Abstract
Background
To determine whether markers of systemic inflammation are associated with the presence of moderate-to-severe obstructive sleep apnea (OSA), and whether this association differs based on HIV and HIV treatment status.
Methods
HIV-uninfected men (HIV−; n=60), HIV-infected men receiving HAART (HIV+/HAART; n=58), and HIV-infected men not receiving HAART (HIV+/ No HAART; n=41) underwent polysomnograpy and measurement of plasma levels of TNF-alpha, soluble TNF-alpha receptors I and II (sTNFRI and sTNFRII) and IL-6. The relationship between moderate-severe OSA (respiratory disturbance index ≥15 apnea/hypopnea events/hour) and inflammatory markers was assessed with multivariable regression models.
Results
Compared to the HIV− men, HIV+/HAART men and HIV+/No HAART men had higher levels of TNF-alpha, sTNFRI, and sTNFRII, independent of age, race, smoking status, obstructive lung disease (OLD), and BMI. Moderate-to-severe OSA was present in 48% of the sample (HIV−:57%; HIV+/HAART: 41%; HIV+/No HAART: 44%). Among the HIV+/No HAART men, but not in the other groups, TNF-alpha, sTNFRII, and IL-6 levels were higher in those with moderate-severe OSA compared to men with no-to-mild OSA after adjustment for age, race, smoking status, OLD, and BMI. Within this group, the association of high TNF-alpha concentrations with moderate-severe OSA was also independent of CD4 cell count and plasma HIV RNA concentration.
Conclusions
Compared to HIV-infected men on HAART and HIV-uninfected men, markers of systemic inflammation were higher in HIV-infected men not receiving HAART. In these men, TNF-alpha was significantly related to obstructive sleep apnea, independent of HIV-related covariates.
INTRODUCTION
Effective antiretroviral therapy has dramatically reduced morbidity and mortality among HIV-infected patients, such that a greater number of HIV-infected individuals are dying from non-AIDS comorbidities.1,2,3 While the etiology of these comorbidities is varied, a common mechanistic link is persistent systemic inflammation and immune activation. With the initiation of HAART, inflammatory markers decrease, but remain elevated compared to HIV-uninfected individuals.4,5 Elevated levels of inflammatory markers in HIV-infected populations have been associated with increased mortality,6,7 and other non-AIDS diseases including cardiovascular disease8 and diabetes.9
Obstructive sleep apnea (OSA) has also been associated with systemic inflammation in the general population.10 While adiposity particularly in the neck and upper abdomen leads to mechanical alterations in upper airway function, adipose tissue secretes inflammatory markers, such as TNF-alpha and IL-1 beta which may impair neuromuscular responses to airway obstruction, further predisposing to upper airway collapse.11,12,13 Furthermore, inflammatory markers are associated with OSA independent of adiposity and may be due to hypoxemia-induced oxidative stress.10,14,15,16
Little is known about whether an association exists between OSA severity and inflammation in HIV-infected persons. In a sub-study of the Multicenter AIDS Cohort Study (MACS) named SIESTA (Study of Immunes Effect on Sleep, HIV Treatment, and Apnea) we found a high prevalence of OSA regardless of HIV-status, and regardless of HAART treatment.15 Interestingly, OSA was associated with higher waist circumference and trunk fat in the HAART-treated men, but not in the HIV-infected men who were not receiving HAART. In an exploratory analysis in a subset of this group, we found that moderate-severe OSA (defined as a respiratory disturbance index (RDI) ≥ 15 events/hour) was independently associated with higher serum hsCRP and higher plasma HIV RNA concentrations. These findings raised the possibility that inflammation may be related to OSA in untreated, HIV-infected persons. In this investigation, our aim was to extend our previous findings to determine whether blood levels of other inflammatory markers (specifically TNF-alpha, soluble TNF-alpha receptors I and II [sTNFRI and sTNFRII], and IL-6, all reportedly elevated in HIV infection and/or OSA5,14,17) were associated with moderate-severe OSA in the SIESTA cohort, particularly among HIV-infected men not receiving HAART.
METHODS
Study Population
HIV-uninfected men (HIV−; n=60), HIV-infected men receiving highly active antiretroviral therapy (HIV+/HAART; n=58), and HIV-infected men not receiving HAART (HIV+/ No HAART; n=41) from the Baltimore/Washington and Pittsburgh sites of the Multicenter AIDS Cohort Study (MACS)18 were recruited for this analysis, as previously described.15 HAART was defined according to the DHHS/Kaiser Guidelines.19 Men who had not received HAART in the previous 12 months were permitted to enroll into the HIV+/No HAART group. All qualified participants signed written informed consents after institutional review board approval of study protocol and forms.
Study Protocol
Participants were admitted to the Johns Hopkins General Clinical Research Center between 6–8 pm and a polysomnography study to assess sleep and breathing was conducted between 11 pm and 7 am. Serum for measurement of inflammatory markers was collected at 7am on the morning after polysomnogram. Other data and specimens were derived from the MACS visit closest to the sleep study visit were used (median interval between MACS visit and sleep study visit: 4 days [Interquartile Range (IQR); 0, 39 days]); these data included demographic and HIV treatment information, HIV serostatus, plasma HIV RNA concentration (Amplicor HIV Monitor Assay, Roche Diagnostics, Nutley, NJ), and T-cell subsets determined by flow cytometry.20 Hepatitis B infection was defined by Hepatitis B positivity at most recent measurement (e.g. most recent Hepatitis B Surface antigen positive or most recent Hepatitis B viral load positive). Hepatitis C infection was defined by Hepatitis C RNA positivity before or at the time of visit.
