Abstract
Background:
The National Emphysema Treatment Trial (NETT) demonstrated that lung volume reduction surgery (LVRS) is an effective treatment for emphysema in select patients. With chronic lower respiratory disease being the third leading cause of death in the US, this study sought to assess practice patterns and outcomes for LVRS on a national level since the NETT.
Methods:
Aggregate statistics on LVRS reported in the Society of Thoracic Surgeons (STS) Database from January 2003 to June 2011 were analyzed to assess procedure volume, pre-operative and operative characteristics, and outcomes. Comparisons to published data from the NETT were made using chi-squared and two sided t-tests.
Results:
In 8.5 years, 538 patients underwent LVRS, with 20 to 118 cases reported in the STS database per year. When compared to NETT subjects, STS patients were younger (p<0.001), a larger proportion underwent the procedure thoracoscopically (p<0.001), and FEV1 was 31% vs. 28% of predicted (p<0.001). When mortality was compared between STS patients and all NETT subjects randomized to surgery, there were no significant differences. However, mortality was 3% higher in STS patients when compared to the non-high risk NETT subset (p=0.005).
Conclusions:
This study demonstrates the importance of patient selection and the need to develop consensus on appropriate benchmarks for mortality rates after lung volume reduction surgery. It underscores the need for dedicated centers to increasingly address the heavy burden of chronic lower respiratory disease in the US in a multi-disciplinary fashion, particularly for preoperative evaluation and postoperative management of emphysema.
Keywords: Lung volume reduction, Emphysema, Outcomes, Database
Introduction
Chronic lower respiratory disease is the third leading cause of death in the United States,1 with chronic obstructive pulmonary disease (COPD) taking approximately 126,000 lives every year.2 At least one third of these COPD cases are related to a diagnosis of emphysema.3 Contemporary treatment options for emphysema include oxygen therapy, beta agonists and anti-cholinergics, oral and inhaled steroids, pulmonary rehabilitation, lung transplantation, experimental endobronchial therapies, and lung volume reduction surgery (LVRS). LVRS has been reported to improve long-term survival and quality of life in appropriately selected patients with emphysema.4-9 But LVRS practice patterns and outcomes have not since been evaluated nationally, outside of a clinical trial.
The National Emphysema Treatment Trial (NETT),4,5 which first published results in 2003, randomized 1218 patients with emphysema to either LVRS or best medical therapy and examined the primary end-points of survival and maximal exercise performance, with secondary end-points of pulmonary function, patient symptom severity, and quality of life.4 The NETT had a large enough subject enrollment to identify the subgroup of emphysema patients with heterogeneous, upper lobe predominant disease and low exercise capacity who have the best short and long-term outcomes after bilateral LVRS, with significant improvements in survival and exercise capacity. The trial also identified that people with a forced expiratory volume in one second of 20 percent or less than predicted, and those with either a homogeneous distribution of emphysema or a carbon monoxide diffusing capacity of 20 percent or less than predicted were at high risk of death after LVRS.4 The NETT thus defined selection criteria for emphysema patients who are appropriate candidates for LVRS by identifying those who are at high risk for poor outcomes. Since closure of the trial, multiple meta-analyses of LVRS outcomes have been performed using NETT data, but there have been few subsequent studies reporting LVRS outcomes in the post NETT era. Almost ten years after the trial results were published, it is worthwhile to evaluate LVRS practice patterns and outcomes on a national level.
Despite the published benefits of LVRS as a treatment option for emphysema, the procedure is reportedly underutilized.10 Reasons for this are unclear, as COPD and emphysema comprise a significant burden of disease in the US population. The National Heart, Lung, and Blood Institute projected that COPD costs $29.6 billion in direct healthcare expenditures and $20.4 billion in indirect morbidity and mortality expenditures annually.11 In this study, we report on comprehensive LVRS data from the Society of Thoracic Surgeons (STS) database beginning in 2003, when the NETT was first published. The STS database provides a geographically diverse national sample, which unlike Medicare claims data, includes patients under 65 years of age. This is a valuable advantage of STS data because approximately half of emphysema patients in the country are between ages 45 and 64.2 By examining the outcomes of LVRS in the STS database, and comparing outcomes to results of the NETT, our study assesses the performance of LVRS compared to the clinical benchmark set by a landmark clinical trial. This study has implications for future identification of determinants of LVRS quality, and development of LVRS-specific quality benchmarks.
Methods
Study Design and Data Sources
This study involved a retrospective review of de-identified aggregate statistics on patients who underwent lung volume reduction surgery (LVRS) reported in the Society of Thoracic Surgeons (STS) Database from 2003 to 2011. Previously published data from the National Emphysema Treatment Trial (NETT)4,5,12,13 was studied for statistical comparison. The University of Wisconsin Institutional Review Board approved this study.
