Abstract
Prior research has established significant relations between measures of sensory ability and cognitive function in adults of different ages, and several explanations for this relation have been proposed. One explanation is that sensory abilities restrict cognitive processing, a second is that cognitive abilities influence assessment of sensory ability, and a third is that both sensory function and cognition are affected by a common, potentially age-based, third factor. These explanations were investigated using mediation and moderation analyses, with near visual acuity as the sensory measure and scores on visual speed tests and auditory memory tests as the cognitive measures. Measures of visual acuity, speed, and memory were obtained from three moderately large samples, two cross-sectional (N = 380, N = 4779), and one longitudinal (N = 2258), with participants ranging from 18 to 90 years of age. The visual acuity and cognitive measures had different age trajectories, and the visual acuity-cognition relations were similar in each 5-year age band. The results suggest that the age-related differences and changes in near visual acuity are unlikely to contribute to the age-related differences and changes in speed and memory measures.
Keywords: sensory function, cognitive change, visual acuity, longitudinal
Previous research has reported correlations between measures of sensory function and measures of cognitive function in adults of different ages (e.g., Baltes & Lindenberger, 1997; Clark, 1960; Lindenberger, & Baltes, 1994; Lindenberger & Ghisletta, 2009; Salthouse, Hambrick, & McGuthry, 1998; Salthouse, Hancock, Meinz & Hambrick, 1996; Henderson et al., 2011; Valentijn et al., 2005). At least three explanations have been proposed to account for these relations. First, sensory capabilities may limit the availability of relevant information, and therefore impair registration and encoding of information in that sensory modality (e.g., Hofer, Berg & Era, 2003; Salthouse et al., 1996; Valentijn et al., 2005). Second, the sensory assessments could be influenced by cognitive factors, as even relatively simple sensory measures require the ability to comprehend and remember instructions, and to sustain attention (e.g. Salthouse et al., 1996). And third, the relation between sensory function and cognition may be attributable to a factor that is common to both sensory and cognitive functioning, such as age or a factor related to age (e.g., Baltes & Lindenberger, 1997; Christensen, Mackinnon, Korten, & Jorm, 2001; Lindenberger & Baltes, 1994).
Because both measures of sensory ability and of cognitive functioning are related to age, there has been considerable interest in determining whether the two sets of relations might be related to one another. Two analytical methods, mediation and moderation, have been used to address the nature of the relations among age, sensory ability, and cognitive performance. Mediation investigates the effects of controlling the variability in one of the variables on the relation between the other two variables (e.g., Salthouse, 2011). For example, if age-related differences in vision were postulated to be partially responsible for the age-related differences in cognition, the relation between age and cognition would be expected to be reduced when the variation in vision was controlled. Indeed, several studies have found a reduction in the relations between age and measures of cognition when the variance in measures of sensory ability was statistically controlled (e.g., Anstey, Luszcz, & Sanchez, 2001; Anstey & Smith, 1999; Baltes & Lindenberger, 1997; Lindenberger & Baltes, 1994; Salthouse et al. 1998; Salthouse et al. 1996).
Although these results are consistent with the hypothesized direction of influence, it is important to consider alternative models when interpreting mediation results because the results could be equally consistent with alternative models. It has therefore been proposed that stronger conclusions might be possible if the results were found to be inconsistent with expectations from other models (Salthouse, 2011). For example, the sensory mediation interpretation would be more convincing if there was reduction in the age-cognition relation after controlling the variation in the vision measure, but little or no reduction in the age-vision relation after controlling the variation in the cognitive measure, and little or no reduction in the vision-cognition relation after controlling the variation in age. Control of the age variation could be achieved either with statistical control procedures, or by high-density cross-sectional comparisons of many groups in narrow age ranges (Hofer et al., 2003).
The second analytical method that can be used to investigate the role of sensory factors on age-cognition relations is moderation, which focuses on whether the sensory-cognition relation varies as a function of age. If declines in sensory ability contribute to declines in cognition, one might expect that sensory-cognition relations would be stronger at older ages when the changes in sensory abilities have accumulated to approach the minimum threshold for adequate functioning. Previous studies examining moderation have been inconsistent, with some reports of stronger sensory function-cognition relations at older ages (e.g., Baltes & Lindenberger, 1997; Dulay & Murphy, 2002), but other reports of nearly constant sensory-cognition relations at all ages (e.g. Salthouse et al. 1996). Moderation can be formally investigated with the interaction term in analyses in which the age and sensory measures are used to predict cognition, and also by examining sensory-cognition relations at different ages. The interaction indicates whether the relations differ according to age, and the age-specific analyses are informative about which specific ages might have different relations.
