Abstract
Early embryonic stem cell (ESC) differentiation is marked by the formation of 3 germ layers from which all tissues types will arise. While conventionally ESCs were differentiated by altering their chemical microenvironment, recently it was established that mechanical microenvironment can also contribute towards cellular phenotype commitment. In this study, we are reporting how the cellular mechanical microenvironment of soft substrates affects the differentiation and phenotypic commitment of ESCs. Mouse ESCs were cultured in a fibrin hydrogel matrix in two- and three-dimensional culture. The gelation characteristics of the substrate were modulated by systematically altering the fibrinogen concentration and the fibrinogen-thrombin cross-linking ratio. Analysis of the embryonic stem cells cultured on different substrate conditions clearly illustrate strong influence which substrate physical characteristics assert on cellular behaviors. Specifically it was found that ESCs have a higher proliferation rate in gels of lower stiffness. Early differentiation events were studied by analyzing the gene and protein expression levels of early germ layer markers. Our results revealed that lower substrate stiffness elicit stronger up-regulation of endoderm related genes Sox17, Afp and Hnf4 when compared to stiffer substrates. While both 2D and 3D cultures show a similar response, the effects were much stronger in 3-dimensional culture as compared to 2-dimensional one. These results suggest that physical cues can be used to modulate ESC differentiation into clinically relevant tissues such as liver and pancreas.
Keywords: Soft substrates, Embryonic stem cells, Mechanical properties, Fibrin hydrogel, Endoderm, Differentiation
1. INTRODUCTION
Embryonic stem cells (ESCs) are pluripotent cells isolated from the inner cell mass of the blastocyst. Aside from their ability to differentiate into any tissue cell type, they can be propagated in vitro in an undifferentiated state indefinitely which makes them perfect candidates for use in the area of regenerative medicine and study of differentiation [De Vos J 2009]. The first stage of embryonic stem cell differentiation is marked by the formation of 3 germ layers with distinct molecular markers from which all tissue types will arise. Ectoderm forms mainly the skin and the nervous system. The mesoderm forms muscle, cartilage, bone as well as hematopoietic cells while endoderm mainly forms the GI tracts and both liver and pancreas [Murry CE 2008].
Research in the last decade has established the possibility of differentiating ESCs in an in-vitro setting to not only early germ layers, but to mature organ specific cells as well. Most of these in-vitro inductions are achieved through modulations of the chemical microenvironment. More recently, researchers are studying the effect of mechanical cues such as matrix elasticity on stem cell differentiation [Chowdhury F 2010, Evans N 2009]. Mesenchymal stem cells, when cultured on substrates of varying stiffness were reported to exhibit significant difference in their lineage commitment, which could be correlated to the physiological stiffness of the differentiated phenotype [Engler AJ 2006]. Similar studies have been performed in embryonic stem cells; however, most of these studies are targeted toward osteogenic differentiation, hence using substrates with stiffness ranges that are a few orders of magnitude higher than the ones used for this study [Evans N 2009].
In this paper, we are reporting how the physical microenvironment of soft substrates affects the early differentiation and phenotypic commitment of embryonic stem cells, with emphasis on endodermal differentiation. In order to demonstrate this effect, fibrin, a biocompatible hydrogel was selected as the substrate for providing the desired variation in the physical microenvironment, primarily by modification of its gelation characteristics. Fibrin was selected primarily because of its known attributes as a biocompatible and biodegradable scaffold, which will enable future translation into clinical studies. Furthermore, fibrin is a biopolymer that plays a key role in the natural process of wound healing [Mosesson M 2001] which makes it a good candidate for stem cell transplantation [Rosso F 2005]. Moreover, it has also been recently studied as a gel carrier system for beta-islet transplantation [Lim J-Y 2009] and can also be easily coupled with chemical cues to aid differentiation [Kidszun A 2006], making it ideal for stem cell differentiation studies. Additionally, the substrate chemistry of fibrin hydrogels can be easily manipulated to achieve a broad range of soft substrates, making it suitable for the present study.
The overall objective of this study is to investigate the effect of physical properties of soft substrates on specific characteristics of embryonic stem cells. The ESC cultures differentiated on fibrin gel were analyzed in detail to ascertain their proliferative potential and differentiation patterning behavior in response to variations of substrate mechanical properties. Two different culture configurations were analyzed: cells seeded on top of pre-formed fibrin gel (2 dimensional) and cells embedded inside the fibrin gel (3 dimensional). To the best of authors’ knowledge this is the first report illustrating the effect of physical properties of soft substrates on endodermal differentiation of embryonic stem cells. This can have a significant impact in the derivation of hepatic or pancreatic cells from ESCs, to be used in the treatment of hepatic disorders and diabetes respectively.
2. MATERIALS AND METHODS
2.1 Gel Synthesis
Soft fibrin hydrogels comprising 1, 2, 4, and 8 mg/ml of fibrinogen, respectively, were synthesized. The fibrinogen to thrombin ratios of 10, 2.5, and 1.25 mg/U (fibrinogen/thrombin) were synthesized for each fibrinogen concentration as previously described [Ko HF]and shown in Table (1). For convenience these ratios are referred to as 0.25X, 1X, and 2X respectively, corresponding to increased cross linking with increased thrombin concentration throughout the text. The fibrinogen to thrombin ratio, rather than simply either the fibrinogen or the thrombin concentration, was altered in order to ensure that a wide of a range of stiffness values could be studied for the fibrinogen concentrations chosen. This was systematically done to ensure a complete range of low, medium and high concentrations of fibrinogen and thrombin, respectively as outlined in Table 1, 1 mg/ml representing a low fibrinogen concentration and 8mg/ml correspondingly representing a high fibrinogen concentration. Thrombin concentrations were selected to represent three F/T ratios of low (1.25), medium (2.5) and high (10) corresponding to low, medium and high units of Thrombin.
Table 1.
Concentration of thrombin in NIH units of activity per ml for all fibrin hydrogel conditions
Fibrinogen/Thrombin = 10
Fibrinogen/Thrombin = 2.5
Fibrinogen/Thrombin = 1.25
2.2 Rheology Measurements
Gel discs of 35 mm diameter, prepared as described for 2D gel synthesis, were deposited onto glass slides which were pre-rinsed with DI water. The samples were then allowed to gel fully at 4 °C. After complete gelation, they were fully immersed in the same media used for differentiation studies. The glass slides were then secured to the Peltier cell of a TA Instruments AR2000 stress-controlled rheometer, which was kept at 37 °C throughout the measurements.
A frequency sweep was then performed, using a 25 mm stainless steel in parallel plate geometry with sandpaper glued to the plate to avoid slippage. The samples were subjected to an oscillatory strain described by equation (1), where γ0 is the amplitude of the oscillatory strain (5%), f is the frequency and t is the time. Frequencies employed ranged from 0.1 to 100 rad/s.
| (1) |
The stress required to achieve the specified strain was measured and the components of the complex modulus, the storage (G’), and loss (G”) moduli were accordingly determined.
2.3 Scanning Electron Microscopy (SEM)
The fibrin gel microstructure was analyzed using SEM. The gels were prepared for SEM analysis as described previously in the 2D gel synthesis section above. Preparation of samples was performed as previously described [Ko HF]. SEM images were collected using a Philips XL30 field emission gun SEM (FEI Company). Further image analysis was then performed using the ImageJ software (acquired from http://rsbweb.nih.gov/ij/).
2.4 Maintenance and differentiation of ES cells
Murine ESD3 cells were cultured according to previously published methods [Banerjee I 2010].
2.5 Cell Culture in 2D
For differentiation of the ESCs on fibrin substrate, the cells were tripsynized, washed and re-plated in appropriate configurations. For the 2D culture 30,000 cells in 200 l media were plated on top of the pre-formed fibrin gels prepared on wells of 48 well plates and polymerized overnight at 4°C temperature.
2.6 Cell Culture in 3D
For 3D cell culture format 100,000 cells were re-suspended in the fibrinogen solution before adding thrombin and plated on wells of 48 well plates. The gel with the entrapped cells was then allowed to polymerize for one hour at a temperature of 4°C , after which the culture media was added and subsequently the culture was incubated. For both cases, the cells were maintained in DMEM medium (Invitrogen) supplemented with 10% FBS, 4mM L-glutamine (Cambrex) and 100U/ml penicillin, with media being changed every day. As control embryoid bodies were formed in rotary culture by adding 100,000 cells in ultra low attachment 35mm dishes with same media described above. The dishes were placed in a rotator in the incubation and maintained at constant speed of 40 RMP
2.7 qRT-PCR analysis
The mouse embryonic stem cells (ESD3) cultured in the two- or three-dimensional configurations were harvested by trypsin after five days of culture and RNA was extracted using the NucleoSpin kit according to the manufacturer's protocol. The sample absorbance at 280nm and 260nm was measured using a BioRad Smart Spec spectrophotometer to obtain RNA concentration and quality. Reverse transcription was performed using ImProm II Promega reverse transcription kit following the manufacturer's recommendation. qRT-PCR analysis was performed for pluripotency and early germ layer markers using the primers listed in Table (2). Each sample was then run in replicates, and average values were accordingly used for analysis.