Study populations
Subjects in the NETT who were randomized to surgery underwent bilateral stapled wedge resection. These patients were subdivided into a non-high risk group and a sub-group of non-high risk patients with upper lobe predominant disease and low exercise tolerance.
Patients included in the analysis of STS patients underwent either bilateral or unilateral resection. Both groups were included due to lack of distinction between unilateral and bilateral LVRS in certain versions of the STS General Thoracic Surgery Database Major Procedure Collection Form.14 Patients with the following procedure codes were included:
Major Procedure Collection Form Version 2.2 (Last revised 2012):“Removal of lung, excision-plication of emphysematous lung(s) for lung volume reduction (LVRS) (32491);” “Thoracoscopy with resection-plication for emphysematous lung (bullous or non-bullous) for lung volume reduction-LVRS, unilateral including any pleural procedure (32672).” Version 2.081 (Last revised 2009): “Removal of lung, excision-plication of emphysematous lung(s) for lung volume reduction (LVRS) (32491).” Versions 2.06 (2004) and 2.07 (2005): “Lung volume reduction.”
Analysis
The STS database yearly annual volume of LVRS was calculated to depict nationwide trends in volume over time, without attempt to capture total national volume. Meta-analysis was required in order to estimate differences between sample means and proportions using null hypothesis significance testing with t-tests and chi-squared tests, respectively. This allowed for estimation of confidence intervals around calculated differences in event rates, while accounting for sample size. Confidence intervals around estimated differences were calculated using the Z statistic, with alpha = 0.05, assuming normal distribution. Pre-operative and operative characteristics were compared between STS patients who underwent LVRS and NETT subjects randomized to surgery. Pre-operative characteristics included: age, sex, race, and pulmonary function tests which included percent of predicted forced expiratory volume in one second (FEV1) and carbon monoxide diffusing capacity (DCLO). Operative characteristics accounted for the surgical approach to lung volume reduction: median sternotomy, video-assisted thoracoscopic surgery, or other.
Descriptive statistics on health status indicators and comorbidities of STS patients were calculated. Published data on NETT subjects’ overall health status and comorbidities were not directly comparable. Therefore, a descriptive comparison was made based on related health indicators and NETT cohort selection criteria. STS health status indicators and comorbidities included: congestive heart failure (CHF), coronary artery disease, pulmonary hypertension, systemic hypertension, peripheral vascular disease, diabetes, steroid use (defined as systemic steroid therapy, inhaled steroid therapy, or preoperative protocol within 30 days before the procedure)14, previous cardiothoracic surgery, lung cancer, smoking history, American Society of Anesthesiologists (ASA) class, and Eastern Clinical Oncology Group (ECOG)/Zubrod score. Related health status indicators in the NETT included the Quality of Well-Being Score and the St. George’s Respiratory Questionnaire score. The Quality of Well-Being score, which ranges from 0 to 1, with higher values indicating better health-related quality of life.15 St. George’s Respiratory Questionnaire score, which ranges from 0 to 100, with lower values indicating better health-related quality of life.16
Outcomes within 30 days of surgery were compared between STS patients and non-high risk NETT subjects. These outcomes included: readmission to the intensive care unit, sepsis, arrhythmia requiring treatment, myocardial infarction, ventilator dependence beyond 48 hours post-operatively, and re-intubation. Mortality within 30 days of surgery was also compared between STS patients and A) all NETT subjects, as well as B) the non-high risk NETT subset, and C) the NETT subset with upper lobe predominant disease and low exercise tolerance.
In all analyses, missing observations in STS data were excluded from the denominator, however patients with missing data were not excluded from the overall STS cohort. Only de-identified aggregate data were available from the STS database, therefore imputation could not be performed. Proportions of missing observations were reported with outcomes to aid interpretation. Additionally, to account for missing data, mortality outcomes were analyzed under two other scenarios: one in which all missing patients were assumed to be alive, and another in which all missing patients were assumed to be deceased. Statistical analyses were performed in STATA (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
From January 2003 to June 2011, 585 patients underwent LVRS reported in the STS database. Yearly annual volume of LVRS is shown in Figure 1. Patients in the STS database were compared to the 608 NETT subjects who were randomized to surgery, of whom 538 were determined to be non-high risk, and 139 had upper lobe predominant disease and low exercise tolerance.
Figure 1.