Study Procedures
Polysomnography Testing
Standard overnight polysomnography was carried out as previously described.15 OSA severity was characterized by the respiratory disturbance index (RDI). The RDI was defined as the total number of apneas or hypopneas per hour of sleep. Apnea was defined as a >90% reduction in thermistor-based airflow for at least 10 seconds. Hypopnea was defined as a > 30% reduction in airflow for at least 10 seconds, which was accompanied by an electroencephalographic arousal or a drop in SaO2 of 4% or more.21 Significant OSA was defined as an RDI ≥ 15 events/hour, which is considered moderate-severe OSA and represents a clinically important threshold at which treatment may be considered.22,23
Inflammatory marker Measurement Methods
Plasma samples, frozen at −80 degrees Celsius at time of collection, were pulled from repository and forwarded to the Johns Hopkins Bayview Advanced Chemistry Laboratory in Baltimore, MD. Markers (including TNF-alpha, soluble TNF-alpha receptors I and II (sTNFRI and sTNFRII) and IL-6) were measured by ELISA by R&D Systems, Minneapolis, MN. Sensitivity, intra-assay and inter-assay variation (expressed as percent coefficient of variation), are expressed in sequence for individual cytokines is as follows: TNF-alpha (0.48 pg/ml, 1.87%, 11.07%), sTNFRI (0.77 pg/ml, 1.31%, 8.95%), sTNFRII (0.60 pg/ml, 2.55%, 5.93%), and IL-6 (0.40 pg/ml, 2.19%, 9.79%). Values were measured in duplicate and averaged for analysis.
Statistical Analyses
Participant characteristics in the 3 groups were compared using the nonparametric testing for continuous variables and chi-squared testing for categorical variables, with post-hoc pairwise comparisons as appropriate.
To determine whether the concentrations of inflammatory markers differed by group, unadjusted concentrations in each of the 3 groups were compared using Kruskall-Wallis testing. Multivariable generalized gamma regression was then used to compare the percentile values of inflammatory markers in each of the two HIV-infected groups to the HIV-uninfected men (reference group) because of the non-normal distribution of the inflammatory markers.24 The relative percentile (RP), obtained from exponentiating the β coefficient, represents the shift in the distribution of the inflammatory markers compared to the reference; for example, a RP of 2 means that the levels in a given group are twice as high as those in the reference group. Since the shape and scales of the distributions were held constant, this shift applies to the entire distribution, and not just the center, i.e., average or median. Models were adjusted for age, race, BMI, smoking status (current, former, never), and obstructive lung disease (OLD) (defined via the Global initiative for Obstructive Lung Disease (GOLD) cutoff of a forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of less than 70%)25, with exclusion of participants for missing data (n=3). Post-bronchodilator testing was not available.
Potential differences in demographic factors, treatment-related variables, and inflammatory markers between those with and without moderate-severe OSA in each group defined by HIV and treatment status were evaluated using Wilcoxon-rank sum and chi-squared tests. Multivariable regression was then used to compare the relative percentiles of inflammatory markers according to the presence of moderate-severe OSA and stratified by group defined by HIV and treatment status (using the HIV-uninfected men with no-mild OSA as the reference group).
The association between individual inflammatory markers and moderate-severe OSA was further explored in the HIV+/No HAART group via bivariate logistic regression models with each of the potential confounding variables. Given the non-normality of their distributions, inflammatory markers were dichotomized as “high” or “low” with respect to the median level of each inflammatory marker.
Statistical analyses were performed using Statistical Analysis Software (SAS, version 9.2).
RESULTS
Study Population Characteristics (Table 1)
Table 1. Study Population Characteristics.
Continuous variables compared using Kruskall-Wallis testing. Categorical values compared using chi-squared analysis. Values with “±” and number following represent mean ± standard deviation. Respiratory Disturbance Index (RDI) is defined as the total number of apneic or hypopneic events per hour of total sleep time. Table as previously seen in: Brown et al. (2010)19.