Table 2.
Primers used for qPCR experiments
| TISSUE | NAME | DIR | SEQUENCE |
|---|---|---|---|
| Control | B-Actin | L | cagcagttggttggagca |
| R | tgggagggtgagggactt | ||
| PLURIPOTENCY | rex1 | L | aaggtcatccacggcaca |
| R | tgggagtcatcgcttggt | ||
| Oct4 | L | ggagaagtgggtggaggaa | |
| R | gctgattggcgatgtgag | ||
| sox2 | L | ctggactgcgaactggaga | |
| R | ttggatgggattggtggt | ||
| Mesoderm | Brachyury T | L | aagaacggcaggaggatg |
| R | gcgagtctgggtggatgta | ||
| FGF8 | L | acggcaaaggcaaggact | |
| R | tgaagggcgggtagttga | ||
| GSC | L | gcaccgcaccatcttca | |
| R | tcgcttctgtcgtctcca | ||
| Endoderm | Sox 17 | L | atccaaccagcccactga |
| R | acaccacggaggaaatgg | ||
| AFP | L | ctctggcgatgggtgttt | |
| R | aactggaagggtgggaca | ||
| HNF4 | L | catcgtcaagcctccctct | |
| R | ccctcagcacacggtttt | ||
| Ectoderm | Nestin | L | ggaggatgtggtggaggat |
| R | ttcccgtctgctctggtt | ||
| FGF5 | L | ttcaagcagtccgagcaa | |
| R | taggcacagcagagggatg | ||
| BMP4 | L | atctggtctccgtccctga | |
| R | cgctccgaatggcacta |
2.8 Cell proliferation assay
The proliferative potential of ES cells was analyzed using the Alamar Blue assay. For the two dimensional culture, 15,000 ESD3 cells were plated on fibrin gels of different configurations. 24 hours after plating, the culture media was replaced by fresh media containing 10% Alamar blue and incubated for 4 hours. Fluorescence reading was obtained from a multi-well reader (BioTek Synergy 2, Winooski, VT) at an excitation wavelength of 570nm and emission wavelength of 585nm according to the manufacturer's instructions. Results were normalized with respect to the values obtained for the gels with the highest thrombin concentration, or most cross-linked (2X) condition.
2.9 Immunohistochemical analysis
Staining was performed on differentiated cells. The fibrin gels containing cells in 3D format were formed on cover-slips in wells of a 24 well plate. Staining was performed following the company recommendations .The antibodies used were AFP goat polyclonal antibody and Sox17 rabbit polyclonal antibody (Santa Cruz). The secondary antibodies used were donkey anti-rabbit IgG Texas Red (Santa Cruz) and Alexa Fluor® 488 donkey anti-goat IgG (Invitrogen). Confocal images were taken using an Olympus Fluoview 1000 system.
2.10 Statistical analysis
Each experiment was performed twice with duplicates each time. Average and standard error were found and Student t-test was performed for significance and correspondingly, reported in the results section.
3. RESULTS
3.1 SEM Characterization of Fibrin Microstructure
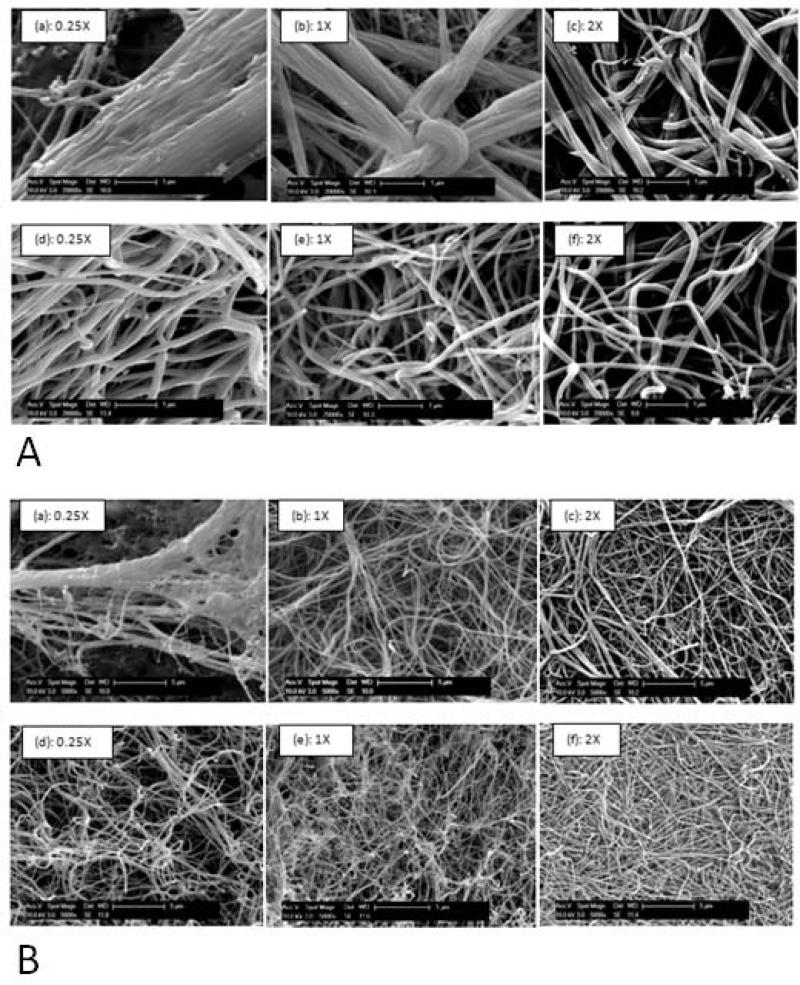
Appropriate characterization of the ESC interaction with substrate microenvironment requires a thorough understanding of the microstructural features of the substrate under these varying conditions. Figure (1, A and B), illustrates SEM images of the fibrin gels taken at two extreme values of fibrinogen concentration, 1mg/ml and 8mg/ml, respectively selected as representative concentrations. It is observed that for a fixed fibrinogen concentration, as the thrombin concentration is increased (Figure (1A (a), (b), and (c))), there is a decrease in fiber diameter and the fibers appear to be less bundled. It can also be seen that there is no significant difference in fiber diameter between 1X and 2X, when compared with 0.25X. The difference in diameter also appears to become less drastic as the fibrinogen concentration is increased.
Figure 1.
(A)Effect of varying the fibrinogen concentration as well as the fibrinogen to thrombin ratios on the microstructure of the gels imaged at a high magnification of 20KX. In (a)-(c) 1 mg/ml of fibrinogen at fibrinogen to thrombin ratios of 0.25X, 1X, and 2X respectively. In (d)-(f) 8 mg/ml of fibrinogen at fibrinogen to thrombin ratios of 0.25X, 1X, and 2X respectively. (B) Effect of varying the fibrinogen concentration as well as the fibrinogen to thrombin ratios on the microstructure of the gels observed at a low magnification of 5KX. In (a)-(c) 1 mg/ml of fibrinogen at fibrinogen to thrombin ratios of 0.25X, 1X, and 2X, respectively. In (d)-(f) 8 mg/ml of fibrinogen at fibrinogen to thrombin ratios of 0.25X, 1X, and 2X, respectively.
As the fibrinogen concentration is decreased, for a fixed fibrinogen to thrombin ratio, the fibers form more bundle type thicker structures, as can be seen in Figure (1A-e) compared to (1A-b). Therefore each fibril appears to have a larger fiber diameter, ~ 0.5-0.75 μm for 1mg/ml fibrinogen compared to ~ 0.1 μm, for a fibrinogen concentration of 8mg/ml at a fixed fibrinogen to thrombin ratio of 1X.