The present study examined mediation and moderation of sensory-cognition relations with three sets of data, each involving moderately large samples of adults ranging from 18 to 90 years of age who were assessed in near visual acuity, and performed visual speed tests and auditory memory tests. Two of the data sets were based on cross-sectional comparisons, and the third was based on two-occasion longitudinal comparisons across an average interval of 3.0 years. One cross-sectional data set is a re-analysis of data reported inSalthouse et al. (1998), and the other two data sets are based on the Virginia Cognitive Aging Project (VCAP), which is an ongoing cross-sectional and longitudinal study focused on cognitive aging (Salthouse, 2013; in press).
The present study is unique in two important aspects. First, the sensory measure used in this study was near visual acuity, chosen because it has a relatively abrupt decline between the ages of about 40 and 50 (Gittings & Fozard, 1986; Salthouse et al., 1998), unlike other sensory measures that decline more gradually. This distinct transition allows analyses of the vision-cognition relations before, during, and after the period when the sensory measure exhibits the most dramatic changes. And second, two types of cognitive measures were used: visually presented speed tests and auditorially presented memory tests1. The use of cognitive measures in different sensory modalities allows a determination of whether the sensory-cognition relationship is modality specific or domain general. Little or no vision-cognition relations would be expected on the memory tests if the relations are attributable to limitations in the initial registration of the material because the tests have no visual requirements. However, comparable relations on the speed and memory measures might be expected if cognitive factors influence sensory assessments, or if both sensory and cognitive measures are influenced by a common third factor.
Method
Participants
Three sets of data were analyzed in this study, two cross-sectional and one longitudinal. The first data set consisted of data from 380 adults between 18 and 87 years of age, first reported inSalthouse et al. (1998). The primary data were obtained from participants in the Virginia Cognitive Aging Project (Salthouse, 2013; in press). Participants in VCAP were recruited from newspaper advertisements, flyers, and referrals from other participants. Eligibility criteria included at least 10 years of education, and sufficient cognitive, physical and sensory abilities to allow independent living. The cross-sectional data available for this study were based on 4799 participants ranging between 18 and 90 years of age who participated at least once, with the longitudinal data consisting of 2258 of those individuals who returned for a second occasion between 1 and 10 years after the initial occasion. Characteristics of the individuals in the once-tested and twice-tested samples are presented in Table 1.
Table 1.
Age, proportion of females, and years of education for once-tested and twice-tested participants in five-year age ranges. Note that the cross-sectional data are based on both samples.
| Only One Occasion | Two or More Occasions | T2-T1 | |||||
|---|---|---|---|---|---|---|---|
| Age Range | N | Fem. | Educ. (SD) | N | Fem. | Educ. (SD) | Change |
| 18–23 | 332 | .54 | 14.2 (1.6) | 149 | .56 | 13.4 (1.4) | −.03 |
| 24–28 | 236 | .62 | 15.8 (2.2) | 86 | .64 | 15.0 (2.2) | −.00 |
| 29–33 | 169 | .62 | 16.0 (2.9) | 73 | .66 | 15.8 (2.5) | −.04 |
| 34–38 | 122 | .73 | 15.6 (3.0) | 102 | .77 | 15.7 (2.4) | −.09* |
| 39–43 | 135 | .69 | 15.2 (3.0) | 145 | .76 | 15.1 (2.7) | −.14* |
| 44–48 | 187 | .74 | 15.2 (2.7) | 215 | .69 | 15.6 (2.4) | −.12* |
| 49–53 | 255 | .69 | 15.5 (2.6) | 304 | .75 | 15.7 (2.5) | −.05* |
| 54–58 | 264 | .70 | 15.7 (2.7) | 297 | .71 | 15.8 (2.7) | −.03* |
| 59–63 | 242 | .65 | 16.2 (3.0) | 236 | .66 | 16.6 (2.4) | −.04* |
| 64–68 | 177 | .67 | 16.3 (3.0) | 214 | .65 | 16.2 (2.8) | −.05* |
| 69–73 | 118 | .58 | 16.1 (3.1) | 168 | .56 | 16.1 (2.7) | −.05* |
| 74–78 | 123 | .58 | 15.8 (3.2) | 144 | .61 | 16.1 (2.8) | −.06* |
| 79–83 | 102 | .56 | 15.6 (2.5) | 82 | .49 | 16.4 (3.5) | −.04 |
| 84–90 | 59 | .49 | 16.3 (3.2) | 41 | .51 | 15.8 (3.0) | −.02 |
| Total | 2521 | .64 | 15.6 (2.7) | 2258 | .67 | 15.7 (2.7) | |
Note:
p<.01. Fem refers to proportion of females. Change in visual acuity was evaluated with paired t-tests comparing the T1 and T2 scores.
