Abstract
Glycogen is the major store of glucose in brain and is mainly in astrocytes. Brain glycogen levels in unstimulated, carefully-handled rats are 10-12 mol/g, and assuming that astrocytes account for half the brain mass, astrocytic glycogen content is twice as high. Glycogen turnover is slow under basal conditions, but it is mobilized during activation. There is no net increase in incorporation of label from glucose during activation, whereas label release from pre-labeled glycogen exceeds net glycogen consumption, which increases during stronger stimuli. Because glycogen level is restored by non-oxidative metabolism, astrocytes can influence the global ratio of oxygen to glucose utilization. Compensatory increases in utilization of blood glucose during inhibition of glycogen phosphorylase are large and approximate glycogenolysis rates during sensory stimulation. In contrast, glycogenolysis rates during hypoglycemia are low due to continued glucose delivery and oxidation of endogenous substrates; rates that preserve neuronal function in the absence of glucose are also low, probably due to metabolite oxidation. Modeling studies predict that glycogenolysis maintains a high level of glucose-6-phosphate in astrocytes to maintain feedback inhibition of hexokinase, thereby diverting glucose for use by neurons. The fate of glycogen carbon in vivo is not known, but lactate efflux from brain best accounts for the major metabolic characteristics during activation of living brain. Substantial shuttling coupled with oxidation of glycogen-derived lactate is inconsistent with available evidence. Glycogen has important roles in astrocytic energetics, including glucose sparing, control of extracellular K+ level, oxidative stress management, and memory consolidation; it is a multi-functional compound.
Keywords: astrocyte, neuron, glycogen, sensory stimulation, in vivo glycogenolysis rates
Introduction
“In physical science the first essential step in the direction of learning any subject is to find principles of numerical reckoning and practicable methods for measuring some quality connected with it. I often say that when you can measure what you are speaking about, and express it in numbers, you know something about it; but when you cannot measure it, when you cannot express it in numbers, your knowledge is of a meagre and unsatisfactory kind; it may be the beginning of knowledge, but you have scarcely in your thoughts advanced to the state of Science, whatever the matter may be.”
Sir William Thomson (Lord Kelvin), Popular Lectures and Addresses, vol. 1, 2nd Edition, “Electrical Units of Measurement”, a lecture delivered at the Institution of Civil Engineers, May 3, 1883; London, Macmillan and Co., 1891, p. 80-81.
Glycogen is a glucose polymer that constitutes the major glucose storage depot in brain, and it is located mainly in astrocytes (Cataldo and Broadwell 1986). However, glycogen is sparsely detectable in some neurons (Sotelo and Palay 1968), and dysregulation of neuronal glycogen metabolism is associated with neurodegeneration in myoclonus epilepsy and Lafora disease (Duran et al. 2012; Valles-Ortega et al. 2011; Vilchez et al. 2007). Glucose is the obligatory fuel for brain, and glycogen levels and turnover, therefore, provide a window for studies of astrocytic energetics under resting and activating conditions; this is the reason that our laboratory studied glycogen in awake rodents. During the past two decades, the conceptual role of brain glycogen has shifted from an emergency energy reserve to a dynamic participant in metabolism during brain activation. However, studies of glycogen mobilization and elucidation of its functions in living brain are very difficult due to its lability during brain tissue sampling and extraction procedures, its sensitivity to sensory stimuli and physiological state, the complexity of its regulation and turnover, and the lack of methods with high temporal-spatial resolution to evaluate changes in glycogen level and labeling. This review emphasizes quantitative aspects of glycogen biology and focuses on brain activation in normal, awake subjects; it highlights advances in understanding glycogen biology in living brain. Comprehensive discussions of important in vivo, in situ, and in vitro studies that have helped reveal the regulatory and signaling mechanisms governing glycogen content, its participation in brain functions, and its relationship to overall astrocytic and brain energetics and physiology are found in recent reviews (Obel et al. 2012; Dinuzzo et al. 2012; DiNuzzo et al. 2011; Schousboe et al. 2010; Brown 2004; Gruetter 2003; Hertz et al. 2007; Tesfaye et al. 2011; Gibbs and Hutchinson 2012).
Paradigm shift for roles of glycogen: From energy failure to sensory stimulation
Emergency fuel
Many studies of glycogenolysis during the 1960-1970s focused on glycogen utilization during pathophysiological conditions, including ischemia, hypoxia, anoxia, seizures, and hypoglycemia (reviewed by (Siesjö 1978)). This work led to the concept of glycogen as a stockpile that can help sustain brain function for a short interval in the absence of glucose or oxygen, or when fuel supplies do not match energy demands. These studies also established that glycogen turned over very slowly in resting brain (Watanabe and Passonneau 1973), and its levels (expressed as glucosyl units) obtained in extracts of brain were similar to those of glucose, about 2-3 mol/g. More recent studies using microwave fixation or funnel-freezing of carefully-handled animals have reported values in the range of about 5-12 mol/g (see Discoveries arising from investigation of the basis for unexpected, discrepant results, below). Thus, if the total pool of carbohydrate fuel in brain comprised of glucose plus glycogen were 7 mol/g it could sustain brain metabolism for about 7 min in normal, resting cerebral cortex that has a rate of glucose utilization (cerebral metabolic rate [CMR] for glucose [glc], or CMRglc) of about 1 mol/g/min (Sokoloff et al. 1977). However, this fuel supply is consumed much more quickly when glycolysis is upregulated during abnormal states, such as ischemia (Lowry and Passonneau 1964; Lowry et al. 1964), and rodents lose consciousness due to energy failure within about 30 seconds after onset of ischemia. Together, this work led to the notion that brain glycogen is quickly consumed during an energy crisis, but it is probably not very important for normal brain function due to its very slow turnover.