| HIV− | HIV+/no HAART | HIV+/HAART | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| n | 60 | 41 | 58 | |
| Age (years) | 54 ± 9 | 49 ± 8 | 51 ± 8a | 0.02 |
| Race (non-black), n(%) | 25 (42%) | 16 (39%) | 28 (58%) | 0.62 |
| BMI (kg/m2) | 28.6 ± 7.2 | 25.4 ± 4.1 | 25.5 ± 4.5ab | <0.01 |
| Obstructive lung disease, n(%) | 6 (10) | 9 (23)Ϯ | 10 (18)* | .45 |
| Smoking | ||||
| Never, n(%) | 11(18%) | 6(15%) | 16(28%) | |
| Former, n(%) | 27(45%) | 18(44%) | 26(45%) | .47 |
| Current, n(%) | 22(37%) | 17(41%) | 16(28%) | |
| HIV-Related Demographics | ||||
| HIV RNA <50 copies/ml, n(%) | 4 (10%) | 47 (81%) | <0.01 | |
| CD4 (cells/mm3) | - | 518 ± 287 | 583 ± 306 | 0.27 |
| Yrs from HAART initiation | - | - | 7.5 ± 2.8 | |
| HAART-naïve, n(%) | - | 21 (51%) | - | |
| HIV Treatment Regimen | ||||
| PI-based, n(%) | - | 3 (7%) | 30 (53%) | |
| NNRTI-based, n(%) | - | 0 (0%) | 23 (40%) | |
| 3 NRTIs, n(%) | - | 0 (0%) | 3 (5%) | |
| Coinfection | ||||
| Hepatitis B Positive | 1 (2%) | 2 (5%) | 2 (3%) | NA |
| Hepatitis C Positive | 8 (13%) | 13 (32%) | 12 (21%) | <0.01 |
| Polysomnography | ||||
| RDI median (IQR) | 18 (6,29) | 11 (4,24) | 11 (4,21) | 0.09 |
| Prevalence of Mod-Sev OSA | ||||
| n (%) | 34 (57%) | 19 (44%) | 24 (41%) | 0.19 |
| BMI< 25 kg/m2, n (%) | 5 of 20 (25%) | 11 of 22 (50%) | 7 of 29 (24%) | 0.10 |
| Inflammatory marker levels (pg/ml) | ||||
| TNF-alpha, median (IQR) | 2.2 (0.7) | 5.2 (4.1)a | 2.7 (1.7)ab | <0.01 |
| sTNFRI, median (IQR) | 2013 (1086) | 2149.6 (1115) | 2148 (1292) | 0.37 |
| sTNFRII, median (IQR) | 4662 (2004) | 6936 (2717)a | 5409 (2909)ab | <0.01 |
| IL-6, median (IQR) | 1.2 (1.7) | 1.5 (1.6) | 1.4 (1.6) | 0.46 |
=p<0.05 vs HIV− Group.
=p<0.05 vs HIV+/No HAART Group.
=(n=39).
=(n=57)
The study population consisted of 159 men; 60 HIV negative (HIV−), 58 HIV-infected on HAART (HIV+/HAART), and 41 HIV-infected not on HAART (HIV+/no HAART). Mean ages were similar in all groups, with the exception of the HIV+/no HAART group which was significantly less than in the HIV− group (p<0.05). Race distribution was similar across groups. Both HIV+ groups had a lower average BMI, compared to the HIV− group (p<0.01). There was no significant difference in prevalence of OLD or smoking status between groups defined by HIV status. The percentage with suppressed HIV RNA levels (below 50 copies/mL) differed between the HAART and no HAART groups (91% versus 17%, respectively; p<0.01), while CD4 cell counts did not differ significantly. Hepatitis C status differed significantly between the three groups, with the highest percentage in the HIV/no HAART group.
The prevalence of moderate-to-severe OSA, as defined by a RDI ≥15 events per hour, was similar across groups, ranging from 41% in the HIV+/HAART group (n=24) to 57% in the HIV− group (n=34) (p=0.19 for difference between no HAART group and other groups), as previously described.15 When only men with a BMI < 25 kg/m2 were evaluated, there was a higher prevalence of OSA in the HIV+/no HAART group (50%, n=22) as compared to 25% for the HIV− group (n=20) and 24% for the HIV+/HAART group (n=29), though these differences were not statistically significant (p=0.10).