Measures
Visual acuity
Presenting (i.e., with usual corrective lenses) near visual acuity was measured using the Lighthouse Near Visual Acuity Card, (2nd Edition, New York). Each eye was measured separately, but because the scores for the two eyes were significantly correlated (i.e., r = .54 in the total sample, with a range of correlations from .30 to .88 across the 14 age groups in Table 1) only the average acuity scores, expressed as a Snellen ratio, were used in the analyses. However, it should be noted that the results reported below were very similar when the analyses were based on the score of the eye with the better vision instead of the presumably more reliable measure based on the average score across the two eyes.
Speed
Speed of processing was measured using the Digit Symbol test (Wechsler, 1997a), and the letter comparison and pattern comparison tests (Salthouse and Babcock, 1991). The Digit Symbol test, administered via paper-and-pencil, requires the participant to write symbols associated with digits in a code table as rapidly as possible. The comparison tests were also administered via paper-and-pencil, and involved the participant judging pairs of patterns or letter sets and writing either S (for same) or D (for different). Performance was assessed as the number of items correctly completed in the specified time (120 sec. for digit symbol, and 30 sec. for the comparison tests).
Memory
Episodic memory was measured using the logical memory and word recall lists from the Wechsler Memory Scale (Wechsler, 1997b), and a paired associates test (Salthouse, Fristoe, & Rhee, 1996). The stories, words, and word pairs in the memory tests were presented auditorially, and participants recalled the material with vocal responses. Only the word recall memory test was administered in theSalthouse et al. (1998) study.
Because prior research has established that the tests have moderate to high loadings on their respective ability factors (e.g. Salthouse, 2004, Salthouse & Tucker-Drob, 2008), the z-scores for the three speed tests were averaged to form a composite speed measure, and the z-scores for the three memory tests were averaged to form a composite memory measure.
Results
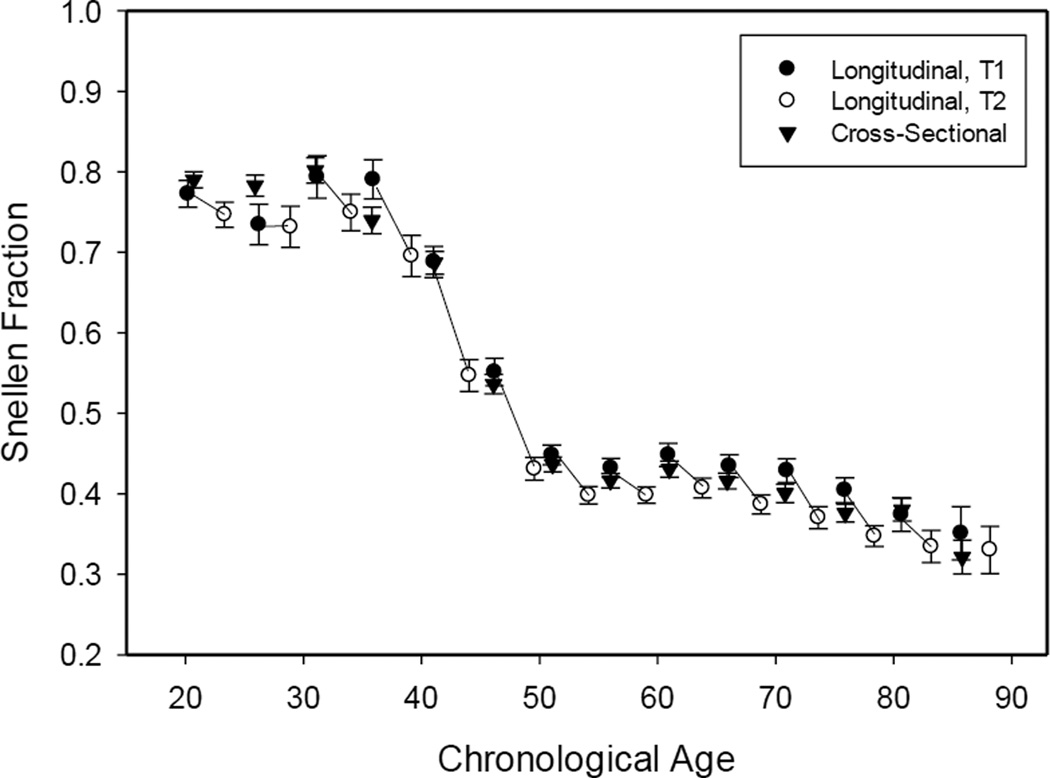
Average visual acuity as a function of age in the cross-sectional and longitudinal comparisons in the VCAP data is portrayed in Figure 1. The average visual acuity score was close to .8 Snellen units between the ages of about 20 and 40, and then decreased over a period of 10 years to an average of about .4. Even at young ages the acuity values were not 1.0, corresponding to 20/20, which may be attributable to conservative assessment, as visual acuity was based on the smallest fonts in which all 5 items in the line were correct instead of the smallest fonts with more than half, or any, of the items correct. The lower scores may also be due to the use of monocular assessment because prior research has demonstrated that individuals have better visual acuity when measured using both eyes than when each eye is measured separately, (e.g., Cagenello, Arditi, & Halpern, 1993).