Physiological roles
Modulation of glycogen mobilization by various neurotransmitters and neuromodulators, particularly the stress-activated noradrenergic system, was well-documented in the 1980-1990s and indicated that astrocytes respond to neuronal signaling (see discussion in (Cruz and Dienel 2002)). In 1992, Swanson and colleagues demonstrated that glycogen was consumed specifically in the activated barrel cortex during whisker stimulation of the conscious rat (Swanson et al. 1992; Swanson 1992). This important discovery was the turning point that directed attention to roles for glycogen in astrocytic energetics when neuronal information processing increases. As noted by the authors, glycogenolysis provides a rapid source of glucose-6-phosphate (Glc-6-P) to astrocytes independent of glucose delivery from blood. Subsequent work in our and other laboratories confirmed and extended these findings by demonstrating that brain glycogen levels are much higher than generally recognized and turnover and net utilization of glycogen rise during and after sensory stimulation of the awake rat.
Very high glycogen concentration in brain
Why does the CMRO2/CMRglc ratio fall during brain activation?
Our first study involving glycogen during sensory stimulation (Madsen et al. 1999) was designed to determine if the ratio of oxygen to glucose utilization (i.e., CMRO2/CMRglc that was measured by arteriovenous differences) fell during sensory stimulation of awake rats, as was previously observed in humans during mental testing and physiological activity (Madsen et al. 1995; Fox et al. 1988). If this metabolic ratio fell in the animal model, the basis for the disproportionately higher consumption of glucose compared with oxygen during brain activation could be studied. The CMRO2/CMRglc did, in fact, fall during generalized stimulation of rats that had been very carefully handled and sequestered to minimize environmental stimuli prior to the experiment. Analysis of the major carbohydrate pools in brain revealed that glucose content was stable, lactate concentration and efflux from brain to blood increased, and glycogen content gradually fell, reaching statistical significance only at 15 min after cessation of stimulation. However, changes in the amounts of glucose, glycogen, and lactate and lactate efflux from brain to blood did not fully account for the excess glucose consumed during sensory stimulation compared with oxygen utilization indicating that the analysis was incomplete.
Impact of glycogen turnover on whole-brain metabolic studies
Arteriovenous assays of brain metabolism are based on global utilization of blood-borne substrates, and are calculated as cerebral blood flow (CBF) times the difference in substrate concentration in arterial (A) and venous (V) blood (i.e., CBF[A-V]), and ratios of metabolic rates are calculated as CMRO2/CMRglc (i.e., [CBF(A-V)O2]/[CBF(A-V)glc]. Under resting conditions, the CMRO2/CMRglc ratio is close to the theoretical maximum of 6.0, indicating that most of the glucose is completely oxidized (i.e., 6 O2 + 1 glucose → 6 CO2 + 6 H2O). When non-oxidative utilization of glucose is enhanced, the CMRO2/CMRglc ratio falls; the basis for this phenomenon is not understood.
Because glycogen is an endogenous substrate that is not taken into account in studies of glucose utilization, an important outcome of our above study was recognition of the following consequences of glycogen turnover. (i) If glycogen were consumed and oxidized during brain activation it would contribute to oxygen consumption, thereby inflating the apparent oxidation of blood-borne glucose. Use of glycogen as oxidative fuel would disproportionately increase CMRO2 or (A-V)O2 compared with CMRglc or (A-V)glc because arteriovenous difference assays and ratios of net uptake of these two fuels into brain does not account for metabolism of other compounds. (ii) If glycogen were glycolytically metabolized with release of the lactate to blood, it would inflate the apparent amount of lactate produced from blood-borne glucose. (iii) Re-synthesis of glycogen after its depletion requires non-oxidative glucose utilization, thereby lowering the whole-brain CMRO2/CMRglc ratio while glycogen level is being restored. Thus, astrocytic glycogen turnover is important because it could influence the overall balance between oxygen and glucose utilization in whole-brain assays, and it could also influence interpretation of routine in vivo glucose utilization assays using labeled precursors by providing an additional source of fuel that is not taken into account. We, therefore, carried out parallel series of in vivo studies of brain activation with emphasis on two pathways specific for astrocytes, glycogen turnover and oxidative metabolism using [14C]acetate as a tool; acetate is preferentially metabolized by astrocytes. Discussion of the acetate studies is beyond the scope of this review, but this work revealed that astrocytic tricarboxylic acid (TCA) cycle activity is upregulated during sensory stimulation and other conditions, and it is high in glial tumors (Dienel et al. 2007b; Cruz et al. 2005; Dienel et al. 2003; Cetin et al. 2003; Dienel et al. 2001a; Dienel et al. 2001b; Dienel et al. 1999). Because activity of the astrocytic oxidative pathway rises during brain activation, it is important to consider both glycolytic and oxidative fates of glycogen carbon within astrocytes that mobilize glycogen, as well as in their gap junction-coupled partners into which products of glycogen metabolism can diffuse.