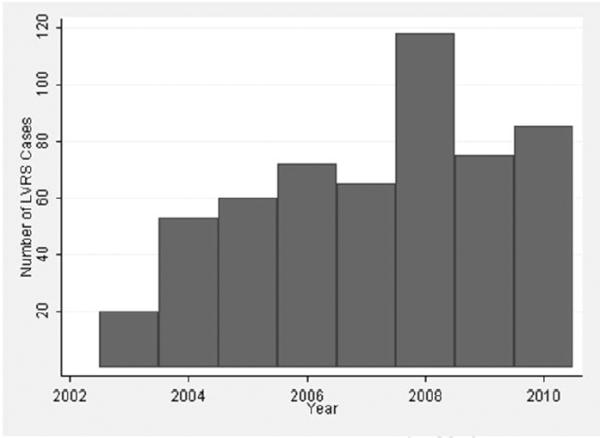
Yearly annual volume of lung volume reduction surgery (LVRS) reported in the Society of Thoracic Surgeons Database from January 2003 through December 2010; 2011 volume is not shown, as yearly data was incomplete at the time of this study.
When STS patients’ pre-operative characteristics were compared to those of all NETT subjects, STS patients were younger and a larger proportion was of a non-White race (Table 1). There were no STS patients with FEV1 or DLCO below 20% of predicted, who would fall into the high risk category deemed by the NETT. While the majority of NETT patients underwent LVRS via a median sternotomy approach, most STS patients underwent the procedure with a thoracoscopic approach. Other approaches included cervical, subxyphoid, thoracotomy, and transverse sternotomy.
Table 1.
Quantitative Comparison of LVRS Patients' Pre-Operative & Operative Characteristics in NETT versus STS Database
| STS | NETT | Mean Difference | P value a | ||
|---|---|---|---|---|---|
| Characteristics | Mean | Mean | Mean |Δ| |
(95%CI) | |
| Age, mean years | 61.3 | 66.5 | 5.2 | (−6.1 to −4.3) | <0.001 |
| Sex | 0.192 | ||||
| Male | 54.6% | 58.4% | 3.7% | (−9.4% to 1.9%) | |
| Female | 45.4% | 41.6% | 3.7% | (−1.9% to 9.4%) | |
| Race | 0.010 | ||||
| White | 91.4% | 95.6% | 4.1% | (−6.9 to 9.7) | |
| Black | 5.1% | 3.1% | 1.9% | (−0.3 to 4.2%) | |
| Other | 3.5% | 1.3% | 2.2% | (0.4% to 3.9%) | |
|
Pulmonary
Function Tests |
|||||
| FEV1 % Predicted | 31.1 | 28.1 | 3.0 | (1.6 to 4.4) | <0.001 |
| DLCO %Predicted | 37.8 | 29.2 | 8.6 | (6.9 to 10.2) | <0.001 |
|
Surgical
Approach |
<0.001 | ||||
| Median sternotomy | 35.8% | 70.0% | 34.2% | (−40.2% to −28.1%) | |
| VATS | 51.3% | 30.0% | 21.3% | (15.1% to 27.5%) | |
| Other | 12.9% | 0% | 12.9% | (9.6% to 16.3%) | |
P values calculated using two sided t-tests for continuous variables and chi-squared tests for differences in proportions.
Overall, fewer than half of STS patients had major comorbidities (Table 2). Approximately 10% of STS patients had previous cardiothoracic surgery, whereas NETT enrollees with previous sternotomy or lobectomy were excluded. One and a half percent of STS patients had a diagnosis of lung cancer, whereas the NETT excluded enrollees with pulmonary nodules or evidence of neoplasia that could potentially interfere with the trial.
Table 2.