Figure 1.
Visual acuity and change in visual acuity as a function of decade. Error bars are standard errors.
The longitudinal changes were evaluated with paired t-tests comparing scores at the first (T1) and second (T2) occasions within each 5-year age band. The mean changes are reported in the right-most column of Table 1, where it can be seen that there was significant longitudinal decline in this measure of near visual acuity beginning in the late 30s.
Segmented regression analyses were conducted on the cross-sectional means and longitudinal changes to estimate the transition points in the age-acuity functions. An analysis with three segments (two transition points) did not yield meaningful estimates, and therefore separate analyses specifying two segments were conducted, one with data from age 18 to 50 and the other with data from age 40 to 90. These analyses revealed estimated transition points of 38.8 years for the drop point and 48.5 years for the leveling off point in the cross-sectional data, and of 44.2 and 52.1 years, respectively, for the longitudinal data.
Mediation
Three mediation models were considered with each cognitive measure in each dataset. The first specified that vision mediated the age-cognition relations, the second that cognition mediated the age-vision relations, and the third, that age mediated the vision-cognition relations. The primary prediction in each model was that the relation between the two primary variables would be reduced when the variation in the hypothesized mediator was statistically controlled. Partial correlations were used to control the variability in the hypothesized mediator, and both the simple and partial correlations for each model are reported in Table 2.
Table 2.
Results of mediation analyses with three models in terms of correlations and partial correlations.
| Age-Cognition | Age-Vision | Vision-Cognition | ||||
|---|---|---|---|---|---|---|
| Alone | After Vision | Alone | After Cognition | Alone | After Age | |
| Salthouse et al. (1998) | ||||||
| Speed | −.55* | −.40* | −.58* | −.45* | .45* | .19* |
| Memory | −.40* | −.27* | −.58* | −.52* | .33* | .13 |
| VCAP Data | ||||||
| Cross-Sectional | ||||||
| Speed | −.60* | −.47* | −.56* | −.41* | .45* | .17* |
| Memory | −.41* | −.28* | −.56* | −.50* | .32* | .12* |
| Longitudinal | ||||||
| Speed | −.15* | −.13* | .02 | .03 | .10* | .09* |
| Memory | −.21* | −.21* | .02 | .02 | .05 | .06 |
p<.01
The patterns in all three models were similar with both sets of cross-sectional results. In each case the partial correlations, when the variability in the hypothesized mediator was controlled, were smaller than the simple correlations. These results are consistent with the expectations from each of the models postulating different patterns of mediation. If anything, the results are most consistent with the interpretation that the vision-cognition relations are attributable to the influence of age on both measures because the differences between the simple and partial correlations were largest with the third model in Table 2. However, analyses of the longitudinal data yielded a different pattern of results. With these data there were significant relations between age and the change in speed and the change in memory, and between the change in speed and the change in vision, but no reduction of the relations when the variance in the hypothesized mediator was controlled in any of the models.
The results of the mediation analyses with the cross-sectional data are therefore ambiguous because the patterns were consistent with the expectations from three models postulating quite different types of mediation. The mediation analyses were interpretable for the longitudinal data, but only because in this case there was no evidence for mediation in any of the models.
Moderation
The initial moderation analyses focused on the interaction between age and vision in predicting speed or memory. In order to minimize collinearity, the age and vision variables were first centered, and the interaction term was created by multiplying the centered age and vision variables. Results of simultaneous regression analyses, with age, vision, and their interaction as predictors, are reported in Table 3.
Table 3.