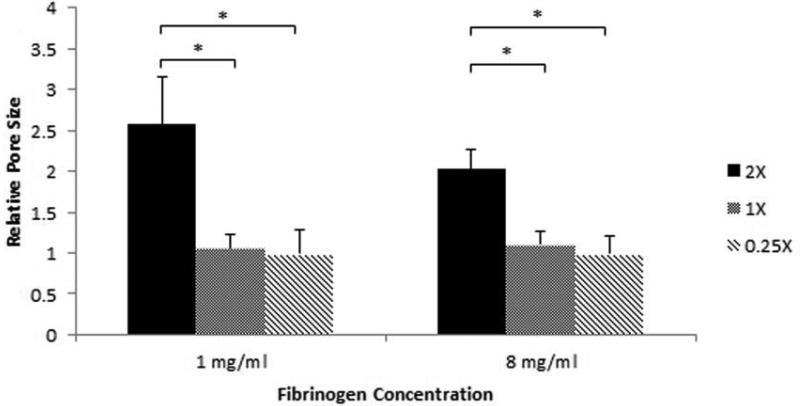
Furthermore, at a fixed fibrinogen concentration, as the thrombin concentration is decreased from 2X to 1X and 0.25X, the substrate appears to have a more open pore structure with a larger pore size as can be seen for both 1 mg/ml and 8 mg/ml fibrinogen concentration in Figure (1B (a)-(c) and (d)-(f)) respectively. To better analyze the effect of thrombin concentration on the porosity of the substrate, ImageJ software was used to quantify the average pore sizes of the gel at fixed fibrinogen concentrations. SEM images were first made binary and the ImageJ software was used to estimate the average size of spaces between fibers. This value was taken for each fibrinogen concentration and was normalized to the values obtained for gels synthesized with the same fibrinogen concentration with a 2X fibrinogen to thrombin ratio. As illustrated in Figure (2), for both 1 mg/ml and 8 mg/ml values of fibrinogen concentrations the pore size decreased with increasing thrombin concentration. However, the effect of thrombin concentration on pore size is much stronger between 0.25X and the higher ratios, while between 1X and 2X the changes are minimal, as can be seen in Figure (1).
Figure 2.
The average pore size calculated using ImageJ for 1 mg/ml and 8 mg/ml fibrinogen concentrations and all three fibrinogen to thrombin ratios normalized to 2X for both fibrinogen concentrations. * p<0.05 compared to highest stiffness group.
3.2 Rheological Characterization of Fibrin Hydrogels
The effect of microstructural variations of the gel on the macroscopic property of the gel substrate is investigated by conducting a rheological characterization of the gel. Table (3A) accordingly illustrates the storage modulus (G’), a measure of energy stored by the sample under oscillatory deformation conditions, while Table (3B) illustrates the loss modulus (G”), a measure of energy dissipated under vibratory conditions. Both the storage and loss modulus showed little dependence on the frequency of oscillation indicative of a solid-like behavior, therefore, their values at a frequency of 0.5 Hz was arbitrarily chosen as a representative value.
Table 3.
(a) G′ values in Pa for various fibrinogen concentrations and all three cross-linking ratios, at a frequency of 0.5 Hz. (b) G″ values in Pa for various fibrinogen concentrations and all three cross-linking ratios, at a frequency of 0.5 Hz.
| Table 3A. | |||
|---|---|---|---|
| 0.25X | 1X | 2X | |
| 1 mg/ml | 4.0 ± 0.9* | 14.1 ± 4.0* | 24.8 ± 4.5 |
| 2 mg/ml | 13.0 ± 0.9* | 35.8 ± 8.7 | 42.0 ± 7.1 |
| 4 mg/ml | 72.1 ± 6.0* | 89.2 ± 9.1 | 97.9 ± 11.9 |
| 8 mg/ml | 171.1 ± 20.3* | 193.9 ± 17.7* | 247.3 ± 15.5 |
| Table 3B. | |||
|---|---|---|---|
| 0.25X | 1X | 2X | |
| 1 mg/ml | 0.7 ± 0.1* | 1.5 ± 0.3 | 2.7 ± 0.6 |
| 2 mg/ml | 3.3 ± 0.4 | 4.6 ± 1.6 | 4.8 ± 1.7 |
| 4 mg/ml | 9.4 ± 1.4 | 10.8 ± 3.0 | 11.5 ± 2.5 |
| 8 mg/ml | 24.7 ± 4.6* | 28.2 ± 2.8 | 33.7 ± 3.9 |
p<0.05 compared to highest stiffness group.
After being cleaved by plasmin, fibrinogen is converted into the monomeric form of fibrin and self assembles to form an insoluble fibrin clot which is stabilized by the crosslinking of factor XIII. In this study, the scaffold storage modulus was varied by three orders of magnitude, from 4 Pa, corresponding to a fibrinogen concentration of 1mg/ml and fibrinogen/thrombin ratio of 0.25X to 247 Pa, corresponding to a fibrinogen concentration of 8 mg/ml and fibrinogen/thrombin ratio of 2X. Furthermore, upon increasing thrombin concentration for a fixed fibrinogen concentration, gel stiffness increased by as much as 76 Pa (8 mg/ml of fibrinogen for fibrinogen to thrombin ratios of 0.25X and 2X). Mechanical stiffness was varied by either keeping the fibrinogen concentration fixed as well as by selecting four different fibrinogen concentrations, 1 mg/ml representing the low range and 8mg/ml representing the high range. Similarly, for a fixed fibrinogen concentrations, the thrombin concentration was varied corresponding to the fibrinogen to thrombin ratios of 0.25X, 1X, and 2X representing a low, medium and high units of thrombin. Following this rationale it was thus possible to prepare fibrin gels which will exhibit a whole range of mechanical stiffness.
Moreover, despite altering the fibrinogen/thrombin ratio, conditions with the same thrombin concentration and different fibrinogen concentrations also existed. Similarly, upon increasing fibrinogen concentration, gel stiffness also increased by as much as 95 Pa (compare 4 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 2X and 8 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 1X). For the three cases in which fibrinogen concentration was doubled (1 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 2X and 2 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 1X; 2 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 2X and 4 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 1X; and 4 mg/ml of fibrinogen with fibrinogen to thrombin ratio of 2X and 8mg/ml of fibrinogen with fibrinogen to thrombin ratio of 1X) while keeping the thrombin concentration fixed, gel stiffness also near doubled, except at low fibrinogen concentrations, as is illustrated in Table 3A.
In comparing this trend to the conditions where thrombin concentration was doubled for a fixed fibrinogen concentration, (1X – 2X for all fibrinogen concentrations) a much lower relative increase in storage modulus was observed, except at lower fibrinogen concentrations. This result suggests that fibrinogen concentration was more influential in the manipulation of gel modulus except under dilute conditions.
Interestingly, the storage modulus for all conditions was not exclusive and an overlap in storage modulus was observed between two conditions. More specifically, the moduli of gels prepared with 1 mg/ml fibrinogen and a 1X fibrinogen/thrombin ratio and 2 mg/ml fibrinogen with a 0.25X fibrinogen/thrombin ratio were both ~14 Pa indicative of the influence of other microstructural aspects also affecting the mechanical stiffness such as the fiber diameter, porosity, and fiber orientation.
3.3 Effect of Substrate Properties on Embryonic Stem Cell Proliferation
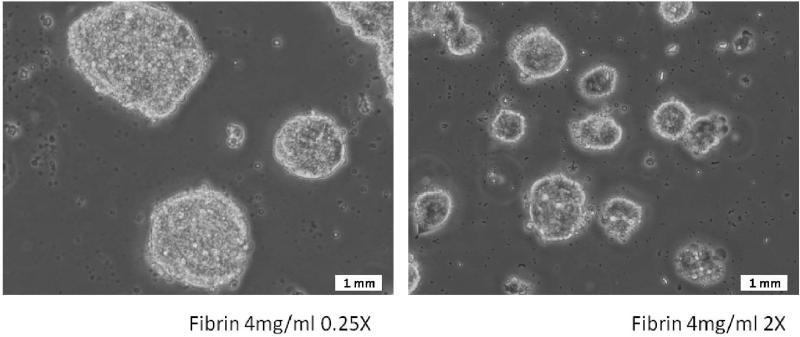
The ESCs when plated on soft fibrin substrates were found to attach well in all the chosen substrate conditions, and were alive and proliferating. However, the cells did not spread out appreciably and retained a spherical, clumped-up morphology. Observation of the cells after 3 days of culture consistently showed formation of cell clusters as shown in Figure (3). When the cell morphology was compared across substrates of different thrombin concentrations for the same fibrinogen concentration, it was typically observed that substrates with lower thrombin concentrations result in larger cell clusters compared to their more cross-linked counterparts.
Figure 3.
Representative images of cells plated on gels of identical fibrinogen concentration, but varying cross-linking fibrinogen/thrombin ratio: 0.25X (Left) and 2X (Right). Cells attach, but do not spread out, instead, they form cell clusters. Cell cluster size greater in gels with lower cross-linking ratio.