Description of LVRS Patients’ Pre-operative Health Status in STS Database Compared with NETT Selected Cohort
| Patient Characteristics | STS % |
NETTa |
|---|---|---|
| Comorbidities | ||
| CHF | 2.6% | Excluded if “Congestive heart failure within 6 months of interview and ejection fraction <45%” |
| Coronary artery disease | 17.4% | |
| Pulmonary hypertension | 0.7% | Excluded if “Pulmonary hypertension: mean PPA on right heart catheterization ≥35 mm Hg (≥38 mm Hg in Denver) or peak systolic PPA on right heart catheterization ≥45 mm Hg (≥50 mm Hg in Denver); right heart catheterization is required to rule out pulmonary hypertension if peak systolic PPA on echocardiogram >45 mm Hg” |
| Systemic hypertension | 45.1% | Excluded if “Uncontrolled hypertension (systolic >200 mm Hg or diastolic >110 mm Hg)” |
| Peripheral vascular disease | 2.6% | |
| Diabetes | 9.4% | |
| Steroids | 17.8% | Excluded if “Daily use of more than 20 mg of prednisone or its equivalent as of randomization” |
| Previous Cardiothoracic Surgery | 9.9% | Excluded if “Previous sternotomy or lobectomy” |
| Lung Cancer | 1.5% | Excluded if “Evidence of systemic disease or neoplasia that is expected to compromise survival over the duration of the trial” or “Pulmonary nodule requiring surgery” |
| Pre-op chemotherapy & radiation | 1.0% | |
| Smoking History | Included if “Nonsmoker (tobacco products) for 4 months prior to initial interview and patient remains a nonsmoker throughout screening (by history)” |
|
| Current Smoker | 4.6% | |
| Quit within year prior | 8.4% | |
| Quit over a year ago | 52.5% | |
| Never Smoked | 34.5% | |
| ASA Class | Surgical Patients’ average daily Quality of Weil-Being score b: 0.58±0.12 Upper Lobe Predominant, Low Exercise Tolerant Quality of Well-Being score: 0.57±0.12 Surgical Patients’ St. George’s Respiratory Questionnaire Score c: 52.5±12.6 Upper Lobe Predominant, Low Exercise Tolerant St. George’s Respiratory Questionnaire Score: 54.3±12.1 |
|
| I | 2.0% | |
| II | 6.8% | |
| III | 63.1% | |
| IV | 28.0% | |
| V | 0.2% | |
| ECOG/ Zubrod Score | ||
| 0-Normal Activity | 9.1% | |
| 1-Symptomatic but ambulatory | 63.1% | |
| 2-Symptomatic, <50% daytime in bed | 20.4% | |
| 3-Symptomatic, >50%<100% daytime in bed |
4.5% | |
| 4-Bedridden | 2.9% |
Referenced from Fishman et al. N Engl J Med 2003; 348:2059-73 (Appendix 1)
Quality of Weil-Being score is on a scale from 0 to 1, with higher scores indicating better health
St. George’s Respiratory Questionnaire score is on a scale from 0 to 100, with lower scores indicating better health
The majority of STS patients had ASA Class III or IV and had ECOG or Zubrod scores of 1 or 2, indicating that most patients were symptomatic and either ambulatory or partially disabled. This parallels with the middle range health-related quality of life scores reported by NETT subjects (Table 2 continued).
Median time from surgery to evaluation of 30-day outcomes was 0.7 month in all NETT subjects, including the non-high risk subset, and 0.8 month in the subset with upper lobe predominant disease and low exercise tolerance.4 Outcomes were assessed at 30 days post-operatively in the STS database. Given the slight difference in time from surgery to evaluation of outcomes in NETT versus the STS database, mortality within 60 days is reported for NETT subjects as a frame of reference.
Mortality within 30 days of LVRS was not significantly different between STS patients and all NETT subjects (Table 3a). When 30-day mortality rates were compared between STS patients and the non-high risk NETT subset (Table 3b), as well as the NETT subset with upper lobe predominant disease and low exercise tolerance (Table 3c), STS patients had a 3 to 4% higher mortality rate that was statistically significant. Mortality assessed at 1.7 months post-operatively was 4.8% among non-high risk NETT subjects and 2.9% among subjects with upper lobe predominant disease and low exercise tolerance, compared to the STS 30-day mortality rate of 5.6%.
Table 3.
a: Comparison of Outcomes After LVRS in STS Database Versus NETT
| Outcomes | STS a | All NETT | Difference | P valueb |
|
|---|---|---|---|---|---|
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 5.6% | 3.6% | 2.0% | (−0.5% to 4.5%) | 0.113 |
| Table 3b: Comparison of Outcomes After LVRS in STS Database Versus Non-High Risk NETT Subset | |||||
| Outcomes | STS a |
Non-high
Risk NETT Subset |
Difference |
P
value b |
|
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 5.6% | 2.2% | 3.4% | (1.0% to 5.7%) | 0.005 |
| Re-admit to ICU ‡ | 7.8% | 11.7% | 3.9% | (−8.8% to 1.0%) | 0.156 |
| Sepsis ‡ | 1.9% | 2.5% | 0.6% | (−2.5% to 1.3%) | 0.544 |
| Arrhythmia ‡ | 16.2% | 18.6% | 2.4% | (−7.5% to 2.6%) | 0.350 |
| MI ‡ | 1.1% | 1.0% | 0.1% | (−1.3% to 1.4%) | 0.910 |
| Ventilator>48hrs ‡ | 4.6% | 13.6% | 9.1% | (−12.7% to −5.4%) | <0.001 |
| Re-intubation ‡ | 12.9% | 21.8% | 8.9% | (−13.8% to −3.9%) | <0.001 |
| Table 3c: Comparison of Outcomes After LVRS in STS Database Versus NETT Subset with Upper-Lobe Predominant Disease & Low Exercise Tolerance | |||||
| Outcomes | STS a |
NETT,
Upper Lobe Disease ↓Exercise Tolerance |
Difference |
P
value b |
|
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 5.6% | 1.4% | 4.2% | (1.4% to 7.0%) | 0.039 |
Missing STS observations excluded;
P values from chi-squared tests;
Referenced from Naunheim et al. 2006 ;
Referenced from Fishman et al. 2003
When other outcomes were compared between STS patients and non-high risk NETT subjects randomized to surgery, there were no significant differences in rates of ICU readmission, sepsis, arrhythmia requiring treatment, or myocardial infarction (Table 3b). STS patients had significantly lower rates of ventilator dependence lasting more than 48 hours post-operatively, and re-intubation.