Discoveries arising from investigation of the basis for unexpected, discrepant results
To evaluate incorporation of label into glycogen compared with labeling of metabolites in other pathways, we next used in vivo pulse-labeling with [6-14C]glucose during rest, sensory stimulation, and recovery. Because we planned to use anion-exchange HPLC to separate the 14C-labeled metabolites, an initial technical concern was that the perchloric acid extraction procedure (Passonneau and Lowry 1993) that is routinely used to inactivate enzymes and preserve levels of labile metabolites would result in high perchlorate levels that would interfere with the HPLC analysis. We, therefore, took advantage of an ethanol extraction method that we previously used to evaluate the source of unexpectedly-high deoxyglucose (DG) levels in acid extracts of brains of hypoglycemic rats. The ethanol procedure led to our ‘re-discovery’ of the acid-lability of deoxyglucose-1-phosphate (DG-1-P), which was first synthesized and characterized by Emil Fischer’s laboratory in the early 1900s (Dienel et al. 1990). Use of the ethanol procedure led to two interesting and important findings derived from changes in levels of labile metabolites in the test tube during routine acid extraction, one related to apparent glucose-6-phosphatase activity in brain that would influence downstream metabolism of glycogen, the other related to apparent glycogen level, turnover, and function.
In the first case, we found that DG-1-P is degraded to DG in the test tube during routine acid extraction but not with ethanol-based extraction (Dienel et al. 1990). Conversion of product (DG-1-P) to precursor (DG) in vitro increased the apparent size of the unmetabolized precursor pool. This artifact interfered with determination of the factor lambda of the lumped constant of the [14C]deoxyglucose method as a function of plasma and brain glucose concentration, and it sparked detailed analyses of further metabolism of deoxyglucose-6-P (DG-6-P) (Dienel and Cruz 1993; Dienel et al. 1993; Dienel et al. 1991; Mori et al. 1989). Because the DG-1-P level is equal to 7% that of DG-6-P and DG-6-P level increases with time after pulse labeling, the magnitude of inflation of the apparent size of the precursor pool in acid extracts progressively increases with time and explains the basis for the artifactual glucose-6-phosphatase activity (Dienel et al. 1993; Dienel et al. 1990). In a previous study, modeling of the time courses of DG and DG-6-P in acid extracts of brain (Hawkins and Miller 1978) was based on precursor levels that were too high and product levels that were too low, leading to their incorrect interpretation of high glucose-6-phosphatase activity in brain. Other studies have also shown that Glc-6-phosphatase activity is negligible in brain in vivo (Dienel et al. 1988; Nelson et al. 1985; Schmidt et al. 1989) and in cultured astrocytes (Gotoh et al. 2000). These findings not only demonstrate that the enzyme does not interfere with the [14C]DG method to assay CMRglc by causing product loss (Sokoloff et al. 1977), they are also highly relevant to the fate of brain glycogen. Without significant activity of glucose-6-phosphatase, Glc-6-P derived from glycogen cannot be converted to glucose, as in the liver; instead it must be metabolized in the cell where generated. In cultured astrocytes nearly all glycogen degraded is released to the culture medium as lactate (Dringen et al. 1993a), but it is important to take into account the fact that culture assays have an extremely high extracellular volume that dilutes the released lactate. Dilution maintains an outward intracellular-to-extracellular lactate concentration gradient, thereby facilitating the formation and release of lactate via equilibrative reactions (lactate dehydrogenase and transporters). Thus, lactate release from cultured cells may be much higher than in vivo.
In the second case, when unlabeled glycogen levels in ethanol extracts of samples labeled by [6-14C]glucose were analyzed (Cruz and Dienel 2002), the mean values during rest, sensory stimulation, and recovery from stimulation were about twice those of our previous study (Madsen et al. 1999) that used acid extraction, whereas differences in glucose and lactate levels in samples extracted by the two procedures were smaller. To determine if the discrepant results were due to biological differences between the two groups of rats or to technical issues, paired samples of the same brain powders were extracted in parallel with both procedures. The acid extracts had lower levels of glycogen and higher levels of lactate, suggesting incomplete inactivation of enzymes by the acid extraction procedure. Subsequent assays of postmortem metabolism in the acid extracts in vitro revealed decrements in glycogen level accompanied by increases in lactate content. Thus, enzymes had enough residual activity in the test tube to cause loss of labile metabolites and accumulation of downstream products.
The important finding in our studies was that glycogen levels were 2-4-fold higher than generally recognized, with mean values of about 10-12 mol/g in resting cerebral cortex (Dienel et al. 2002; Cruz and Dienel 2002). Unfortunately, the high animal-to-animal variability of baseline glycogen levels in ethanol compared with acid extracts (coefficients of variation [SD/mean] of 30-40%, i.e., many animals had very high glycogen levels) made it difficult to obtain statistically-significant results in our studies. Nevertheless, two independent studies carried out about a year apart in separate groups of rats obtained similar glycogen levels during rest and similar percent decreases during generalized sensory stimulation and recovery, indicating that the results were biologically reproducible, even if some were not statistically significant (Dienel et al. 2002; Cruz and Dienel 2002). At about the same time, the Geiger laboratory (Kong et al. 2002) used high-power microwave fixation of brain and also found high glycogen levels in cerebral cortex (~9 mol/g) one experiment, whereas in other experiments they obtained lower values, in the range of ~3-6 mol/g that is similar to those in biochemical or magnetic resonance assays, some obtained after prolonged infusion of [13C]glucose into anesthetized rats ((van Heeswijk et al. 2012; Choi et al. 1999; Choi and Gruetter 2003; van Heeswijk et al. 2010; Morgenthaler et al. 2008)). The Kong et al. (2002) study also emphasized sensitivity of glycogen content to microwave-fixation power (i.e., 3.5 and 6 kW power gave lower cerebral cortical glycogen levels than 10 kW, implying incomplete enzyme denaturation) and they reported substantial regional differences in glycogen level (notably very high in corpus callosum white matter, >20 mol/g), consistent with previous findings by Sagar et al. (1987) of a 2.5-fold range in glycogen level in various brain regions (2.7-6.9 mol/g, assuming 100 mg protein/g). Also of interest is the fact that various anesthetics depress brain metabolic rate and raise rodent brain glycogen level by 1.7-3-fold within 6 h (Brunner et al. 1971; Nelson et al. 1968). Glycogen levels in human brain samples obtained during surgery for temporal lobe epilepsy were 5-6 mol/g in ‘normal’ cortical tissue, but much higher in pathological hippocampus (13 mol/g (Dalsgaard et al. 2007)). Thus, brain glycogen levels can vary considerably depending on the physiology and history of the subject and exact experimental conditions and assays. Glycogen content will be grossly underestimated unless considerable care is taken with the enzyme inactivation and extraction procedures; decapitation and freezing is not adequate. It is also critical to minimize sensory stimuli and stresses that are commonly associated with animal handling, environmental conditions, and experimental and euthanasia (e.g., during immobilization for freeze-blowing or microwave fixation) procedures because they can quickly accelerate glycogenolysis in vivo prior to tissue sampling (Cruz and Dienel 2002). The point of this discussion is not to belabor differences in values obtained in various studies. Rather, it is intended to emphasize the bottom line that assays using improved technical and experimental methods yield brain glycogen concentrations that are much higher than those historically reported and glycogen level is very labile and more important than previously recognized. A take-home message is that exploration and resolution of the basis for unexpected and apparently-conflicting results can take a lot of time and work but can lead to important advances.