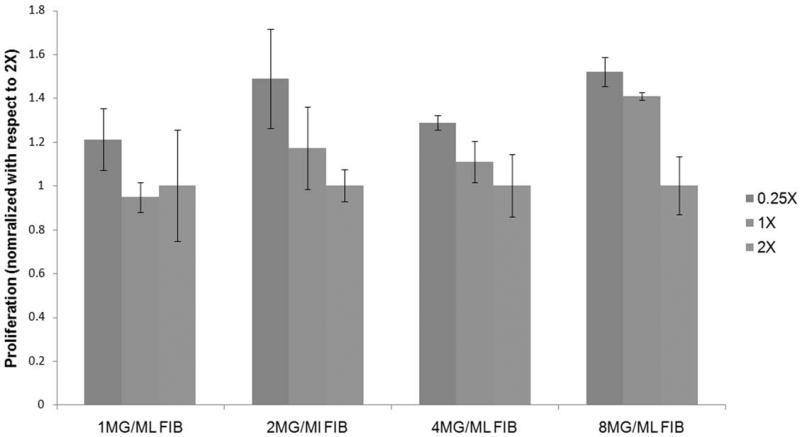
Since the difference in cell cluster size can be attributed to difference in cell proliferation rate, proliferation analysis was performed by alamar blue assay 24 hours after plating the cells on fibrin gels. The time frame of 24 hours was chosen primarily to minimize the effect of fibrin degradation by the cells and to avoid the effect of differentiation on proliferation. The effect of thrombin concentration of the substrates on cellular proliferation is illustrated in Figure (4). For each fibrinogen concentration the proliferation rate is normalized with respect to the condition with the lowest fibrinogen/thrombin ratio. It is consistently observed that for a specific fibrinogen concentration, ESCs cultured on lower crosslinked substrates exhibit a higher proliferation rate compared to parallel cultures on a more crosslinked structure.
Figure 4.
Comparison of the proliferation of mouse ES cells plated on gels of different fibrinogen concentration and varying cross-linking ratio after 24 hours of plating. Y-Axis represents alamar blue fluorescence. All results normalized with respect to the lowest cross-linking ratio for each group. At all fibrinogen concentrations proliferation was found to decrease as the cross-linking increased. * p<0.05 compared to highest stiffness group.
3.4 Effect of Substrate Physical Properties on Embryonic Stem Cell Differentiation
Substrate Stiffness
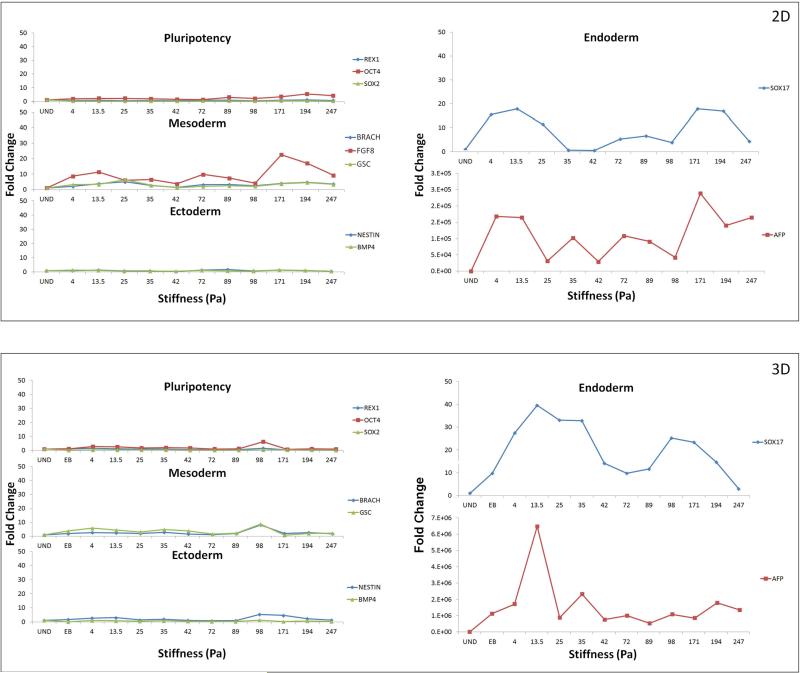
The ESCs were plated on the gels and allowed to spontaneously differentiate for 5 days, following which the cell samples were analyzed by qRT-PCR, for three different markers for each of the germ layers and pluripotency assessment. The chosen markers were Rex1, Oct4 and Sox2 (undifferentiation markers) [Ellis P 2004, Jin GP 2002, Palmqvist L 2005], AFP, Sox17 and Hnf4 (early endoderm markers) [Dziadek M 1978, Hudson C 1997, Weber H 1996] Brachyury T, Gsc and Fgf8 (early mesoderm markers) [Blum M 1992, MacArthur CA 1995, Wilkinson DG 1990] and Nestin, Fgf5 and BMP4 (early ectoderm markers) [Lobo MT 2004, Monsoro-Burq AH 1996, Rathjen J 1999]. During the course of differentiation the cells were cultured in DMEM/ FBS media without any other lineage specific induction. The effect of substrate properties on ESC differentiation is analyzed by comparing the relative variation of all germ layer markers across the entire range of substrate stiffness considered. Figure (5A) illustrates the sensitivity of each germ layer along with pluripotency to changes in substrate stiffness. Overall it was observed that pluripotency markers maintained similar expression levels in all the different substrates considered. Regarding individual germ layers, Figure (5A) shows that mesoderm and ectoderm markers were relatively insensitive to changes in substrate stiffness in the specific stiffness range considered in this study. Endoderm markers, however, were found to respond strongly to changes in substrate property It was observed that substrates of lower stiffness, around the range of 13 Pa were resulting in a stronger endoderm upregulation. However, the higher range of stiffness of 171 Pa was also observed to upregulate both AFP and Sox17. Interestingly, the other germ layers were also slightly elevated under higher stiffness condition, indicating an overall increase in differentiation under those stiffness conditions. Moreover, preferential upregulation of endoderm marker was observed only at lower stiffness conditions. Of the endoderm markers, while both Sox17 and AFP elicited a similar trend in expression, the magnitude of upregulation of AFP was thousands of folds stronger than that of Sox 17.
Figure 5.
The effect of changing substrate stiffness on the embryonic stem cells pluripotency and germ layer commitment in 2-dimensional (a) and 3-dimensional (b) configurations. Results are normalized with respect to undifferentiated cells. Most significant effect was observed in the up-regulation of endoderm markers SOX17 and AFP in lower range stiffness gels. Effect was more prominent in 3-dimensional configurations.
Substrate Composition
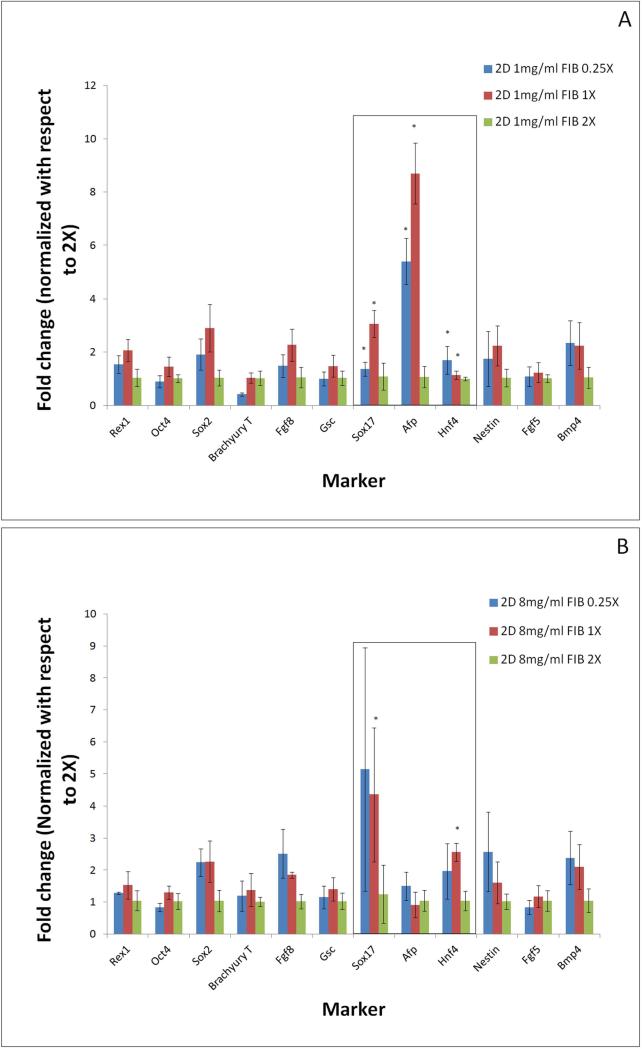
As detailed earlier, fibrin substrate properties were modified by changing both fibrinogen concentration and fibrin to thrombin ratio. Hence it will be important to analyze the effect of gel composition on stem cell differentiation. Differentiation pattern is thus compared across different thrombin concentrations for a fixed value of fibrinogen concentration (Figures 6 a,b). Extreme fibrinogen concentrations of 1mg/ml and 8mg/ml were chosen to assess differentiation patterning across a wide spectrum of fibrinogen concentrations. For ease of comparison the fold changes in gene expression levels are represented by normalizing with respect to 2X crosslinking for each fibrinogen concentration.