Mortality data were missing for 83 (14%) of the STS patients. There were 213 to 214 (36-37%) missing observations for each of the other outcome variables; missing observations were excluded from sample proportions. When all missing patients were assumed to be alive, rather than excluded, no significant differences in mortality were identified between LVRS patients in the STS database versus the NETT (Appendix 1a and 1b). Mortality rates were significantly higher for LVRS patients in the STS database compared to those in the NETT when all missing patients were assumed to be deceased (Appendix 1c and 1d).
Appendix 1
| Appendix Ia: Comparison of Outcomes After LVRS in STS Database Versus NETT (Missing STS patients assumed to be alive) | |||||
|---|---|---|---|---|---|
| Outcomes | STS a | All NETT | Difference | P valueb |
|
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 4.8% | 3.6% | 1.2% | (−0.1% to 3.5%) | 0.306 |
| Appendix Ib: Comparison of Outcomes After LVRS in STS Database Versus NETT Subjects with Upper-Lobe Predominant Disease & Low Exercise Tolerance (Missing STS patients assumed to be alive) | |||||
| Outcomes | STS a |
NETT,
Upper Lobe Disease ↓Exercise Tolerance |
Difference | P value b | |
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 4.8% | 1.4% | 3.4% | (0.8% to 6.0%) | 0.072 |
| Appendix Ic: Comparison of Outcomes After LVRS in STS Database Versus NETT (Missing STS patients assumed to be deceased) | |||||
| Outcomes | STS a | All NETT | Difference |
P
value b |
|
| % | % | %| Δ| | (95%CI) | ||
| 30day Mortality † | 19.0% | 3.6% | 15.4% | (11.9% to 18.9%) | <0.001 |
| Appendix Id: Comparison of Outcomes After LVRS in STS Database Versus NETT Subjects with Upper-Lobe Predominant Disease & Low Exercise Tolerance (Missing STS patients assumed to be deceased) | |||||
| Outcomes | STS a |
NETT,
Upper Lobe Disease ↓Exercise Tolerance |
Difference |
P
value b |
|
| % | % | %| Δ| | (95%CI) | ||
| 3Oday Mortality † | 19.0 | 1.4% | 17.6% | (13.8% to 21.3%) | <0.001 |
Missing STS observations excluded;
P values from chi-squared tests;
Referenced from Fishman et al. 2003
Comment
This study of lung volume reduction surgery in the Society of Thoracic Surgeons database from 2003 to 2011 is the first longitudinal, population-level assessment of LVRS since the National Emphysema Treatment Trial. Our study demonstrates that since the NETT was published in 2003, surgeons have performed LVRS for a broader group of patients (including smokers, younger patients, and people with previous cardiac surgery); techniques have evolved (with greater use of thoracoscopic surgery); and outcomes differ in some areas while remaining the same in others.
The annual volume of LVRS in the STS database increased substantially from 2003 to 2004 but did not steadily increase thereafter. This suggests that LVRS is underutilized, as reported in a study of Medicare claims from 2004 to 2005 in which there were only 258 claims for LVRS over 21 months.10 When considering that emphysema is reported to affect 4.7 million Americans,2 it appears that only a small proportion of these patients are pursuing LVRS as a treatment option. Reasons for this remain unclear. Overly restrictive patient selection criteria do not appear to be the cause. Overall differences in patient characteristics between the STS database and the NETT demonstrate that patient selection was less restrictive in the STS database. With underutilization unlikely to be related to restrictive selection criteria, it may instead be related to restrictive referral patterns and limited access to this specialized surgery.
When compared to NETT subjects, more STS patients underwent LVRS via a thoracoscopic approach, which is likely a consequence of an increase in surgeons’ comfort with thoracoscopy and video-assisted thoracoscopic surgery.17 The fact that 10% of STS patients had previous cardiothoracic surgery is a testament to surgeons’ increased comfort with the technical aspects of the lung volume reduction procedure. The younger mean age of STS patients may relate to increasing numbers of insurers offering coverage of LVRS,18,19 following after the Centers for Medicare & Medicaid Services (CMS) published criteria for expanded coverage of LVRS in 2003.20 However, CMS has restricted the types of facilities that are eligible for reimbursement of LVRS to those approved for the NETT, credentialed by the Joint Commission on Accreditation of Healthcare Organizations under their Disease Specific Certification Program for LVRS, and those approved by Medicare for lung or heart and lung transplants.21 While this policy maintains the quality of LVRS by requiring that the appropriate infrastructure is in place, it likely restricts access to this surgery that could potentially benefit over a million Americans. To address underutilization of LVRS, perhaps an increase in the number of healthcare teams dedicated to treatment of advanced pulmonary disease is needed, similar to but distinct from healthcare teams dedicated to heart and lung transplantation or thoracic oncology.