Glycogen mobilization during brain activation
Turnover exceeds net consumption
Label incorporation from [6-14C]glucose into glycogen isolated from ethanol extracts was similar during rest and generalized sensory stimulation and it doubled during recovery, but the net label incorporation was always very low, i.e., less than 0.7% of the total recovered in all metabolites (Dienel et al. 2002). In a subsequent study in which the glycogen was pre-labeled before sensory stimulation, the percentage of label released during sensory stimulation from glycogen in different dissected brain regions exceeded the corresponding percent decrease in unlabeled glycogen concentration in the same region (Dienel et al. 2007a), indicating that glycogen turnover (degradation and re-synthesis) increased more than net consumption of glycogen during stimulation. Understanding the basis for these findings is complicated because synthesis and degradation may take place in a different regions of the same glycogen molecule, in different molecules or regions in the same astrocyte, in different astrocytes, or all of these alternatives. Unfortunately, current technology cannot distinguish among these possibilities.
Compensatory rise in CMRglc evoked by blockade of glycogenolysis
To further evaluate the quantitative aspects of regional glycogenolysis during sensory stimulation, we reasoned that if glycogen had key roles in astrocytic energetics during brain activation, more blood-borne glucose would be consumed when glycogenolysis is inhibited. Thus, during blockade of glycogen mobilization the magnitude of the compensatory rise in glucose utilization would reflect contributions of glycogenolysis that may otherwise be masked by re-synthesis or averaging when assayed in tissue samples that were dissected from funnel-frozen brain. Inhibition of glycogen phosphorylase had no effect on CMRglc in all brain regions of interest during rest, whereas there were large, regionally-selective increases in CMRglc, particularly in sensory and parietal cortex, during sensory stimulation involving auditory, visual, and whisker pathways (Dienel et al. 2007a). The net compensatory increases in CMRglc in these structures in phosphorylase-inhibited rats ranged from about 0.2-0.4 mol/g/min or about 25-50% above the respective CMRglc in stimulated vehicle-treated controls; these activated controls were, in turn, about 30-60% higher than the corresponding CMRglc baseline values. In other words, glycogen turnover appears to be quite high during physiological sensory stimulation, and it is not detectable unless special experimental approaches are used. Remarkably, the net (activation - rest) compensatory rise in CMRglc in layer 4 plus surrounding tissue in the parietal cortex was greater in magnitude than the stimulus-induced net increase in CMRglc in the same region of vehicle-treated rats. In several other structures, the compensatory response was about half of the stimulus-induced rise in CMRglc. These findings indicate that a very large fraction (50-100%) of the increase in CMRglc in specific structures is associated with physiological functions that predominate during sensory stimulation and are served by or related to glycogen mobilization. A notable implication of the large compensatory CMRglc responses to glycogen phosphorylase blockade is that the commonly-used measures of glycogen contributions to brain energetics (i.e., concentration change, label incorporation, and label release) either cannot detect or greatly underestimate the role of glycogen during brain activation.
The compensatory CMRglc responses to deficits in glycogen utilization may occur solely in astrocytes and involve various subcellular regions (e.g., peri-synaptic processes, large processes, soma, and endfeet). If this is true, astrocytes account for most of the increase in CMRglc in specific structures during sensory stimulation. The compensatory responses may, however, also involve neurons. For example, impaired K+ clearance from extracellular fluid (Xu et al. 2012) by perisynaptic processes during blockade of glycogenolysis could raise extracellular K+ levels and evoke depolarizations that increase neuronal ion pumping and energy demand. Blood flow increases in parallel with CMRglc during activation, and loss of glycogen as fuel in astrocytic endfeet may require more blood-borne glucose for various processes. For example, ammonia rapidly diffuses from blood across the endothelium into brain and its detoxification occurs in endfeet with incorporation of ammonia into glutamine in an ATP-requiring reaction (Cooper 2012). Disturbances of glycogen-related neurotransmitter homeostasis and activities (Schousboe et al. 2010; Walls et al. 2009) when phosphorylase activity is inhibited may also contribute to metabolic changes in astrocytes and neurons. More work is required to establish the cellular and subcellular sites of compensatory CMRglc.