Results of moderation analyses in terms of unstandardized (b) and standardized (β) regression coefficients.
| Speed | Memory | |||
|---|---|---|---|---|
| b | β | b | β | |
| Salthouse et al. (1998) | ||||
| Age | −.024 (.003) | −.435* | −.019 (.004) | −.310* |
| Vision | .511 (.136) | .196* | .421 (.169) | .144* |
| Age*Vision | −.003 (.007) | −.016 | −.009 (.009) | −.044 |
| VCAP Data | ||||
| Cross-Sectional | ||||
| Age | −.025 (.001) | −.511* | −.015 (.001) | −.329* |
| Vision | .593 (.046) | .186* | .468 (.055) | .149* |
| Age*Vision | .012 (.002) | .066* | .007 (.003) | .040* |
| Longitudinal | ||||
| ΔSpeed | ΔMemory | |||
| b | β | b | β | |
| Age | −.004 (.001) | −.142* | −.006 (.001) | −.211* |
| ΔVision | .202 (.041) | .104* | .124 (.050) | .055 |
| Age*ΔVision | −.001 (.003) | −.008 | .001 (.003) | .004 |
p<.01
Inspection of the entries in the table reveals that there was a similar pattern with both speed and memory variables in the two sets of cross-sectional data. In each case there were moderately large relations of age and vision on both cognitive measures, but only small or nonexistent interactions of age and vision. The interactions in the VCAP data were significant, in the direction of weaker (rather than stronger) vision-cognition relations at older ages, but they were still quite small.
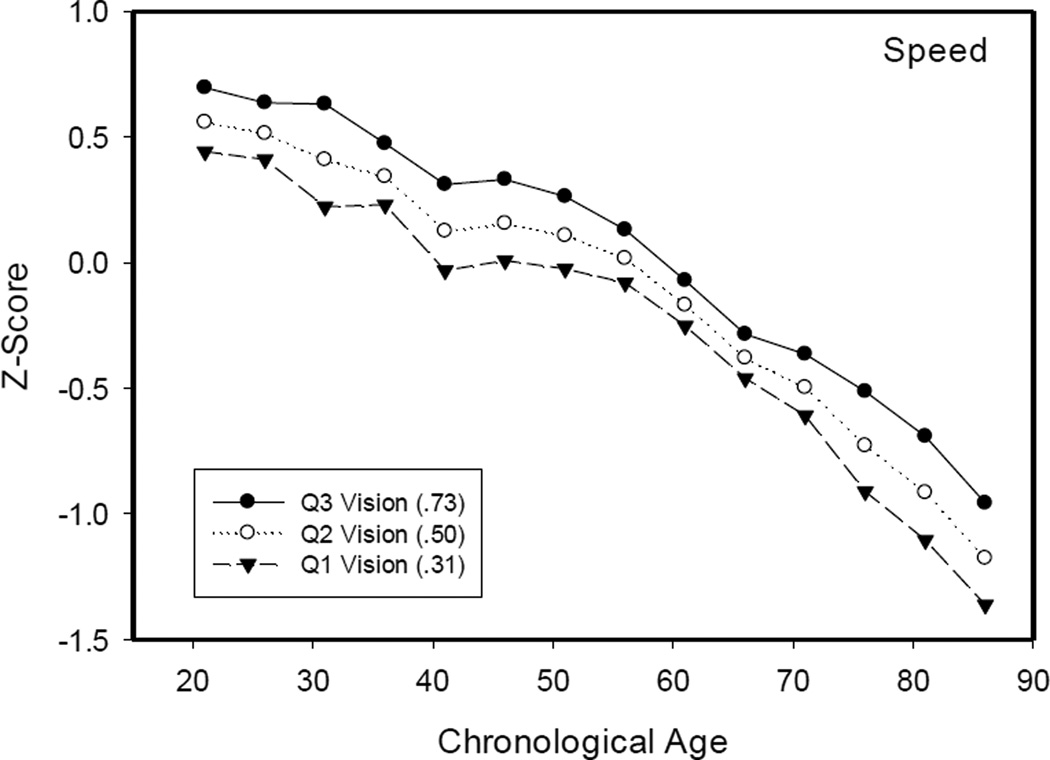
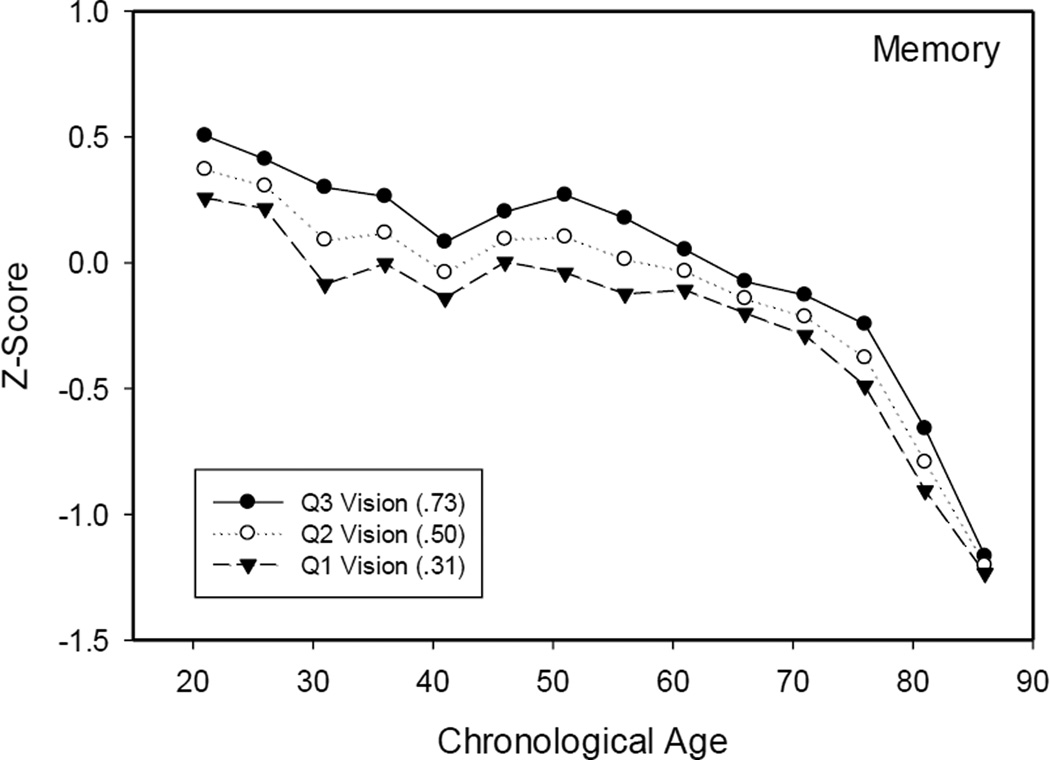
In order to explore the basis for the interactions in the VCAP data, the relations between vision and cognitive measures were determined in groups within the narrow (5-year) age ranges specified in Table 1. Separate regression analyses relating the speed or memory measures to vision were conducted in each group, and then the parameters of the equations were used to determine the cognitive scores expected at the 25th, 50th, and 75th percentiles (as determined in the entire sample) of the vision variable. These predicted scores are plotted in Figure 2 for the speed measure and in Figure 3 for the memory measure.
Figure 2.
Composite speed scores for adults in the 25th, 50th, and 75th percentiles for near visual acuity as a function of age decade.
Note: The z-scores were computed based on the means and standard deviations of the entire sample.