One and a half percent of STS patients in this study were reported to have lung cancer. This may have been a result of mis-coding of the surgical procedure as a lung volume reduction rather than a cancer resection. But, the surgery may also have been performed for the dual purpose of lung volume reduction and cancer resection, as previously published.22 Coding errors are always possible when collecting administrative data. However, given the stringent reporting requirements for re-imbursement of LVRS 18-21, there is a low likelihood that coding errors would be frequent enough to skew the results in our large sample size of over 500 patients.
Outcomes after LVRS were similar between the STS database and the NETT, with key exceptions. Lower rates of re-intubation and prolonged ventilation in the STS database may be related to nationwide efforts to improve these outcomes over the past decade. The Society of Thoracic Surgeons, the American College of Surgeons-National Surgical Quality Improvement Program, 23 and the Physician Quality Reporting System in conjunction with CMS 24 use re-intubation and prolonged ventilation as healthcare quality indicators. Hospitals throughout the country have developed quality improvement programs to address these indicators, with increasing success.25,26
Analysis of outcomes further demonstrated that STS patients had a similar 30-day mortality rate when compared to all NETT subjects. But when compared to non-high risk NETT subjects and those with upper lobe predominant disease and low exercise tolerance, STS patients had a higher mortality rate. This may be related to differences in patient selection, and it may be related to differences in surgical care outside of a clinical trial. Given that 30-day mortality has been demonstrated to be higher in patients undergoing bilateral resection, compared to unilateral resection, 27 the inclusion of STS database patients who had unilateral LVRS was expected to have skewed the 30-day mortality rate in STS to a rate lower than those reported in NETT. However, this was not the case.
As public attention to quality reporting increases and pay-for-performance policies are increasingly implemented, thoracic surgeons may be compelled to decide upon thresholds for mortality. It may be unreasonable to expect the outcomes in practice to be as good as the best subgroup of outcomes in a randomized controlled clinical trial. But it is reasonable to expect a certain standard of care, and decide upon a threshold at which mortality risk cannot outweigh potential benefits.
This study measured outcomes with respect to an absolute benchmark, set by the NETT. Current pay-for-performance models are based on both absolute and relative performance measurements.28 Therefore, future investigation of relative performance measurements for LVRS would allow hospitals to compare their performance with others, and potentially learn from high performing outliers’ patient selection, follow-up care, and other potential determinants of quality.
Although the less restrictive patient selection demonstrated in the STS cohort may contribute to the higher mortality rate when compared to the selected NETT subjects, a small difference in mortality rates may not warrant restricting marginal non-high risk candidates from access to this potentially life-saving procedure that has been shown to provide substantial improvements in quality of life.4-9
It is worth mentioning that the mortality rates reported in the NETT were captured under a slightly narrower time window (0.7 month in all NETT patients including the non-high risk subset, and 0.8 month in the upper lobe predominant low exercise tolerant subset) compared to the 30-day mortality in the STS database. Given that the non-high risk NETT subset mortality rates at 1.7 months remained below the STS 30-day mortality rate, it is unlikely that the 3 to 4% difference in mortality rates can be wholly attributed to the difference in time windows. Although there were differences in data collection between the STS database and the NETT, as described, comparison remains informative with this limitation in mind.
This study was also limited by a lack of long term data on outcomes of patients in the STS database. Although 30-day outcomes were useful for assessing quality of care in the short term, previous studies have shown that the measurement of survival benefits and improvement in quality-adjusted life after LVRS require long term data collection.5,9 Measurement of 90-day and one-year outcomes after LVRS in quality assessment databases such as that of the STS would facilitate future studies. This study of the STS database was also limited by missing data, which comprised 14% to 37 % of observations. Results should be interpreted while considering proportion of missing observations (Appendix 1). Overall, limitations of this study were largely due to limitations of observational data. The STS database provides a national sample that is likely to be biased toward higher participation by major academic centers and hospitals with sufficient data collection resources. This study was not designed to capture total national LVRS volume in the US or prescribe policy to address LVRS quality. Rather, the study was designed to provide a description that facilitates further investigation into quality assessment and quality assurance for lung volume reduction surgery. As we have demonstrated, this goal was achieved and the STS database proved to be useful in providing an unadjusted assessment of volume and outcomes for LVRS.