Shunting of glucose through glycogen
Advantages of storing glucose as glycogen include (i) the glucose polymer has low osmotic activity compared with an equivalent number of ‘free’ glucose molecules, (ii) both the synthesis and degradation of glycogen are tightly regulated, (iii) Glc-6-P can be rapidly generated from glycogen without using ATP at the hexokinase step, and (iv) Glc-6-P is a very poor substrate for passage through astrocytic gap junctions (Gandhi et al. 2009b), so it serves as fuel for the astrocyte where it was generated and it also regulates hexokinase activity in that same cell. Thus, glycogen synthesized when glucose supply exceeds demand has an energetic advantage for astrocytes during brain activation when demands for glucose and ATP increase.
The notion that a fraction of blood-derived glucose is incorporated into glycogen, then released and metabolized further is called the glycogen shunt (Fig. 1), i.e., some blood-borne glucose flows through the astrocytic glycogen pool before entering the glycolytic or pentose phosphate shunt pathways (Shulman et al. 2001). The existence of the glycogen shunt pathway is self-evident because blood glucose is the predominant precursor for glycogen, although gluconeogenesis does occur in cultured astrocytes (Schmoll et al. 1995; Dringen et al. 1993b). However, the concept is important because the crux of the shunting glucose through glycogen is that glycogen turnover could become an energetic drain for astrocytes if glucose simply cycles through glycogen without having some functional advantage. In other words, shunting could constitute a futile cycle in which ATP is wasted because it costs more ATP to make the glycogen and metabolize the same glucosyl molecule compared with direct glycolytic metabolism of glucose. Thus, to evaluate the overall cost-benefit of glycogen shunting it is necessary to know where and when the glycogen is consumed and where and when it is synthesized; cellular localization and subcellular compartmentation of glycogen turnover are the key issues. The existence of glucoseglycogen shunting (i.e., glycogen turnover) above the basal rate is supported by high glycogen mobilization during brain activation and by the fascinating studies in cultured astrocytes demonstrating complex roles of glycogen metabolism in astrocyte function (Walls et al. 2009).
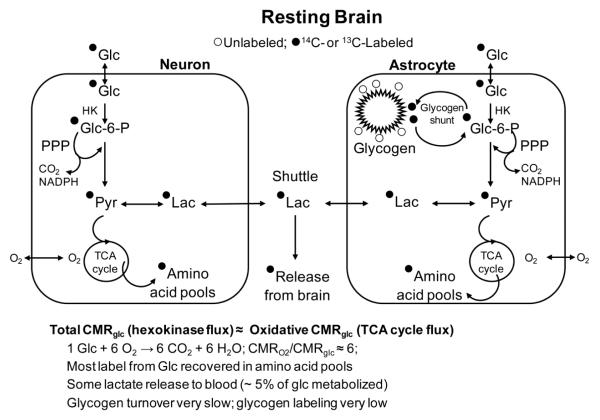
Fig. 1. Glycogen turnover in resting brain.
The glycogen shunt involves passage of blood-borne glucose (Glc) through glycogen before the glucose-6-phosphate (Glc-6-P) is metabolized further via the glycolytic pathway to pyruvate (Pyr) or by the pentose phosphate shunt pathway (PPP). The PPP causes decarboxylation of carbon one of glucose and generates 2 NADPH per glucose for management of oxidative stress; the rest of the carbon re-enters the glycolytic pathway. Incorporation of label from blood-borne glucose into glycogen is low and mainly labels the outer tiers; the inner tiers are mainly unlabeled unless glycogen is depleted prior to labeling. Most of the label derived from glucose is recovered in the tricarboxylic acid (TCA) cycle-derived amino acids, mainly glutamate. However, lactate (Lac) is generated in neurons or astrocytes or both, and some is released from brain under resting conditions. Most of the glucose phosphorylated by hexokinase (HK) in brain (i.e., total glucose consumed) is oxidized, and the ratio of the rate of glucose to oxygen utilization (CMRO2/CMRglc) is close to the theoretical maximum of 6; measured values are slightly lower than 6 due to lactate efflux and non-oxidative biosynthetic activity.
Fate of glycogen carbon during brain activation
The ultimate metabolic fate of the glycogen carbon in activated brain in vivo is not known, and it is extremely difficult to establish experimentally. As illustrated in Fig. 2, glycogen can be metabolized by various routes: (i) it may fuel astrocytes via glycolytic and oxidative metabolism, with oxidation taking place in the cell where glycogen was degraded or in other gap junction-coupled astrocytes when glycogen-derived lactate is shuttled among astrocytes; (ii) lactate may be generated from glycogen via glycolysis and released from the cell; (iii) the released lactate may be discharged from brain to blood or diffuse to nearby neurons (or astrocytes) where it may serve as a supplemental oxidative fuel, as proposed by Dringen et al. (1993a) based on quantitative efflux of glycogen-derived lactate to the culture medium; (iv) glycogen may serve as precursor for de novo synthesis of glutamate via the astrocytic oxidative pathways, as in chick brain during memory consolidation (Gibbs et al. 2007; Gibbs et al. 2006); or (v) all of these possibilities occur to some extent. The major alternative fates of glycogen carbon have predicted phenotypes that can be compared with established metabolic characteristics of brain activation to evaluate whether a specific fate is dominant during brain activation (Fig. 2).