Figure 3.
Composite memory scores for adults in the 25th, 50th, and 75th percentiles for near visual acuity as a function of age decade.
Note: The z-scores were computed based on the mean and standard deviation of the entire sample.
Several points should be noted about the results in Figures 2 and 3. First, although not portrayed to maximize legibility of the figures, the functions for the observed speed and memory scores (based on the average visual acuity at each age), were slightly higher at the young ages and slightly lower at the older ages than the function based on the median (Q2) values. These differences account for the smaller age-cognition relations after control of the variance in the near visual acuity measure in Table 2. Second, the functions were continuous across adulthood, with a possible acceleration after about 70 for memory. Importantly, there was no evidence of an abrupt transition in the decade of the 40s corresponding to the drop in near visual acuity apparent in Figure 1. And third, the difference between predicted cognitive scores at the first and third quartiles provides an estimate of the influence of vision at each age. Although differences between the expected scores at the first and third quartiles of vision were apparent at all ages, indicating that people with better visual acuity had higher scores on the speed and memory tests, the magnitude of the differences was similar at most ages, including the period in the 40s when there was the largest decrease in visual acuity.
Results of the moderation analyses with the longitudinal data are reported in the bottom of Table 3. There were negative relations between age and change in both speed and memory, indicating that the longitudinal change was more negative at older ages, but change in visual acuity was positively associated only with change in speed. Importantly, there was no evidence of an interaction of age and change in vision for the change in either speed or memory. Furthermore, analyses similar to those in Figures 2 and 3 revealed very small relations of the change in vision to the change in speed or memory, with almost completely overlapping functions for the expected changes at the three quartiles of visual change.