Keeping thoracic surgeons apprised of unadjusted quality assessments is essential to involving surgeons in the identification of surgery-specific determinants of quality, the development of surgery-specific quality measures, and therefore the evolution of quality improvement databases such as that of the STS. This study highlights the need to invest in future analyses that identify determinants of outcomes after LVRS so that future quality assessments can adjust for: patient characteristics, payer status, location, et cetera. This is an iterative process that is best conducted with surgeons’ involvement.
Overall, the major findings of this study demonstrate that mortality rates are higher in the STS database than they were in selected NETT patients, and about the same compared to the overall NETT LVRS arm. Interpretation of differences in mortality rates is complicated by lack of consensus on appropriate benchmarks for mortality rates after lung volume reduction surgery. Our results and conclusions, like those of any observational study, are limited to description and interpretation of the available data. We maintain that the STS database provides a geographically diverse national sample of outcomes after LVRS that may capture national trends. This study demonstrates the importance of patient selection and the need to develop consensus on appropriate benchmarks for morbidity and mortality rates after lung volume reduction surgery. Our study also underscores the need for primary care providers, pulmonologists, and thoracic surgeons to address the heavy burden of chronic lower respiratory disease in the US by more frequently engaging a multi-disciplinary team in discussions with patients regarding the treatment options for emphysema, taking into consideration individual patient risk factors and weighing risks and benefits in an evidence-based fashion. More dedicated centers for treatment of advanced respiratory disease are needed to improve access to lung volume reduction surgery, while refining preoperative evaluation and postoperative management of emphysema in a coordinated way.
Acknowledgements and Disclosures
Financial support: The work of Marquita R Decker is financially supported by a National Institutes of Health Research Training Grant in Surgical Oncology (2T32 CA090217).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at the CHEST 2012 Scientific Program in Atlanta, GA.
Author Contributions: Study design & conception (MRD, JDM); Data analysis (MRD, GEL); Manuscript drafting (MRD, WAJ, JDM); Final review and approval (all authors).
References
- 1.Kochanek KD, Xu J, Murphy SL, Minino AM, Kung H. National vital statistics reports. National Vital Statistics Reports. 2011;59(4):1. [PubMed] [Google Scholar]
- 2.Brown DW, Croft JB, Greenlund KJ, Giles WH. Deaths from Chronic Obstructive Pulmonary Disease — United States, 2000–2005. Morbidity & Mortality Weekly Report. 2008;57(45):1229–1232. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5745a4.htm. Accessed April 12, 2013. [PubMed] [Google Scholar]
- 3.American Lung Association Trends in COPD (emphysema and chronic bronchitis): morbidity and mortality. 2010 Feb; [cited 2010 Dec 30]. Available at: http://www.lungusa.org/finding-cures/our-research/trend-reports/copd-trend-report.pdf Accessed April 12, 2013.
- 4.Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348:2059–2073. doi: 10.1056/NEJMoa030287. [DOI] [PubMed] [Google Scholar]
- 5.Naunheim KS, Wood DE, Mohsenifar Z, Sternberg AL, Criner GJ, DeCamp MM, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82:431. doi: 10.1016/j.athoracsur.2006.05.069. [DOI] [PubMed] [Google Scholar]
- 6.Geddes D, Davies M, Koyama H, Hansell D, Pastorino U, Pepper J, et al. Effect of lung-volume-reduction surgery in patients with severe emphysema. N Engl J Med. 2000;343:239–45. doi: 10.1056/NEJM200007273430402. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein RS, Todd TR, Guyatt G, Keshavjee S, Dolmage TE, van Rooy S, et al. Influence of lung volume reduction surgery (LVRS) on health related quality of life in patients with chronic obstructive pulmonary disease. Thorax. 2003;58:405–10. doi: 10.1136/thorax.58.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillerdal G, Lofdahl CG, Strom K, Skoogh BE, Jorfeldt L, Nilsson F, et al. Comparison of lung volume reduction surgery and physical training on health status and physiologic outcomes: a randomized controlled clinical trial. Chest. 2005;128:3489–99. doi: 10.1378/chest.128.5.3489. [DOI] [PubMed] [Google Scholar]
- 9.Miller JD, Malthaner RA, Goldsmith CH, Goeree R, Higgins D, Cox PG, et al. A randomized clinical trial of lung volume reduction surgery versus best medical care for patients with advanced emphysema: A two-year study from Canada. Discussion. Ann Thorac Surg. 2006;81:314–321. doi: 10.1016/j.athoracsur.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 10.Ramsey SD, Shroyer AL, Sullivan SD, Wood DE. Updated evaluation of the cost effectiveness of lung volume reduction surgery. Chest. 2007;131:823–832. doi: 10.1378/chest.06-1790. [DOI] [PubMed] [Google Scholar]
- 11.National Heart Lung and Blood Institute Morbidity and Mortality: 2009 Chart Book on Cardiovascular. Lung and Blood Diseases. Available at: http://www.nhlbi.nih.gov/resources/docs/2009_ChartBook.pdf Accessed May 31, 2013.