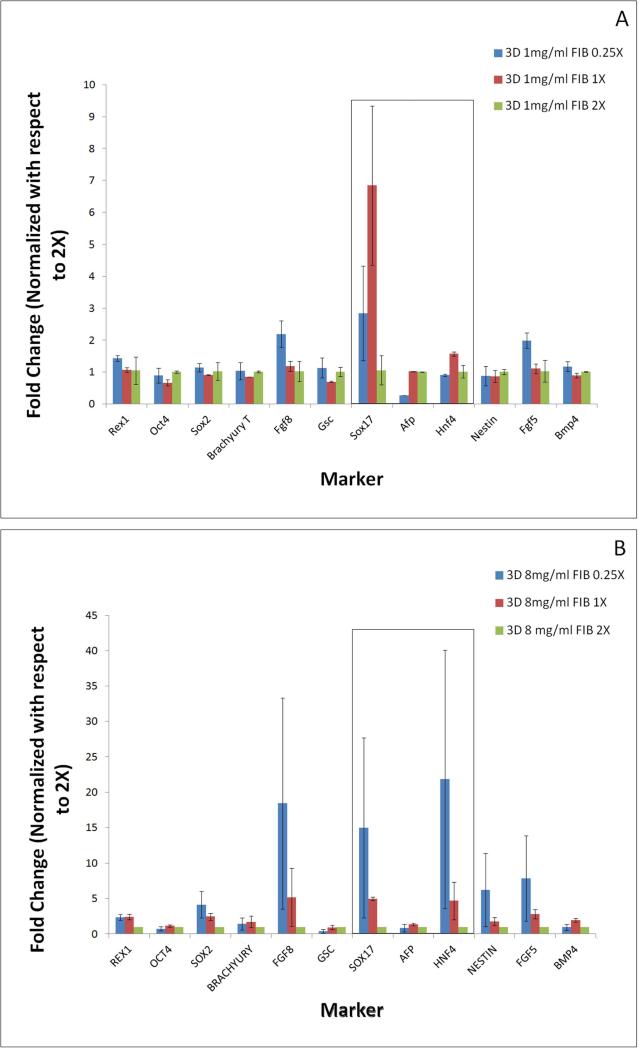
Figure 6.
The effect of changing substrate cross-linking ratios on the embryonic stem cell pluripotency and germ layer commitment, analyzed at extreme fibriniogen concentrations of 1mg/ml (a) and 8mg/ml 1mg/ml (b) in 2-dimensional configuration. Results are normalized with respect to highest cross-linking ratio of 2X. Most significant effect was observed in the up-regulation of endoderm markers when compared between cells differentiated on gels of lower cross-linking (0.25X, 1X) and higher cross-linking (2X) ratio. * p<0.05 compared to highest stiffness group.
Analogous to Figure (5) the mesoderm and ectoderm markers were found to be relatively insensitive to changes in crosslinking ratio in the 1mg/ml fibrinogen concentration (Figure 6a). In contrast, the endodermal markers, particularly Sox17 and AFP, were found to be extremely sensitive to changes in crosslinking ratio. For 1mg/ml fibrinogen concentration we observed approximately 5 and 9 folds increase in AFP expression levels for softer gel conditions of ratios 0.25X and 1X respectively, compared to the stiffer gels synthesized at ratios of 2X. The Sox17 expression levels exhibited a moderate 3-fold and 5-fold increase when the thrombin concentration was lowered from 2X to 1X in both fibrinogen concentration groups respectively. Similar patterns were found across all fibrinogen concentrations where lowering the crosslinking ratio resulted in stronger expression of endoderm markers (Supplementary figures 1 and 2).
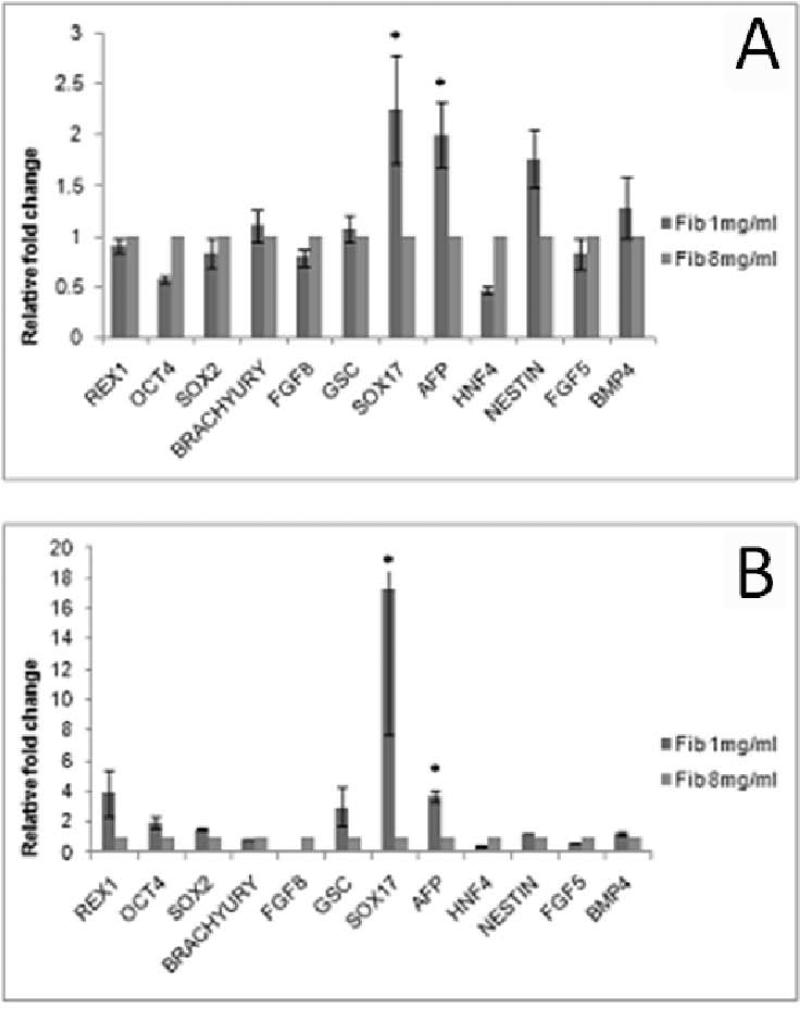
While the above analysis concentrates on the effect of changing crosslinking ratio for a fixed fibrinogen concentration, it will be worthwhile to compare the effect of changing fibrinogen concentration for a fixed cross-linking ratio since both cases result in differences in substrate stiffness (Table 3A). Figure (9) represents the changes in gene expression levels when the ratio of fibrinogen/thrombin is maintained constant (1X) but the concentration of fibrinogen is varied at 1 mg/ml and 8 mg/ml (Figure 9A). Overall it is observed that lower values of fibrinogen concentration preferentially favor endoderm differentiation.
Figure 9.
Pluripotency and germ layer markers of ES cells differentiated in substrates of varying and extreme fibrinogen concentration but same cross-linking ratio of 1X, in (A) 2-dimensional and (B) 3-dimensional culture. Significant upregulation of endoderm markers was found when cells were differentiated on gels obtained at lower fibrinogen concentration. * p<0.05 compared to highest stiffness group.