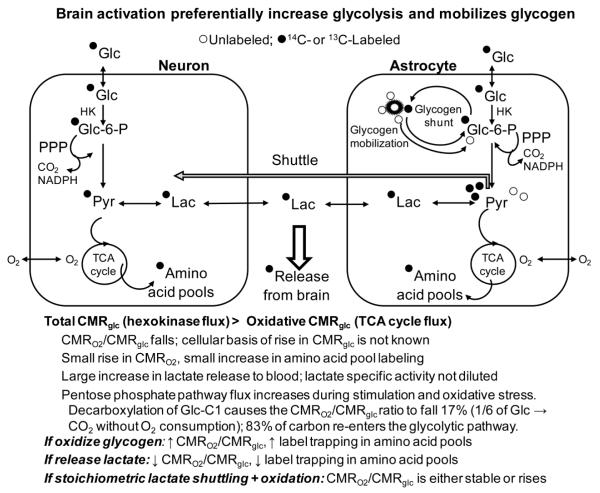
Fig. 2. Glycogen mobilization during brain activation.
Sensory stimulation increases utilization of glucose more than oxygen, causing the CMRO2/CMRglc ratio to fall, along with the other listed characteristics of brain activation. Glycogen is also mobilized, causing release of labeled and unlabeled glucosyl units, with net consumption of glycogen during strong activating conditions. The fate of the glycogen remains to be established, and predicted predominant metabolic outcomes are shown for (i) glycogen oxidation, (ii) lactate release, and (iii) tightly-coupled shuttling of glycogen-derived (or glucose-derived) lactate to neurons with oxidation leading to label incorporation into the TCA cycle-derived amino acid pools. Lactate release is the outcome that agrees best with observed metabolic changes during brain activation (see text). Abbreviations are the same as in Fig. 1.
Net consumption and oxidation of unlabeled glycogen during brain activation
This pathway predicts predominant flow of glycogen carbon into the TCA cycle, causing an increase in CMRO2 over and above that of CMRglc (Fig. 2). One potential cause for the compensatory increased blood-borne glucose utilization during blockade of glycogen mobilization is that the glycolysis fuels the activated astrocyte and lactate generated serves as an oxidative fuel for nearby neurons and/or astrocytes so that the lactate-recipients must now consume more blood-borne glucose. However, if net glycogen utilization coupled with glycogen-derived lactate shuttling plus oxidation took place at a substantial rate during brain activation, the CMRO2/CMRglc ratio is predicted to rise, whereas almost all studies show that it falls (Dienel and Cruz 2008). The reason for a disproportionate rise in CMRO2 is that the CMRO2/CMRglc ratio is based on arteriovenous differences for the two substrates, and oxidation of endogenous glycogen would require more oxygen relative to uptake and oxidation of blood-borne glucose, causing the metabolic ratio to increase during stimulation. We did observe a rise in the metabolic ratio during the recovery phase at 15 min after cessation of sensory stimulation (Madsen et al. 1999), but the biochemical and cellular basis of the fall and subsequent rise in the CMRO2/CMRglc ratio remain to be established; oxidation of various endogenous substrates during recovery could contribute to CMRO2.
High glycogen shunting coupled with lactate shuttling and oxidation in neurons during brain activation
This fate pathway predicts that labeled blood-borne glucose quickly cycles through glycogen during brain activation and labeled lactate generated from labeled glycogen enters the oxidative pathway in neurons. Thus, the CMRO2/CMRglc ratio would not change from rest to activation because the incoming glucose is oxidized at the same rate as during rest after a detour through glycogen. Also, trapping of label in TCA cycle-derived amino acids would be high (Fig. 2). If this process occurs at the magnitude of the compensatory rise in CMRglc during glycogen phosphorylase inhibition, there would have to be enormous fluxes of labeled blood-borne glucose into glycogen and almost immediate release of the recently-incorporated blood-derived glucose molecules from glycogen (i.e., shunting), coupled with their conversion to lactate, release from the cells, oxidation in other cells, and label incorporation into amino acids. The reasons the newly-added outer-tier glucose molecules would have to be involved in the turnover-shuttling-oxidation process are that the compensatory CMRglc rate is quite high (0.2-0.4 mol/g/min) (Dienel et al. 2007a), whereas net labeling of (presumably only outer tiers) glycogen by [6-14C]glucose in brief (5 min) experiments in sensory stimulated rats is extremely low (<0.7% of the total label in metabolites, corresponding to a glucose incorporation rate of 0.002-0.005 mol/g/min (Dienel et al. 2002)), and release of label from pre-labeled glycogen exceeds net consumption of unlabeled glycogen (Dienel et al. 2007a). Thus, there is very low net incorporation (or retention) of label from blood-borne glucose into glycogen, more rapid release of previously-incorporated label compared with net decreases in glycogen content in the same structure and same experimental interval, and a 100-fold higher rate of compensatory utilization of blood glucose when glycogenolysis is impaired compared with the rate of net blood glucose incorporation into glycogen under similar activating conditions. These results infer rapid on-off trafficking of the same labeled glucosyl moieties in the outer tiers of glycogen during activation. In contrast, if label incorporation rate were high and residence time were long, more label incorporation into glycogen than the measured amount is expected, and the percent decreases in pre-labeled and unlabeled glycogen during activation should be similar in magnitude. To sum up, results of in vivo glycogen labeling studies discussed above are consistent with rapid shunting during brain activation, as predicted by this pathway for the fate of glycogen.