- 12.DeCamp MM, Blackstone EH, Naunheim KS, Krasna MJ, Wood DE, Meli YM, et al. Patient and surgical factors influencing air leak after lung volume reduction surgery: lessons learned from the National Emphysema Treatment Trial. Ann Thorac Surg. 2006:82–197. doi: 10.1016/j.athoracsur.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Naunheim KS, Wood DE, Krasna MJ, DeCamp MM, Jr, Ginsburg ME, McKenna RJ, Jr, et al. Predictors of operative mortality and cardiopulmonary morbidity in the National Emphysema Treatment Trial. J Thorac Cardiovasc Surg. 2006;131:43–53. doi: 10.1016/j.jtcvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 14.STS General Thoracic Database, Data Collection Forms. Available at: http://www.sts.org/quality-research-patient-safety/national-database/database-managers/general-thoracic-surgery-databa-1 Accessed October 1, 2013.
- 15.Kaplan RM, Atkins CJ, Timms R. Validity of a quality of well-being scale as an outcome measure in chronic obstructive pulmonary disease. J Chronic Dis. 1984;37:85–95. doi: 10.1016/0021-9681(84)90050-x. [DOI] [PubMed] [Google Scholar]
- 16.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George's Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 17.Boffa DJ, Gangadharan S, Kent M, Kerendi F, Onaitis M, Verrier E, et al. Self-perceived video-assisted thoracic surgery lobectomy proficiency by recent graduates of North American thoracic residencies. Interact Cardiovasc Thorac Surg. 2012;14:797–800. doi: 10.1093/icvts/ivr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blue Cross Blue Shield Association policy # 7.01.71. Available at: http://www.bcbsms.com/index.php?q=provider-medical-policy-search.html&action=viewPolicy&path=%2Fpolicy%2Femed%2FLung_Volume_Reduction_Surgery.html Accessed May 31, 2013.
- 19.Aetna SM. Clinical Policy Bulletin: Lung Volume Reduction Surgery. Number: 0160. Available at http://www.aetna.com/cpb/medical/data/100_199/0160.html Accessed May 31, 2013.
- 20.Medicare National Coverage Determinations. Department of Health and Human Services; Washington, DC: Chapter 1, Part 4 (Sections 200-310.1), Rev. 62. Section 240.1–Lung Volume Reduction Surgery. In: CMS Manual System. [Google Scholar]
- 21.Centers for Medicare & Medicaid Services Lung Volume Reduction Surgery. Available at http://www.cms.gov/Medicare/Medicare-General-Information/MedicareApprovedFacilitie/Lung-Volume-Reduction-Surgery-LVRS.html Accessed April 22, 2013.
- 22.Choong CK, Meyers BF, Battafarano RJ, Guthrie TJ, Davis GE, Patterson GA, et al. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg. 2004;127(5):1323–31. doi: 10.1016/j.jtcvs.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 23.American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP®) Available at: http://site.acsnsqip.org Accessed April 12, 2013.
- 24.Physician Quality Reporting System Available at: http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/PQRS/ Accessed April 12, 2013.
- 25.Robertson TE, Sona C, Schallom L, Buckles M, Cracchiolo L, Schuerer D, et al. Improved extubation rates and earlier liberation from mechanical ventilation with implementation of a daily spontaneous-breathing trial protocol. J Am Coll Surg. 2008;206(3):489–495. doi: 10.1016/j.jamcollsurg.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J, Shapiro M, Grozovski E, Fox B, Lev S, Singer P. Prediction of extubation outcome: a randomised, controlled trial with automatic tube compensation vs. pressure support ventilation. Crit Care. 2009;13(1):R21. doi: 10.1186/cc7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotloff RM, Tino G, Palevsky HI, Hansen-Flaschen J, Wahl PM, Kaiser LR, et al. Comparison of short-term functional outcomes following unilateral and bilateral lung volume reduction surgery. CHEST. 1998;113(4):890–895. doi: 10.1378/chest.113.4.890. [DOI] [PubMed] [Google Scholar]
- 28.Eijkenaar, F. Key Issues in the Design of Pay for Performance Programs. Eur J Health Econ. 2013;14:117–131. doi: 10.1007/s10198-011-0347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