Markers of Systemic Inflammation
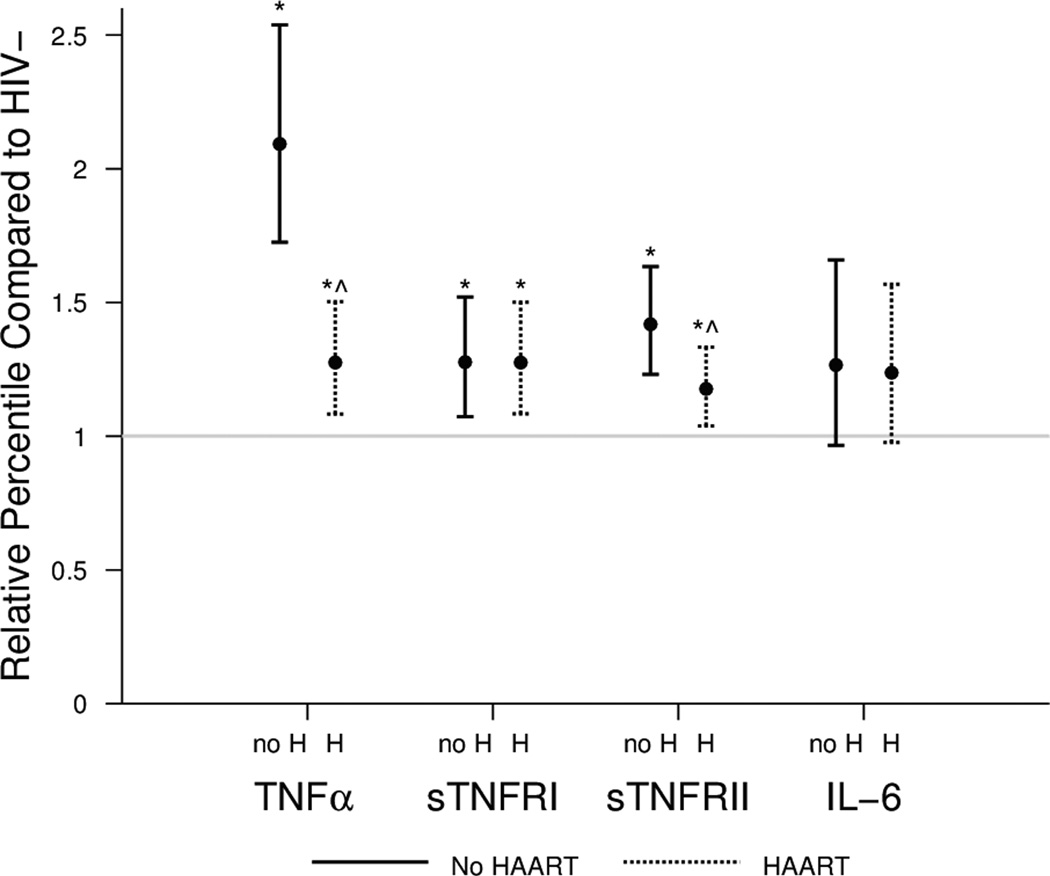
Table 1 shows the unadjusted inflammatory marker levels and their distribution by HIV status and HAART treatment. TNF-alpha and sTNFRII values were significantly higher in both HIV+ groups versus the HIV− group. The values for these two markers were also significantly higher in the HIV+/No HAART group as compared to the HIV+/HAART group. No significant differences were found for IL-6 or sTNFRI. After adjustment for age, race, smoking status, OLD, and BMI, TNF-alpha, sTNFRI, and sTNFRII levels were all significantly higher in the two HIV+ groups than in the HIV− group (Figure 1, p≤0.01). In addition, the TNF-alpha and sTNFRII levels were significantly higher in the HIV+/No HAART group as compared to the HIV+/HAART group.
Figure 1. Inflammatory marker levels vary by HIV and HAART status.
Relative percentiles (RP) of inflammatory marker levels and 95 percent confidence intervals in HIV+ men (HAART (H) and no HAART (no H) groups) as compared to HIV− men (reference group, indicated by a horizontal line at 1.0), adjusted for age, race, smoking status, OLD, and BMI in a generalized gamma model. RPs are interpreted as follows: the relative percentile is the multiplicative factor by which the group of interest differs from the reference group. For example, a RP of 2 means that the levels are twice as high as those in the reference group. (* p < 0.05 vs. HIV−; ^ p <0.05 vs. HIV+/No HAART)
Relationship of Inflammatory Markers to Obstructive Sleep Apnea
Table 2 shows the characteristics and unadjusted inflammatory marker concentrations of each group stratified by the presence or absence of moderate-severe sleep apnea. In both of the HIV− and HIV+/HAART groups, those with moderate-severe sleep apnea had a significantly higher median BMI compared to those with no-mild OSA. Advanced age was also significantly associated with moderate-severe OSA in the HIV+/HAART group. In the HIV− group, sTNFRII levels, but not other inflammatory markers, were significantly higher in those with moderate-severe OSA group compared to those with no-mild OSA, while in the HIV+/No HAART both TNF-alpha and sTNFRII were significantly higher in those with moderate-severe (versus no-mild OSA). Also in the HIV+/No HAART group, those with moderate-severe OSA had a higher percentage with HIV-RNA > 10K copies/ml, were older, and had increased levels of IL-6 compared to those with no-mild OSA, but these differences were of borderline significance (0.05<p<0.10).
Table 2. Comparison of characteristics in HIV−, HIV+/no HAART, and HIV+/HAART groups by presence or absence of moderate/severe OSA.
Inflammatory markers are unadjusted. P-values were obtained using the nonparametric Kruskall-Wallis testing for continuous variables and chi-squared testing for categorical variables. Data are expressed as median (IQR) unless otherwise noted.