Substrate Microstructure
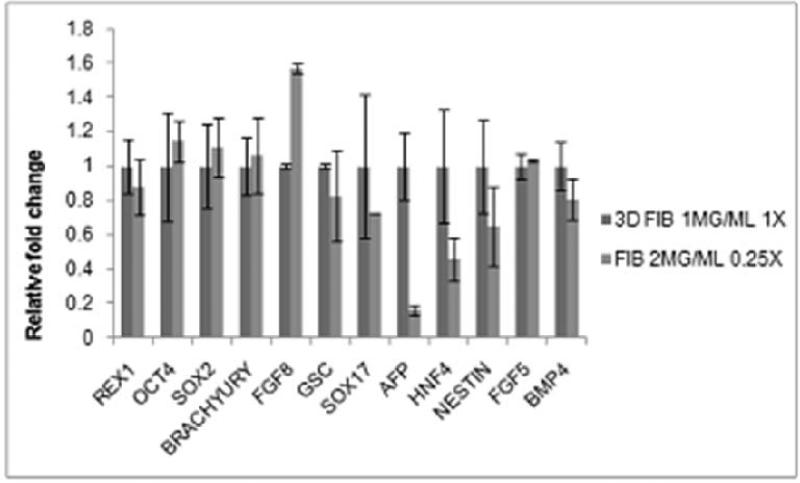
In all of the above mentioned analysis we consistently observed that endoderm upregulation correlated with lower substrate stiffness conditions, achieved either by lowering crosslinking ratio for fixed fibrinogen concentration or by lowering fibrinogen concentration for fixed crosslinking ratio. In order to further analyze if substrate stiffness is uniquely controlling the differentiation patterning, we considered two fibrin substrates of similar stiffness but varying fibrinogen and thrombin concentration, and analyzed the ESC differentiation patterning on them. As illustrated in Table (1) and Table (3A), gels synthesized with 1mg/ml fibrinogen with a fibrinogen to thrombin ratio of 1X and 2mg/ml fibrinogen with a fibrinogen to thrombin ratio of 0.25X result in a similar gel stiffness value of ~14 Pa. ESCs differentiated on these two conditions were analyzed for their differentiation patterning, as illustrated in Figure (10). Quite interestingly it is observed that the mesoderm and ectoderm markers elicited quite a similar behavior under these two conditions, although the endoderm markers were strongly altered. These results indicate that while substrate stiffness is strongly influencing differentiation patterning, clearly it is not the sole player in this complex process. Careful observation of the fibrin gel under these two conditions by SEM (Supplementary Figure 3) revealed significant differences in their microstructural characteristics in spite of comparable stiffness values. Analysis revealed that some of the major structural differences include difference in fiber density, fibrin diameter and pore size. Following this analysis it is reasonable to suggest that along with macroscopic stiffness, microstructural features are also likely to influence differentiation patterning. A more detailed characterization of the gel microstructural features and its possible correlation with ESC differentiation is currently being investigated by our group.
Figure 10.
Marker expression comparison between 1mg/ml fibrinogen obtained at fibrinogen/thrombin cross-linking ratio of 1X and fibrinogen concentration of 2mg/ml obtained atfibrinogen/thrombin cross-linking ratio of 0.25X. Figure shows expression of most markers to be equivalent in both groups except for that of endoderm markers. Results normalized with respect to 1mg/ml Fibrinogen 1X cross-linking ratio.
Culture Configuration
The above experiments were performed by plating cells on pre-formed fibrin gels, in 2-dimensional configurations. However, in order to better elucidate the effects of stiffness, composition and microstructure in ESC differentiation, the ESCs were also cultured in three dimensional configurations where ESCs were suspended within the fibrin substrate during the synthesis of the gels.
Similar to 2D experiments, the effect of substrate properties on ESC differentiation was analyzed by comparing the relative variation of all germ layer markers across the entire range of substrate stiffness considered. Figure (5B) illustrates the sensitivity of each germ layer along with pluripotency to changes in substrate stiffness. Akin to 2D configurations, pluripotency, mesoderm and ectoderm markers were relatively insensitive to changes in substrate stiffness in the specific stiffness range considered in this study. Endoderm markers were found to respond strongly to changes in substrate property. It was observed that substrates of lower stiffness, around the range of 13 Pa were resulting in a stronger endoderm upregulation. In contrast to 2D configurations, the positive effect of higher stiffness range was less obvious in the 3D cultures, where the strongest effect was in the lower range of 13Pa. Both Sox17 and AFP elicited a similar trend in expression; the magnitude of upregulation of AFP was thousands of folds stronger than that of Sox 17, with the upregulation under 3D culture being more dominant than 2D configuration. An additional control of ESC differentiation through EB formation was included in the 3D cultures to account for the possible effect of biochemical induction arising from differential swelling of the gels. As illustrated in Fig (5) the magnitude of upregulation in softer substrates were much stronger than that of EB which suggests that the substrate is playing a more significant role than biochemical induction through the media.
We also studied the effect of substrate composition in 3D configurations (Figure 7). When the fibrinogen concentration was maintained constant and we varied thrombin ratios, the 3-dimensional culture resulted in a much stronger effect on Sox17 expression levels, which was up-regulated by 3 and 7 folds for gel conditions of 0.25X and 1X respectively, as compared to the stiffer gels synthesized at a ratio of 2X for the 1mg/ml fibrinogen gels. In the case of the 8mg/ml gels an even more pronounced effect was found with 5 and 15 fold upregulation in the 1X and 0.25X conditions respectively. Furthermore, in the 8mg/ml fibrinogen conditions the endoderm marker Hnf4 also exhibited a significant upregulation comparable to Sox17. Additionally, while the 2-dimensional cultures leads to somewhat elevated expression levels of many of the germ layer markers; the 3-dimensional cultures were consistently more specific for endoderm markers, along with Fgf and Nestin. Hence while the overall effect of softer substrate (~13Pa) was more pronounced than the stiffer ones, in the entire range of substrate stiffness considered in this study, lower crosslinking always resulted in stronger endoderm expression as compared to higher crosslinking, for invariant fibrinogen concentration.
Figure 7.
The effect of changing substrate cross-linking ratios on the embryonic stem cell pluripotency and germ layer commitment, analyzed at extreme fibriniogen concentrations of 1mg/ml (a) and 8mg/ml 1mg/ml (b) in 3-dimensional configuration. Results are normalized with respect to highest cross-linking ratio of 2X. Most significant effect was observed in the up-regulation of endoderm markers when compared between cells differentiated on gels of lower cross-linking (0.25X, 1X) and higher cross-linking (2X) ratio. * p<0.05 compared to highest stiffness group.
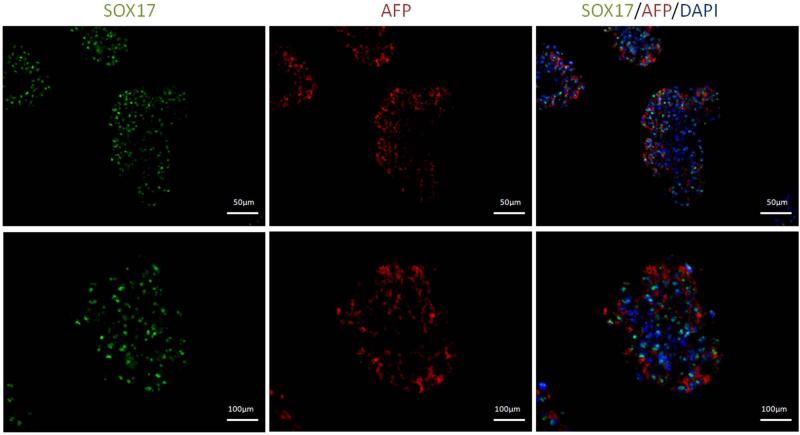
In order to further analyze the differentiated cell population for its protein expression levels, immunohistochemical analysis was performed for the case eliciting the strongest up-regulation in gene expression: the 3-dimensional culture for Sox17 and AFP expression. As illustrated in Figure (8), the differentiated cells cultured in the softer substrates that resulted in highest upregulation of both AFP and SOX17 (1mg/ml, 1X) stained strongly for both Sox17 and AFP. High co-expression of these markers is also observed in the cell clusters formed. Parallel analysis of differentiated cells on gels of higher thrombin concentrations of 2X showed negligible stain (data not included).
Figure 8.
Immunocytochemistry images of cells plated at 1mg/ml and 1X. High expression of endoderm markers AFP (Red) and Sox17 (Green) was observed in the cell clusters which were also found to be highly co-expressed.
When the cross linking ratios were maintained constant, while the fibrinogen concentrations were modified, we once again found that lower values of fibrinogen concentration (Figure 9 b) preferentially favor endoderm differentiation, and such effect is more accentuated in the 3-dimensional culture as compared to the 2-dimensional culture with upregulation of endoderm Afp and Sox17 being 4 and 17 higher in 1mg/ml fibrinogen gels respectively.
4. DISCUSSION
In this paper we present an analysis of the effect of mechanical microenvironment on the embryonic stem cell (ESC) culture and differentiation. ESCs were maintained on soft fibrin substrates of varying physical characteristics, modulated by altering both of its components – fibrinogen and thrombin involved in the formation of fibrin gels.
Analysis of the microscopic architecture of the fibrin gels using SEM reveal a strong effect of both fibrinogen concentration and crosslinking on fiber diameter, fiber bundling and relative pore size, as demonstrated in Figure (1) and (2). Such findings are in agreement with previously published data [Blombäck B 2004, Weisel JW 2004]. Mechanical characterization of the fibrin gels was performed using plate rheometer to measure both the storage and loss modulus as summarized in Table (3). In comparing Table (3A) and (3B), it should be noted that G’>>G” for all of the samples considered which suggests as expected a more solid-like than liquid-like behavior for all the conditions consistent with low loss characteristics of the solids. The results illustrate that increase in fibrinogen or thrombin concentration results in an increase in substrate stiffness. While a higher response is observed by increasing the fibrinogen concentration, chemical variation is also greater, which lead us to focus on the effects of varying thrombin concentration for most of our observations.
