.2002.030205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagashima K, et al. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002;517(1–3):233–238. doi: 10.1016/s0014-5793(02)02634-0. [DOI] [PubMed] [Google Scholar]
- 30.Fukuda M, Kuroda TS, Mikoshiba K. Slac2-a/melanophilin, the missing link between Rab27 and myosin Va: implications of a tripartite protein complex for melanosome transport. J Biol Chem. 2002;277(14):12432–12436. doi: 10.1074/jbc.C200005200. [DOI] [PubMed] [Google Scholar]
- 31.Strom M, et al. A family of Rab27-binding proteins. Melanophilin links Rab27a and myosin Va function in melanosome transport. J Biol Chem. 2002;277(28):25423–25430. doi: 10.1074/jbc.M202574200. [DOI] [PubMed] [Google Scholar]
- 32.Wu XS, et al. Identification of an organelle receptor for myosin-Va. Nat Cell Biol. 2002;4(4):271–278. doi: 10.1038/ncb760. [DOI] [PubMed] [Google Scholar]
- 33. Yoshida-Amano Y, et al. Essential role of RAB27A in determining constitutive human skin color. PLoS One. 2012;7(7):e41160. doi: 10.1371/journal.pone.0041160. This paper showed that the expression levels of Rab27a and melanophilin, the Rab27a effector that links the Rab to myosin Va, correlate positively with melanogenic ability in human skin samples from variety of skin types. In addition, the number of melanosomes transferred to keratinocytes in co cultures and human skin substitutes correlated positively with increasing pigment content in the melanocyte. Finally, knockdown of Rab27a in melanocytes reduced melanosome transfer. Together, these data further confirm the essential role played by Rab27a in determining human skin color.
- 34. Beaumont KA, et al. The recycling endosome protein Rab17 regulates melanocytic filopodia formation and melanosome trafficking. Traffic. 2011;12(5):627–643. doi: 10.1111/j.1600-0854.2011.01172.x. This paper showed that the recycling endosome-associated Rab GTPases Rab17, Rab 11a and Rab11b appear to promote the exocytosis of melanosomes from melanocyte filopodia.
- 35.Menager MM, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8(3):257–267. doi: 10.1038/ni1431. [DOI] [PubMed] [Google Scholar]
- 36.Hsu VW, Prekeris R. Transport at the recycling endosome. Curr Opin Cell Biol. 2010;22(4):528–534. doi: 10.1016/j.ceb.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boissy RE. Melanosome transfer to and translocation in the keratinocyte. Exp Dermatol. 2003;12(Suppl 2):5–12. doi: 10.1034/j.1600-0625.12.s2.1.x. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen T, Wei ML. Hermansky-Pudlak HPS1/pale ear gene regulates epidermal and dermal melanocyte development. J Invest Dermatol. 2007;127(2):421–428. doi: 10.1038/sj.jid.5700566. [DOI] [PubMed] [Google Scholar]
- 39.Han CZ, Ravichandran KS. Metabolic connections during apoptotic cell engulfment. Cell. 2011;147(7):1442–1445. doi: 10.1016/j.cell.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]