Discussion
The primary goal of this study was to investigate how the relations of age with measures of visual acuity are related to the relations of age with speed and memory measures of cognitive functioning. Our results suggest that these relations are very weak.
With the exception of the longitudinal data, in which there was no evidence of mediation with any model, the mediation analyses were not very informative. There was a decrease in the age-cognition relation when the visual measure was statistically controlled, which is consistent with several earlier reports (e.g., Anstey & Smith, 1999; Baltes & Lindenberger, 1997; Lindenberger & Baltes, 1994; Salthouse et al. 1998, Salthouse et al. 1996). However, a limitation of mediation analyses is that the results are merely consistent or inconsistent with a particular model, and should not be considered definitive. It has therefore been suggested that inferences from mediation models would be stronger if they were based on the patterns across alternative models (Salthouse, 2011), and particularly a discovery that the results were inconsistent with models postulating different mediational patterns. The results in Table 2 indicate that this was not the case with either set of cross-sectional data, as there was support for visual acuity mediating the age-cognition relation, but also for cognition mediating the age-visual acuity relation and for age mediating (or at least indirectly responsible for) the visual acuity-cognition relation.Henderson et al. (2011) also found support both for sensory function as a mediator of the relations between age and cognition, and for cognition as a mediator of the relations between age and sensory function. The longitudinal data were not consistent with any of the models, and this finding is similar to the results of earlier studies reporting weaker relations between measures of sensory function and cognition in longitudinal comparisons data when compared to those same relationships in cross-sectional data (e.g., Lindenberger & Ghisletta, 2009; Sternäng, Jonsson, Wahlin, Nyberg, & Nilsson, 2010).
The results of the moderation analyses also did not support the idea that an accumulation of deficits in visual acuity contributes to the relations between age and cognition. Not only were the interactions of age and vision on cognition weak in the cross-sectional data and non-existent in the longitudinal data, but the analyses in narrow age groups in Figures 2 and 3 revealed similar relations of visual acuity with speed and memory at all ages. Furthermore, the results in Figures 2 and 3 indicate that pronounced age relations in speed and memory are still apparent when the comparisons at different ages are at the same level of visual acuity. Although there is some attenuation at the youngest and oldest ages when visual acuity is allowed to vary with age, it is relatively small. These results are more consistent with the earlier findings ofSalthouse et al. (1996) than those of Baltes and Lindenberger (1997) and Dulay and Murphy (2002), who reported stronger sensory-cognition relations at older ages.
In addition, despite the substantial decrease in visual acuity from about 40 to 50 years of age, there was no evidence of a discrete shift in the magnitude of the vision-cognition relations, or in the average level of speed or memory during that same period. The absence of a discontinuity in the age-speed or age-memory functions during the period when there is a marked decline in visual acuity indicates that there was a weak coupling of the change in vision with the changes in speed and memory.
Finally, although the relations with visual acuity were somewhat stronger with the visually-based speed measures than with the auditorially-based memory measures, the significant relations with the memory measures implies that the sensory-cognition linkage is at least partially domain-general. Cross-modal associations could be interpreted as support for a common cause hypothesis that age-sensory and age-cognition relations are attributable to a common factor related to age (e.g., Baltes & Lindenberger, 1997; Lindenberger & Baltes, 1994). However, it is important to recognize that the vision-cognition relations were evident to nearly the same degree at all ages, and thus the cross-modal sensory-cognition relation does not appear to be relevant to the role of sensory factors on the relations of age with cognition.
The present study was limited in several ways. First, only a single relatively crude measure of sensory functioning was examined, and the results could be different with more sensitive visual measures, or measures in other modalities. Second, the longitudinal interval averaging about 3 years may have been too short for some sensory and cognitive changes to be manifested. And third, the sample of participants was generally healthy, and stronger sensory-cognition relations may be apparent in individuals with pathological conditions such as dementia.
In conclusion, although we confirmed prior findings of moderate relations between sensory ability and measures of cognitive functioning, our results are not consistent with the hypothesis that age-related declines in sensory ability contribute to age-related declines in cognitive functioning. Results of mediation analyses were ambiguous, there were substantial relations between age and measures of speed and memory when visual acuity was held constant statistically, and the relations between visual acuity and speed and memory were similar at nearly every age in adulthood, including the period when visual acuity exhibited the greatest age-related change.
Acknowledgments
Funding
This work was supported by the National Institute of Aging, (grant number R37AG024270). The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institute of Aging or the National Institutes of Health.