The ESCs were cultured for 5 days on these substrates, at the end of which the cells were analyzed for their proliferative and differentiation potential. Since we are interested in early germ layer commitment, differentiation was performed over a relatively short period of time. Our first observation after cell plating was that instead of spreading out, the cells form clusters which vary in size depending on the thrombin concentration, as seen in Figure (3). An explanation can be that the fiber thickness decreases as the substrate becomes more cross-linked, thus decreasing pore size, and exhibiting a higher modulus as shown in Figures (1) and (2), and Tables (3A) and (3B). A similar trend is consistently observed across all four fibrinogen concentrations analyzed.
Cell proliferation assay also confirmed that gels synthesized with lower thrombin concentrations facilitated increased proliferation in comparison to stiffer substrates (Figure (4)). A similar trend has also been reported with mesenchymal stem cells and fibroblasts grown in fibrin gels [Duong H 2009, Ho W 2006]. Studies using neural stem cells showed that when plated in 3D hydrogel cultures, there was a significant increase in proliferation as the stiffness of the scaffolds decreased [Banerjee A 2009]. Similar results were obtained for neural cells in 2D matrices with varying elasticity [Engler AJ 2006]. Analysis of differentiation patterning of the cells cultured on the substrates revealed that while mesoderm and ectoderm remain relatively insensitive to changes in substrate physical properties, the endoderm markers elicit quite a strong response. More specifically, substrates in the range of 13Pa show strongest preferential upregulation of the endoderm markers under both 2D and 3D culture configurations. The overall effect of the substrate, however, was more pronounced in 2D than in 3D cultures. At this point we would like to note that a similar study has been recently reported, however, the mentioned study used substrates with stiffness ranges that were four orders of magnitude higher, and their results were targeted towards osteogenic differentiation [Evans N 2009]. While evaluating the effect of the substrate composition, it was observed that for a fixed fibrinogen concentration, softer gels are preferentially up-regulating endoderm related markers as compared to more cross-linked counterparts. Figures (6-7) are extreme representative case study for fibrinogen concentration of 1 mg/ml and 8mg/ml while similar analysis on substrates of different fibrinogen concentrations consistently exhibited a similar trend of softer substrates leading to a stronger upregulation of endoderm related markers. Notwithstanding the variability in the extent of the particular effect, overall there was remarkable consistency in preferential upregulation of endodermal expression in less crosslinked substrates for both two- and three-dimensional culture configurations. For 2D configuration, the ESCs cultured on 0.25X showed stronger upregulation compared to 1X, while the difference between 1X and 2X is more subtle. The three dimensional culture overall shows a clearer trend of higher upregulation with softer substrates.
A careful observation of the gels synthesized with lower thrombin concentrations show that even though no mesoderm markers exhibit significant upregulation, in some cases FGF8 is quite strongly upregulated. An explanation can be that FGFs, apart from being a mesodermal marker, also plays a role in the endothelial development and are important angiogenic factors [Antoine M 2005, West AF 2001]. The upregulation of these markers can probably be attributed to differentiation into endothelial cells, explained by the fact that softer matrices result in larger cell clusters. Larger cell clusters results in insufficient transport of nutrients and oxygen to the center of the structures, which results in hypoxia and may trigger the creation of blood vessels, resulting in upregulation of FGF markers. Overall we consistently observe that for a particular fibrinogen concentration, gels obtained at lower thrombin concentrations favor endodermal differentiation of the cultured embryonic stem cells. As revealed by the microstructural characterization of the gels, increasing the concentration of thrombin results in a dense network of thinner fibers which are less bundled (Figure (2)). Such microstructural features manifest in increased substrate stiffness as a result of increased cross-linking for the same fibrinogen concentration (Table (3A)). Based on the mechanical characteristics of the gel and the biological characterization of the cells on the fibrin substrates, it is reasonable to suggest without any uncertainty that lower substrate stiffness values preferentially favor ESC differentiation towards the endodermal lineage.
Even though substrate stiffness is clearly playing an important role in cellular lineage commitment, it is certainly not the only factor, as revealed in Figure (10). Comparison of two substrates of varying fibrinogen concentration and fibrinogen to thrombin ratios but comparable substrate stiffness revealed that mesoderm and ectoderm markers were almost invariant under these two conditions, indicating these germ layers to be insensitive to the substrate properties at the initial stage of differentiation and within the substrate ranges presented in this study. Endoderm layer however varied significantly between these conditions, which establish that substrate stiffness, although important, is not the sole player in the process of germ layer commitment. Careful analysis of the substrate micorstructural features revealed that even though the macroscopic stiffness properties are similar, the gels differ substantially in their microstructural features. These results warrant a more detailed analysis of the effect of substrate microstructural features on differentiation, which are currently being investigated by the authors. It is important to note that there is variability in the results obtained from different experiments as observed by the error bars attributed to population heterogeneity and variable response to global inductive cues [Canham MA 2010, Gibson JD 2009]. However, despite the variability, all experiments consistently showed the similar trend of softer substrates preferentially favoring differentiation towards endodermal lineage.
5. CONLCUSION
This paper presents a thorough analysis of the effects of varying substrate mechanical properties on the embryonic stem cell proliferation and differentiation potential. The mechanical properties of the fibrin substrate are varied by systematically altering (i) the fibrinogen concentration and (ii) the thrombin concentration. SEM analysis of the synthesized gels illustrate the variation of microscopic gel microstructure, such as the fiber thickness, porosity and the overall effective area of fibrin strand available for cell anchorage as a result of altered fibrinogen and thrombin concentrations . Detailed mechanical characterization of the different substrates reveals that variation of substrate concentration or cross-linking results in substantial modulation of the substrate stiffness. Analysis of the embryonic stem cells cultured on different substrate conditions clearly illustrate the strong influence which substrate mechanical properties assert on cellular proliferation and differentiation potential. Overall, the ESCs cultured on softer substrates, as a result of lower thrombin concentrations, were found to be more conducive to proliferation as well as differentiation. For both the 2-dimensional and 3-dimensional culture configurations analyzed in this work, the substrates with lower thrombin concentrations elicited a somewhat elevated expression level of most of the gene expression markers tested. However, the level of up-regulation strongly depended on the specific germ layer. It was consistently observed that differential expression of endodermal markers are the strongest in gels obtained with lower cross-linking compared to highly cross-linked gels, an effect which was further accentuated in 3-dimensional culture as compared to 2-dimensional culture. The presented results are indicative of the fact that lower substrate stiffness values are favoring the preferential differentiation of the ESCs towards endodermal lineage. However, substrate stiffness is not the sole player controlling differentiation, which is probably dependant on both gel microstructure and chemical composition. Furthermore, among the germ layers tested, early mesoderm and ectoderm layers were found to be less responsive to substrate properties.
Supplementary Material
Supplementary Figure 1. qPCR results for cells plated on gels of 2mg/ml fibriniogen and varying thrombin ratios in 2D(A) and 3D culture formats (B). Expression levels of ectoderm and mesoderm markers remain relatively constant for all cross-linking ratios. There is significant upregulation of endoderm markers AFP in the 2D culture and Hnf4 in the 3D culture for lower cross-linking groups. * p<0.05 compared to highest stiffness values
Supplementary Figure 2. qPCR results for cells plated on gels of 4mg/ml fibrinogen and varying thrombin ratios in 2D(A) and 3D culture formats (B). Most striking difference of marker expression found in 3D formats where all endoderm markers are significantly upregulated in substrates of lower cross-linking ratios when compared to that of highest cross-linking ratio. Fgfs are also upregulated, but no other mesoderm or ectoderm markers show a significant difference. * p<0.05 compared to highest stiffness values
Supplementary Figure 3. SEM images of 1mg/ml gels 1X cross-linking ratio (A) and 2mg/ml gels 0.25X cross-linking value (B). Rheology measurements showed comparable stiffness ranges, however, microstructural analysis showed significant differences in characteristics including node density, pore size distribution and fiber diameter.
AKNOWLEDGEMENTS
IB acknowledges support from NIH New Innovator Award DP2 116520 and ORAU Ralph Powe Junior Faculty Enhancement Award. PNK acknowledges support of the Edward R. Weidlein Chair Professor funds as well as the Center for Complex Engineered Multifunctional Materials (CCEMM) for partial support of this work.