| HIV− | HIV+/No HAART | HIV+/HAART | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RDI < 15 n=26 |
RDI ≥ 15 n=34 |
p | RDI < 15 n=23 |
RDI ≥ 15 n=18 |
p | RDI < 15 n=34 |
RDI ≥ 15 n=24 |
p | |
| Age (years) | 49.7 (13.4) | 54.4 (17.1) | 0.10 | 47.3 (9.5) | 51.9 (8.6) | 0.06 | 47.4 (9.9) | 55.4 (9.5) | 0.02 |
| Race (non-Black), n(%) | 7 (27%) | 18 (53%) | 0.06 | 9 (39%) | 7 (39%) | 1.00 | 16 (47%) | 12 (50%) | 1.00 |
| BMI (kg/m2) | 24.4 (6.4) | 29.9 (5.6) | <0.01 | 25 (5) | 23.7 (4.6) | 0.46 | 23.4 (5.2) | 27.4 (8.2) | <0.01 |
| Obstructive Lung Disease, n(%) | 4 (15%) | 2 (6%) | 0.39 | 6 (26%) | 3 (19%)Ϯ | 0.71 | 5 (15%)* | 5 (21%) | 0.73 |
| Smoking: Never | 6 (23%) | 5 (15%) | 3 (13%) | 3 (17%) | 9 (26%) | 7 (29%) | |||
| Former | 8 (31%) | 19 (56%) | 0.15 | 8 (35%) | 10 (56%) | 0.30 | 17 (50%) | 9 (38%) | 0.63 |
| Current | 12 (46%) | 10 (29%) | 12 (52%) | 5 (28%) | 8 (24%) | 8 (33%) | |||
| CD4 cell count (cells/mm3) | 954 (410) | 1009 (402) | 0.30 | 428 (428) | 440 (343) | 0.95 | 562 (395) | 604 (349) | 0.73 |
| HIV Duration (yrs) | 3.7 (7.8) | 4.6 (16) | 0.93 | 4.8 (15.2) | 14.1 (16.9) | 0.16 | |||
| HIV RNA > 100K copies/ml, n(%) | 3 (13%) | 5 (28%) | 0.27 | ||||||
| HIV RNA > 10K copies/ml, n(%) | 9 (39%) | 13 (72%) | 0.06 | 2 (6%) | 1 (4%) | 1.00 | |||
| HAART Naïve, n(%) | 13 (57%) | 8 (44%) | 0.54 | ||||||
| TNF-alpha (pg/ml) | 2.2 (0.8) | 2.3 (0.9) | 0.23 | 3.7 (3.1) | 6 (4.3) | <0.01 | 2.9(1.9) | 2.6 (1.3) | 0.92 |
| sTNFRI (pg/ml) | 1982 (1096) | 2041 (1224) | 0.34 | 2220 (1703) | 2126 (1042) | 0.64 | 2137 (1055) | 2199 (1579) | 0.86 |
| sTNFRII (pg/ml) | 4306 (1349) | 5123 (2453) | 0.03 | 6488(2514) | 7786 (4005) | 0.04 | 5573 (2992) | 5226 (2491) | 0.43 |
| IL-6 (pg/ml) | 1.1 (1.6) | 1.6 (1.8) | 0.38 | 1.4 (1.7) | 1.8 (2.6) | 0.09 | 1.4 (1.4) | 1.4 (1.8) | 0.59 |
=(total n=16).
=(total n=33)
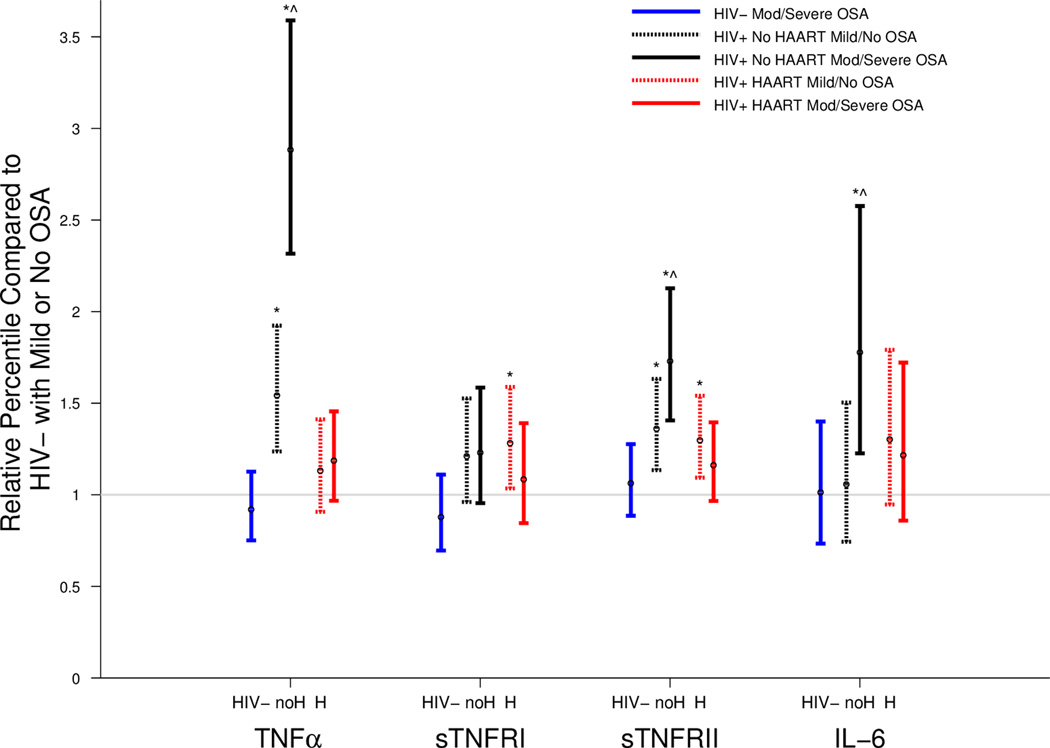
We then compared the distributions of the inflammatory markers in each group defined by HIV status dichotomized by the presence or absence of moderate-severe OSA, using the HIV-uninfected men without moderate-severe OSA as a reference group. Analyses were adjusted for race, age, smoking status, OLD, and BMI (Figure 2). In the HIV− men, inflammatory marker concentrations were similar, regardless of OSA status. In the HIV+/No HAART men both with and without moderate-severe OSA, TNF-alpha and sTNFRII concentrations were higher than the HIV− reference group (no-mild OSA), whereas IL-6 concentrations were higher than the reference group in the HIV+/No HAART group with moderate-severe OSA. Among the HIV+/no HAART men, TNF-alpha, sTNFRII, and IL-6 concentrations were significantly higher in the moderate-severe OSA group than the no-mild OSA groups. In the HIV+/HAART group, men both with and without moderate-severe OSA generally had similar concentrations of inflammatory markers to the HIV− men with no-mild OSA, with the exception of sTNFRI and sTNFRII, both of which were higher in the HIV+/HAART group with no-mild OSA (p=0.02 and p<0.01, respectively).