The second aspect of the shunt-shuttle-oxidation pathway for the fate of glycogen (Fig. 2) is related to metabolic studies with 14C- or 13C-labeled glucose that take advantage of label incorporation into metabolites in different pathways. Dilution of label into the large unlabeled pools of TCA cycle-derived amino acids (Fig 2) traps the label, and 13C- labeling of neuronal glutamate is used to calculate glucose oxidation rates (de Graaf et al. 2011; Rothman et al. 2011). High rates of shunting of labeled blood glucose through astrocytic glycogen during brain activation followed by metabolism via the glycolytic or pentose phosphate shunt pathways would label pyruvate and lactate, and stoichiometric lactate shuttling plus its oxidation would cause high label trapping in amino acids (Fig. 2). Similarly, shuttling of lactate derived from glycolytic metabolism of blood glucose tightly coupled with lactate oxidation would also cause high label retention in tissue. Because oxidation of lactate requires oxygen consumption, the CMRO2/CMRglc ratio would not fall from rest to activation if blood glucose-derived lactate oxidation stoichiometrically matched lactate production, whether generated via high flux through the glycogen shunt or directly by glycolytic metabolism. Thus, this scenario predicts (i) stability of the CMRO2/CMRglc ratio from rest to activation because blood glucose would be oxidized to the same extent as during rest, whether it shunts through glycogen or flows directly to the TCA cycle (i.e., total glucose utilization would equal oxidative metabolism of glucose), and (ii) high trapping of glucose label in metabolites (Fig. 2). As discussed below, neither criterion is fulfilled.
Release of glycogen-derived lactate from brain during activation
This fate (Fig. 2) is compatible with any magnitude of glycogen shunting, and it predicts release of labeled and unlabeled lactate from activated brain regions, a fall in the CMRO2/CMRglc ratio during brain activation (i.e., total glucose utilization exceeds oxidative metabolism), a relatively small rise in CMRO2 above the resting rate due to predominant lactate release, and much lower retention of labeled metabolites of glucose in amino acid pools than expected based on assays of the hexokinase step (Fig. 2). The following independent lines of evidence strongly support predominant lactate release from brain of normal awake subjects.
(i) Trapping of [6-14C]glucose-derived label during brain activation is incomplete, and the rise in glucose-derived label retention in tissue corresponds to less than half of the rise in total glucose utilization determined with [14C]deoxyglucose when assayed in parallel experiments in many laboratories (reviewed in (Dienel 2012b)). This means about 50% of the label in metabolites of glucose is quickly (within 5 min) released from brain. Because lactate shuttling-oxidation would result in label incorporation into TCA cycle-derived amino acids, the rise in label trapping represents a maximal level of shuttling. However, this amount will be too high and must be corrected downwards for label incorporated into metabolites via other pathways and directly from pyruvate.
(ii) Lactate carries all of the label in the precursor glucose except that lost via the pentose phosphate pathway, and its efflux from brain would proportionately diminish label trapping in amino acid pools and eliminate pyruvate/lactate as oxidative fuel (Fig. 2). During spreading depression, labeled and unlabeled lactate are quickly released from brain to venous blood in amounts equal to about 20% of the 14C-labeled and unlabeled glucose entering the brain in the same arteriovenous samples (Cruz et al. 1999; Adachi et al. 1995). This lactate efflux accounted for about half the magnitude of the underestimation of CMRglc with [6-14C] glucose (reflecting mainly oxidative metabolism) compared with [14C]DG (reflecting total glucose utilization at the hexokinase step). Lactate efflux from brain also rises after ammonia treatment (Hawkins et al. 1973), during sensory stimulation of rats (Madsen et al. 1999), and mental testing of humans (Madsen et al. 1995), and large amounts of lactate, ranging from 20-100% of glucose influx, are released from the eye of normal animals (see Table 6 in (Dienel 2012a)). Our subsequent follow-up studies identified a novel route for metabolite release, the perivascular-lymphatic drainage system that removes glucose, lactate, other compounds, and proteins from interstitial fluid (Cruz et al. 2013; Ball et al. 2010). These data are direct proof for rapid, substantial lactate efflux from brain by at least two routes, perivascular-lymphatic flow and release to blood. Lactate diffusion from interstitial to perivascular fluid and discharge from astrocytic endfeet can feed lactate into both of these pathways. Metabolite release from brain via the perivascular-lymphatic drainage system is consistent with clearance of various small molecules, fluorescent tracers, and proteins from brain interstitial fluid to lymph nodes in the neck and spine (Bradbury and Cserr 1985; Weller et al. 2009; Carare et al. 2008; Nagra et al. 2006; Koh et al. 2005) that is driven by arterial pulsations (Rennels et al. 1990; Rennels et al. 1985) and other factors (Bradbury and Westrop 1983) and is linked to water flow across astrocytic endfeet (Iliff et al. 2013; Iliff et al. 2012).
(iii) The CMRO2/CMRglc ratio falls during activation. The magnitude of the fall varies under different experimental conditions, but the findings rule out stoichiometric formation, shuttling, and oxidation of lactate that require CMRO2 to rise in proportion (i.e., 6:1) with CMRglc (Figs. 1, 2).
(iv) The rise in CMRO2 during activation is quite small, generally <20-30%, compared with much larger increases in CMRglc, consistent with loss of oxidative fuel due to lactate efflux. The magnitude of the net rise in CMRO2 during activation also sets the upper limit for any lactate shuttling coupled with its oxidation that requires oxygen. However, this value is too high and must be corrected downward for direct oxidation of glucose- or glycogen-derived pyruvate and oxidation of other endogenous substrates. Estimates of maximal lactate shuttling contribution to total oxidation are in the range of 10% or so (Dienel 2013; Dienel 2012a, b).
(v) The specific activity of blood glucose-derived lactate in brain during [6-14C]glucose labeling experiments equals the theoretical maximum (i.e., half that of glucose because one lactate is labeled and one unlabeled), meaning that the labeled lactate is not diluted by unlabeled lactate entering brain from blood or generated in brain from glycogen during rest, sensory stimulation, and recovery and during spreading depression (Dienel and Cruz 2009). This implies that the pool of pyruvate/lactate derived from labeled blood glucose and the pool from unlabeled glycogen are segregated into different compartments that do not readily mix, as observed in cultured astrocytes (Sickmann et al. 2005). Lack of lactate specific activity dilution means that label incorporation from glucose- or glycogen-derived lactate into glutamate should be high if lactate shuttling were tightly coupled with shunting and oxidation. The rise in metabolite labeling by glucose during activation is, however, modest. Instead, release of highly-labeled and unlabeled lactate to blood would account for experimental findings documenting incomplete trapping of labeled products of glucose, the greater rise in CMRglc compared with CMRO2, and the relatively small rise in CMRO2.