Footnotes
The participants also performed visually presented tests of higher-order fluid cognition involving reasoning and spatial visualization, and mediation and moderation analyses with those measures yielded results similar to the results with the speed and memory measures.
There are no conflicts of interests.
References
- Anstey KJ, Luszcz MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory function, and cognitive function in older adults. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2001;56B:3–11. doi: 10.1093/geronb/56.1.p3. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Smith GA. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychology and Aging. 1999;14:605–618. doi: 10.1037//0882-7974.14.4.605. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: A new window to the study of cognitive aging? Psychology and Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Cagenello R, Arditi A, Halpern DL. Binocular enhancement of visual acuity. Journal of Optical Society America A. 1993;10:1841–1848. doi: 10.1364/josaa.10.001841. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten A, Jorm AF. The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychology and Aging. 2001;16:588–599. doi: 10.1037//0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- Clark JW. The aging dimension: A factorial analysis of individual differences with age on psychological and physiological measurements. Journal of Gerontology. 1960;15:183–187. doi: 10.1093/geronj/15.2.183. [DOI] [PubMed] [Google Scholar]
- Dulay MF, Murphy C. Olfactory acuity and cognitive function converge in older adulthood: Support for the common cause hypothesis. Psychology and Aging. 2002;17:392–404. [PubMed] [Google Scholar]
- Gittings NS, Fozard JL. Age related changes in visual acuity. Experimental Gerontology. 1986;21:423–433. doi: 10.1016/0531-5565(86)90047-1. [DOI] [PubMed] [Google Scholar]
- Henderson RD, Allerhand M, Patton N, Pattie A, Gow AJ, Dhillon B, Starr JM, Deary IJ. Vision and intelligence at age 83 in the Lothian Birth Cohort 1921. Intelligence. 2011;39:148–154. [Google Scholar]
- Hofer SM, Berg S, Era P. Evaluating the interdependence of aging-related changes in visual and auditory acuity, balance, and cognitive functioning. Psychology and Aging. 2003;18:285–305. doi: 10.1037/0882-7974.18.2.285. [DOI] [PubMed] [Google Scholar]
- Lighthouse Near Visual Acuity Test. 2nd Ed. New York, New York: Lighthouse International; Available from: http://www.lighthouse.org/ [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychology and Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Ghisletta P. Cognitive and sensory declines in old age: Gauging the evidence for a common cause. Psychology and Aging. 2009;24:1–16. doi: 10.1037/a0014986. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. Localizing age-related individual differences in a hierarchical structure. Intelligence. 2004;32:541–561. doi: 10.1016/j.intell.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137:753–784. doi: 10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Effects of age and ability on components of cognitive change. Intelligence. 2013;41:501–511. doi: 10.1016/j.intell.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Correlates of cognitive change. Journal of Experimental Psychology: General. doi: 10.1037/a0034847. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Developmental Psychology. 1991;27:763–776. [Google Scholar]
- Salthouse TA, Fristoe N, Rhee SH. How localized are age-related effects on neuropsychological measures? Neuropsychology. 1996;10:272–285. [Google Scholar]
- Salthouse TA, Hambrick DZ, McGuthry KE. Shared age-related influences on cognitive and non-cognitive variables. Psychology and Aging. 1998;13:486–500. doi: 10.1037//0882-7974.13.3.486. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Hancock HE, Meinz EJ, Hambrick DZ. Interrelations of age, visual acuity, and cognitive functioning. The Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 1996;51B:317–330. doi: 10.1093/geronb/51b.6.p317. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Pink JE, Tucker-Drob EM. Contextual analysis of fluid intelligence. Intelligence. 2008;36:464–486. doi: 10.1016/j.intell.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Tucker-Drob EM. Implications of short-term retest effects for the interpretation of longitudinal change. Neuropsychology. 2008;22:800–811. doi: 10.1037/a0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternäng O, Jonsson B, Wahlin Å, Nyberg L, Nilsson L-G. Examination of the common cause account in a population-based longitudinal study with narrow age cohort design. Gerontology. 2010;56:553–563. doi: 10.1159/000279754. [DOI] [PubMed] [Google Scholar]
- Valentijn SAM, van Boxtel MPJ, van Hooren SAH, Bosma H, Beckers HJM, Ponds RWHM, Jolles J. Change in sensory functioning predicts change in cognitive functioning: Results from a 6-year follow-up in the Maastricht aging study. Journal of American Geriatrics Society. 2005;53:374–380. doi: 10.1111/j.1532-5415.2005.53152.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. San Antonio, TX: Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Third Edition. San Antonio, TX: Psychological Corporation; 1997b. [Google Scholar]