Figure 2. Inflammatory marker levels vary by OSA category when men are stratified by HIV and HAART status.
This figure displays relative percentiles of inflammatory marker levels by HIV and OSA groups using the HIV− with no to mild OSA (RDI<15) group as a reference. Generalized gamma models adjusted for age, race, and BMI. HIV− = uninfected men with moderatesevere OSA; H=HAART; no H=no HAART. Vertical lines represent 95 percent confidence intervals. (* p < 0.05 vs. HIV−, no to mild OSA; ^ p <0.05 vs. HIV status-matched, no to mild OSA group).
Exploratory Analysis in the HIV+/No HAART group
We undertook a series of exploratory analyses using logistic regression to better understand the relationship between inflammatory markers and moderate-severe OSA in the HIV+/No HAART group. High TNF-alpha (above the median) (OR 5.94, 95%CI: 1.52, 23.18; p=0.01) and sTNFRII (OR 3.75, 95% CI: 1.02, 13.80; p=0.05) levels were significantly associated with moderate-severe OSA, whereas there was no significant association with sTNFRI or IL-6. The presence of HIV RNA > 10,000 copies/ml was the only factor apart from the inflammatory cytokines that was associated with moderate/severe OSA in univariate analyses (OR 4.04, 95% CI: 1.07, 23.17; p=0.01).
We then used bivariate logistic regression to evaluate the relationships between inflammatory markers and the presence of moderate/severe OSA, adjusting for individual demographic and HIV-related variables. High TNF-alpha levels were significantly associated with an increased odds of moderate-severe OSA both with and without adjustment for individual variables. The effect was strengthened by adjustment for smoking status (OR 7.09, 95%CI: 1.65, 30.40: p<0.01) and was only slightly attenuated by adjustment for the presence of HIV RNA > 10,000 copies/mL (OR 4.36, 95% CI: 1.03, 18.53: p=0.05). The significant association of high sTNFRII level with moderate-severe OSA persisted after individually adjusting for race, BMI, smoking status, OLD, CD4 count, and HIV duration. The effect size persisted, but with borderline significance, when adjusting separately for age (OR 3.54, 95% CI:0.93, 13.52; p=0.06), HIV viral load >100K (OR 3.36, 95% CI: 0.34,9.85, p=0.08) and prior HAART exposure (OR 3.85, 95% CI: 0.91,16.18; p=0.07), and was non-significant with adjustment for HIV viral load>10K. Neither high sTNFRI level nor high IL-6 level were associated with an increased odds of moderate-severe OSA (p> 0.05 in all analyses).
DISCUSSION
In this sub study of the MACS, elevated levels of the inflammatory markers TNF-alpha, sTNFRII, and IL-6 were associated with moderate-severe sleep apnea in HIV-infected men not on HAART therapy. Importantly, after adjustment for multiple HIV-related variables, the association between TNF-alpha and OSA remained significant, suggesting an independent effect. These novel findings suggest a link between systemic inflammation and obstructive sleep apnea in untreated HIV-infected persons, although a causal role cannot be established in this study.
Systemic inflammation has also been associated with OSA in the general population, but a direction of the association is not clear. Several studies in HIV-uninfected individuals have demonstrated association between OSA and higher levels of inflammatory markers, including CRP, IL-6, and TNF-alpha, independent of obesity.26,27,28 Meta-analyses have demonstrated that treatment of OSA with CPAP decreases levels of inflammatory markers.29,30 Karamanli et al. demonstrated that CPAP treatment decreased levels of inflammatory markers in exhaled breathing condensates, but not consistently in the serum.31 Conversely, Vgontzas et al. showed that HIV-uninfected individuals treated with etanercept, a direct TNF-alpha inhibitor, demonstrated significant reductions in daytime sleepiness and OSA severity in a placebo-controlled, double-blind study.32 These findings suggest a causal role for inflammation in the pathogenesis of obstructive sleep apnea.
Although the association between TNF-alpha and OSA has been described in the general population,26,33,34,35 these studies variably examined total and regional adiposity, one of the major sources of TNF-alpha and a potential confounder in the described relationship.36,37 Among HIV-infected men not receiving HAART, the source of TNF-alpha is less likely to be adipose tissue,15 but rather activated immune cells. We hypothesize that elevated inflammatory markers(e.g. TNF-alpha) resulting from immune activation in untreated HIV-infected patients centrally depress neuromuscular control of the upper airway, leading to airway collapse and OSA.11 The importance of TNF-alpha as a causative agent is also supported by a recent genetic study in the general population showing that a polymorphism which leads to increased transcription of the TNF-alpha gene (TNF-α-308/GA) increases the risk of developing OSA.38 Our hypothesis regarding the relationship between TNF-alpha and OSA in untreated HIV-infection can be specifically tested by evaluating the effect of HAART initiation on OSA severity, as HIV treatment is consistently associated with reductions of TNF- alpha and its soluble receptors.5,39,40 It is unclear why moderate-severe OSA in the HIV-uninfected and HIV+/HAART groups was not associated with increased inflammation. It is conceivable that if higher levels of TNF-alpha lead to reduced airway control, then there might be a threshold level of TNF-alpha below which no association is present. In other words, it is possible that the plasma TNF-alpha concentration must be above a certain level before a central effect on neuromuscular control of the upper airway occurs and an association between TNF-alpha and OSA can be detected.