Footnotes
7. AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- 1.Antoine M, Wirz W, Tag CG, Mavituna M, Emans N, Korff T, et al. Expression pattern of fibroblast growth factors (FGFs), their receptors and antagonists in primary endothelial cells and vascular smooth muscle cells. Growth Factors. 2005;23(2):87–95. doi: 10.1080/08977190500096004. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Arha M, Choudhary S, Ashton RS, Bhatia SR, Schaffer DV, et al. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials. 2009;30(27):4695–4699. doi: 10.1016/j.biomaterials.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee I, Sharma N, Yarmush M. Impact of co-culture on pancreatic differentiation of embryonic stem cells. Journal of Tissue Engineering and Regenerative Medicine. 2010 doi: 10.1002/term.317. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 4.Blombäck B, Bark N. Fibrinopeptides and fibrin gel structure. Biophysical Chemistry. 2004;112(2-3):147–151. doi: 10.1016/j.bpc.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Blum M, Gaunt SJ, Cho KWY, Steinbeisser H, Blumberg B, Bittner D, et al. Gastrulation in the mouse: The role of the homeobox gene goosecoid. Cell. 1992;69(7):1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 6.Canham MA, Sharov AA, Ko MSH, Brickman JM. Functional Heterogeneity of Embryonic Stem Cells Revealed through Translational Amplification of an Early Endodermal Transcript. PLoS Biol. 2010;8(5):e1000379. doi: 10.1371/journal.pbio.1000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chowdhury F, Li Y, Poh Y-C, Yokohama-Tamaki T, Wang N, Tanaka TS. Soft Substrates Promote Homogeneous Self-Renewal of Embryonic Stem Cells via Downregulating Cell-Matrix Tractions. PLoS ONE. 2010;5(12):e15655. doi: 10.1371/journal.pone.0015655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vos J, Assou S, Tondeur S, Dijon M, Hamamah S. Les cellules souches embryonnaires humaines : de la transgression de l'embryon humain à la médecine régénératrice de demain. Gynécologie Obstétrique & Fertilité. 2009;37(7-8):620–626. doi: 10.1016/j.gyobfe.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Duong H, Wu B, Tawil B. Modulation of 3D Fibrin Matrix Stiffness by Intrinsic Fibrinogen–Thrombin Compositions and by Extrinsic Cellular Activity. Tissue Engineering Part A. 2009;15(7):1865–1876. doi: 10.1089/ten.tea.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dziadek M, Adamson E. Localization and synthesis of alphafoetoprotein in post-implantation mouse embryos. Journal of Embryology and Experimental Morphology. 1978;43(1):289–313. [PubMed] [Google Scholar]
- 11.Ellis P, Fagan B, Magness S, Hutton S, Taranova O, Hayashi S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Developmental Neuroscience. 2004;26:18. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- 12.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 13.Evans N, Minelli C, Gentleman E, LaPointe V, Patankar S, Kallivretak iM, et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 2009;18:15. doi: 10.22203/ecm.v018a01. [DOI] [PubMed] [Google Scholar]
- 14.Gibson JD, Jakuba CM, Boucher N, Holbrook KA, Carter MG, Nelson CE. Single-celltranscript analysis of human embryonic stem cells. Integrative Biology. 2009;1(8-9):540–551. doi: 10.1039/b908276j. [DOI] [PubMed] [Google Scholar]
- 15.Ho W, Tawil B, Dunn JCY, Wu BN. The Behavior of Human Mesenchymal Stem Cells in 3D Fibrin Clots: Dependence on Fibrinogen Concentration and Clot Structure. Tissue Eng. 2006;12(6):9. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 16.Hudson C, Clements D, Friday RV, Stott D, Woodland HR. Xsox17[alpha] and -[beta] Mediate Endoderm Formation in Xenopus. Cell. 1997;91(3):397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 17.Jin GP, Chang ZY, Scholer HR, Pei D. Stem cell pluripotency and transcription factor Oct4. Cell Res. 2002;12(5-6):321–329. doi: 10.1038/sj.cr.7290134. [DOI] [PubMed] [Google Scholar]
- 18.Kidszun A, Schneider D, Erb D, Hertl G, Schmidt V, Michael E, et al. Isolated Pancreatic Islets in Three-Dimensional Matrices Are Responsive to Stimulators and Inhibitors of Angiogenesis. Cell Transplantation. 2006;15:489–497. doi: 10.3727/000000006783981774. [DOI] [PubMed] [Google Scholar]
- 19.Ko HF. Design, Synthesis, and Optimization of Nanostructured Calcium Phosphates (NanoCaPs) and Natural Polymer Based 3D Non-Viral Gene Delivery Systems [Ph. D. Thesis] Department of Biomedical Engineering, Carnegie Mellon University; Pittsburgh, Pa: 2008. [Google Scholar]
- 20.Lim J-Y, Min B-H, Kim B-G, Han H-J, Kim S-J, Kim C-W, et al. A fibrin gel carrier system for islet transplantation into kidney subcapsule. Acta Diabetologica. 2009;46(3):243–248. doi: 10.1007/s00592-008-0073-4. [DOI] [PubMed] [Google Scholar]
- 21.Lobo MT, Arenas M, Alonso FJ, Gomez G, Bazán E, Paíno C, et al. Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell and Tissue Research. 2004;316(3):369–376. doi: 10.1007/s00441-003-0848-4. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur CA, Lawshe A, Xu J, Santos-Ocampo S, Heikinheimo M, Chellaiah AT, et al. FGF-8 isoforms activate receptor splice forms that are expressed in mesenchymal regions of mouse development. Development. 1995;121(11):3603–3613. doi: 10.1242/dev.121.11.3603. [DOI] [PubMed] [Google Scholar]
- 23.Monsoro-Burq AH, Duprez D, Watanabe Y, Bontoux M, Vincent C, Brickell P, et al. The role of bone morphogenetic proteins in vertebral development. Development. 1996;122(11):3607–3616. doi: 10.1242/dev.122.11.3607. [DOI] [PubMed] [Google Scholar]
- 24.Mosesson M, Siebenlist K, DA. M. The structure and biological features of fibrinogen and fibrin. Annals of the New York Academy of Science. 2001;936:19. doi: 10.1111/j.1749-6632.2001.tb03491.x. [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Keller G. Differentiation of Embryonic Stem Cells to Clinically Relevant Populations: Lessons from Embryonic Development. Cell. 2008;132(4):661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Palmqvist L, Glover CH, Hsu L, Lu M, Bossen B, Piret JM, et al. Correlation of Murine Embryonic Stem Cell Gene Expression Profiles with Functional Measures of Pluripotency. Stem Cells. 2005;23(5):663–680. doi: 10.1634/stemcells.2004-0157. [DOI] [PubMed] [Google Scholar]
- 27.Rathjen J, Lake J, Bettess M, Washington J, Chapman G, Rathjen P. Formation of a primitive ectoderm like cell population, EPL cells, from ES cells in response to biologically derived factors. J Cell Sci. 1999;112(5):601–612. doi: 10.1242/jcs.112.5.601. [DOI] [PubMed] [Google Scholar]
- 28.Rosso F, Marino G, Giordano A, Barbarisi M, Parmeggiani D, Barbarisi A. Smart materials as scaffolds for tissue engineering. Journal of Cellular Physiology. 2005;203(3):465–470. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 29.Weber H, Holewa B, Jones EA, Ryffel GU. Mesoderm and endoderm differentiation in animal cap explants: identification of the HNF4-binding site as an activin A responsive element in the Xenopus HNF1alpha promoter. Development. 1996;122(6):1975–1984. doi: 10.1242/dev.122.6.1975. [DOI] [PubMed] [Google Scholar]
- 30.Weisel JW. The mechanical properties of fibrin for basic scientists and clinicians. Biophysical Chemistry. 2004;112(2-3):267–276. doi: 10.1016/j.bpc.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 31.West AF, O'Donnell M, Charlton RG, Neal DE, Leung HY. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer. 2001;85(4):576–583. doi: 10.1054/bjoc.2001.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343(6259):657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. qPCR results for cells plated on gels of 2mg/ml fibriniogen and varying thrombin ratios in 2D(A) and 3D culture formats (B). Expression levels of ectoderm and mesoderm markers remain relatively constant for all cross-linking ratios. There is significant upregulation of endoderm markers AFP in the 2D culture and Hnf4 in the 3D culture for lower cross-linking groups. * p<0.05 compared to highest stiffness values
Supplementary Figure 2. qPCR results for cells plated on gels of 4mg/ml fibrinogen and varying thrombin ratios in 2D(A) and 3D culture formats (B). Most striking difference of marker expression found in 3D formats where all endoderm markers are significantly upregulated in substrates of lower cross-linking ratios when compared to that of highest cross-linking ratio. Fgfs are also upregulated, but no other mesoderm or ectoderm markers show a significant difference. * p<0.05 compared to highest stiffness values
Supplementary Figure 3. SEM images of 1mg/ml gels 1X cross-linking ratio (A) and 2mg/ml gels 0.25X cross-linking value (B). Rheology measurements showed comparable stiffness ranges, however, microstructural analysis showed significant differences in characteristics including node density, pore size distribution and fiber diameter.