There are several limitations in our current study. Our sample size was small and therefore not powered to detect subtle differences in inflammatory markers between groupsMen not on HAART varied in their past exposure to HAART, but prior HAART was not related to the presence of moderate-severe OSA. Our study included men only and cannot be applied to women, who may have different inflammatory marker responses. Furthermore, our study was cross-sectional, and therefore not designed to examine the effects of HAART initiation or therapy for obstructive sleep apnea.
For the first time, we demonstrate a significant link between inflammatory markers and obstructive sleep apnea in a population of untreated HIV-infected individuals. Given the known relationship between OSA and increased morbidity and mortality due to cardiovascular disease, stroke, and diabetes, as well as significant impairment in quality of life,41 treatment of OSA in people with HIV is important. It is possible that reduction is inflammation may provide a new target for treatment of OSA. Our findings also suggest that HIV and its treatment might provide a suitable model to better understand the contribution of systemic inflammation on the pathogenesis of OSA.
Acknowledgements
Sources of Funding:
This work was supported in part by NIH (NCAAM) 5K23AT2862 (TTB), NIH (NHLBI) K23HL077137 (SPP), Grant Number UL1 RR 025005 from the National Center for Research Resources (NCRR), NIH (NHLBI) HL79554 (PLS), (5-MO1-RR-00052) UO1-AI-35042, UO1-AI-35043, UO1-AI-35041,T32HL007534.
Footnotes
Data presented prior as an oral abstract at the 11th Intl Workshop on Adverse Drug Reactions. 26–28 October 2009, Philadelphia. Oral abstract O-25. Antiviral Therapy 2009; 14 Suppl 2: A19.
Disclosure Statement:
Dr. Todd Brown has affiliations with EMD-Serono, Gilead, ViiV Healthcare, Merck, Abbott, and GSK. Dr. Lisa Jacobson serves on the NIH Office of AIDS Research Advisory Council. These affiliations did not affect the authors’ analyses or reporting of results.
References
- 1.Grinsztejn B, Luz PM, Pacheco AG, et al. PLOS One. 4. Vol. 8. San Francisco, CA: PLOS; 2013. Apr, [Accessed September 02, 2013]. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: Shifting from AIDS to Non-AIDS related conditions in the HAART era; p. e59768. [serial online] Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart A, Carusone SC, To K, et al. AIDS Research and Treatment. New York, NY: Hindawi Publishing Corporation; [Accesses September 2, 2013]. Causes of death in HIV patients and the evolution of an AIDS hospice: 1988–2008. [serial online]. Volume 2012, Article ID: 390406, 7 pages. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Antiretroviral Therapy Cohort Collaboration. Causes of Death in HIV-1-Infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clinical Infectious Diseases. 2002;186(7):1023–1027. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reqidor DL, Detels R, Breen EC, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25(3):303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keating SM, Golub ET, Nowicki M, et al. The effect of HIV infection and HAART on inflammatory biomarkers in a population-based cohort of women. AIDS. 2011;25(15):1823–1832. doi: 10.1097/QAD.0b013e3283489d1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr. 2010;55(3):316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuller LH, Tracy R, Belloso W, et al. PLoS Med. 10. Vol. 5. San Francisco, CA: PLOS; 2008. Oct, [Accessed September 02, 2013]. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection; p. e203. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross AC, Rizk N, O’Riordan MA, et al. Relationship between Inflammatory Markers, Endothelial Activation Markers, and Carotid Intima-Media Thickness in HIV-Infected Patients Receiving Antiretroviral Therapy. Clin Infect Dis. 2009;49:1119–1127. doi: 10.1086/605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown TT, Tassiopoulos K, Bosch RJ, et al. Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care. 2010;33(10):2244–2249. doi: 10.2337/dc10-0633. Epub 2010 Jul 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciftci TU, Kokturk O, Bukan N, et al. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz AR, Patil SP, Squier S, et al. Obesity and upper airway control during sleep. J Appl Physiol. 2010;108(2):430–435. doi: 10.1152/japplphysiol.00919.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang J, Wang Y, Krueger JM. Effects of interleukin-1β on sleep are mediated by the type I receptor. Am J Physiol Regul Integr Comp Physiol. 1998;274:655–660. doi: 10.1152/ajpregu.1998.274.3.R655. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi S, Kapas L, Fang J, et al. Somnogenic relationships between tumor necrosis factor and interleukin-1. Am J Physiol Regul Integr Comp Physiol. 1999;276:1132–1140. doi: 10.1152/ajpregu.1999.276.4.R1132. [DOI] [PubMed] [Google Scholar]
- 14.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 15.Brown TT, Patil SP, Jacobson LP, et al. Anthropometry in the prediction of sleep disordered breathing in HIV-positive and HIV-negative men. Antivir Ther. 2010;15(4):651–659. doi: 10.3851/IMP1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamauchi M, Kimura H. Oxidative stress in obstructive sleep apnea: putative pathways to the cardiovascular complications. Antiox. Redox Signal. 2008;10(4):755–768. doi: 10.1089/ars.2007.1946. [DOI] [PubMed] [Google Scholar]
- 17.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144(2):480–484. [PubMed] [Google Scholar]
- 18.Kaslow RA, Ostrow DG, Detels R, et al. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310. [DOI] [PubMed] [Google Scholar]
- 19.Dybul M, Fauci AS, Bartlett JG, et al. Guidelines for using antiretroviral agents among HIV-infected adults and adolescents. Ann Intern Med. 2002;137(5 Pt 2):381–433. doi: 10.7326/0003-4819-137-5_part_2-200209031-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hultin LE, Menendez FA, Hultin FA, et al. Assessing immunophenotyping performance: proficiency-validation for adopting improved flow cytometry methods. Cytometry B Clin Cytom. 2007;72(4):249–255. doi: 10.1002/cyto.b.20176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 22.Gould GA, Whyte KF, Rhind GB, et al. The sleep hypopnea syndrome. Am Rev Respir Dis. 1988;137(4):895–898. doi: 10.1164/ajrccm/137.4.895. [DOI] [PubMed] [Google Scholar]
- 23.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 24.Stacy EW. A generalization of the gamma distribution. Ann Math Statist. 1962;33(3):1187–1192. [Google Scholar]
- 25.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 26.Vgontzas AN, Papanicolaou DA, Bixler EO, et al. Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab. 2000;85(3):1151–1158. doi: 10.1210/jcem.85.3.6484. [DOI] [PubMed] [Google Scholar]
- 27.Punjabi NM, Beamer BA. C-reactive protein is associated with sleep disordered breathing independent of adiposity. Sleep. 2007;30(1):29–34. doi: 10.1093/sleep/30.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lui M, Lam J, Mak H, et al. C-reactive protein is associated with obstructive sleep apnea independent of visceral obesity. Chest. 2009;135(4):950–956. doi: 10.1378/chest.08-1798. [DOI] [PubMed] [Google Scholar]
- 29.Guo Y, Pan L, Ren D, et al. Impact of continuous positive airway pressure on C-reactive protein in patients with obstructive sleep apnea: a meta-analysis. Sleep Breath. 2012;17(2):495–503. doi: 10.1007/s11325-012-0722-2. [DOI] [PubMed] [Google Scholar]
- 30.Baessler A, Nadeem R, Harvey M, et al. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers – a meta-analysis. J Inflamm. 2013;10(1):13. doi: 10.1186/1476-9255-10-13. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karamanlı H, Ozol D, Ugur KS, et al. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. 2012 Sep 4; doi: 10.1007/s11325-012-0761-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Vgontzas AN, Zoumakis E, Lin HM, et al. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89(9):4409–4413. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 33.Bhushan B, Guleria R, Luthra K, et al. TNF-alpha level is directly associated with obstructive sleep apnea in obese Asian-Indians. Poster session presented at: Chest Annual Meeting; 2007 Oct 20–25; Chicago, IL. [Google Scholar]
- 34.Minoguchi K, Tazaki T, Yokoe T, et al. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest. 2004;126:1473–1479. doi: 10.1378/chest.126.5.1473. [DOI] [PubMed] [Google Scholar]
- 35.Hatipoglu U, Rubenstein I. Inflammation and obstructive sleep apnea syndrome: how many ways do I look at thee? Chest. 2004;126:1–2. doi: 10.1378/chest.126.1.1. [DOI] [PubMed] [Google Scholar]
- 36.Kern PA, Saghizadeh M, Ong JM, et al. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Invest. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hotamisligil G, Arner P, Caro J, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J, Liao N, Huang QP, Xie ZF. Association Between Tumor Necrosis Factor-α-308G/A Polymorphism and Obstructive Sleep Apnea: A Meta-Analysis. Genet Test Mol Biomarkers. 2011;16(4):246–251. doi: 10.1089/gtmb.2011.0170. [DOI] [PubMed] [Google Scholar]
- 39.Brazille P, Dereuddre-Bosquet N, Leport C, et al. Decreases in plasma TNF-alpha level and IFN-gamma mRNA level in peripharl blood mononuclear cells (PBMC) and an increase in IL-2 mRNA level in PBMC are associated with effective highly active antiretroviral therapy in HIV-infected patients. Clin Exp Immunol. 2003;131(2):304–311. doi: 10.1046/j.1365-2249.2003.02064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aukrust P, Muller F, Lien E, et al. Tumor necrosis factor (TNF) system levels in human immunodeficiency virus-infected patients during highly-active antiretroviral therapy: persistent TNF activation is associated with virologic and immunologic treatment failure. J Infect Dis. 1999;179(1):74–82. doi: 10.1086/314572. [DOI] [PubMed] [Google Scholar]
- 41.Patil SP, Schneider H, Schwartz AR, Smith PL. Adult obstructive sleep apnea: pathophysiology and diagnosis. Chest. 2007;132(1):325–337. doi: 10.1378/chest.07-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]