(vi) Astrocytes have high lactate trafficking capacity, and are poised to clear lactate from activated brain regions. Astrocytes in adult rat brain slices have 2-4-fold higher rates and capacities for lactate uptake from extracellular fluid than neuronal uptake and for dispersal of lactate throughout the syncytium of gap junction-coupled astrocytes compared with lactate transfer from astrocytes to neurons (Gandhi et al. 2009a). Thus, lactate formed in astrocytes from glycogen or glucose or taken up after release from neurons can be quickly discharged to perivascular fluid, meninges, and blood from endfeet (Cruz et al. 2013; Ball et al. 2010) without mixing with lactate generated by and retained in neurons. On the other hand, lactate produced by neurons may be relatively sequestered intracellularly due to near-saturation of their low Km MCT2 transporters, leading to restricted lactate efflux from neurons (Chih and Roberts Jr 2003; Chih et al. 2001). Near-saturation of MCT2 during activation also limits lactate entry into neurons, facilitating its clearance from brain via gap junction-coupled astrocytes.
In sedentary subjects, the blood lactate level is lower than that in brain, and the outwardly-directed brain-to-blood lactate concentration gradient originating in activated brain cells would act to ‘pull’ lactate out of the brain. ‘Pulling’ is due to concentration-driven transport, dilution of lactate in the large blood volume of the whole body, and fast cerebral blood flow rates that maintain the lower venous blood lactate level compared with brain tissue. Conversely, raising blood lactate level by muscular activity (or lactate infusion) creates a different physiological condition than during sensory stimulation of sedentary subjects. In this case, the elevated blood lactate level drives lactate into the entire brain, thereby diminishing intracerebral lactate gradients and pushing blood-derived lactate into all brain cells. Use of blood lactate as a supplemental oxidative fuel by brain under hyperlactemic conditions is important for glucose sparing during intense physical activity when large amounts of lactate are generated by muscles and released to blood. Some investigators have used lactate loading experiments (in vivo lactate infusions or high lactate levels in tissue culture media) to evaluate neuronal lactate oxidation and they interpreted the results as evidence for lactate as neuronal fuel to support the existence of astrocyte-to-neuron lactate shuttling during brain activation. However, the physiology of each experimental condition must be taken into account, and hyperlactemic conditions during which ‘lactate muscles its way into consciousness’ (Dienel 2004, 2012b) is not compelling evidence for substantial astrocyte-to-neuron lactate shuttling during brain activation in sedentary subjects; lactate oxidation itself is not proof of shuttling. Indeed, when plasma lactate is < 3 mM, oxidation of blood-borne lactate by human brain contributes <10% to the total oxidation of glucose plus lactate (van Hall et al. 2009; Boumezbeur et al. 2010), sharply contrasting the 45-75% in immature cultured neurons at 1 mM lactate (Bouzier-Sore et al. 2006; Bouzier-Sore et al. 2003).
(vii) Glycogen is mobilized by oxidative stress to generate NADPH via the pentose phosphate shunt pathway in cultured astrocytes, even in the presence of high extracellular glucose (Rahman et al. 2000). Flux through the pentose phosphate shunt pathway in vivo rises from about 7% at rest to 25% of glucose oxidized in the inferior colliculus in our acoustic stimulation studies (see Fig 6c-1 in (Dienel 2012b). Pentose phosphate pathway activity causes label loss from C1-labeled glucose and, therefore, depresses the CMRO2/CMRglc ratio. Decarboxylation of carbon one of glucose means that 1/6 (17%) of the glucose carbon is converted to CO2 without consumption of an equivalent amount of oxygen, causing the oxygen/glucose ratio to fall by the same proportion. The remaining 83% of the glucose carbon re-enters the glycolytic pathway for further metabolism (if this did not occur, phosphate would be trapped in pentose pathway intermediates and interfere with ADP/ATP turnover). If oxidation of unlabeled glycogen carbon occurred to the same extent as the rise in pentose phosphate shunt flux to manage oxidative stress, the CMRO2/CMRglc ratio would be relatively stable from rest to activation instead of the predicted increase discussed above. Thus, interactive effects of changes in fluxes in different pathways that can influence the global metabolic ratio illustrates one of the complexities in evaluation of alternative fates of glycogen.
To sum up, the functional roles of glycogen are important for the energetics of brain activation, but the details of its metabolism, compartmentation, and fate are quite complex and very difficult to assess experimentally. Furthermore, simultaneous changes in different pathways complicate interpretation of results. Nevertheless, available evidence can exclude or support major alternatives, and the in vivo data derived from studies discussed above strongly support predominant release of glycogen-derived lactate (and blood glucose-derived lactate) from brain, not direct oxidation or shuttling plus oxidation. However, the fate of glycogen need not be exclusively lactate release because small or transient fluxes involving oxidation may occur and their detection may be masked by higher efflux rates. In addition, metabolic fates of glycogen and functions served by glycogen metabolism may vary among brain regions, even during the same activation event. For example, memory consolidation in the hippocampus or other structures may involve different uses of glycogen carbon compared with somatosensory cortex that processes input from the event (e.g., foot shock or adverse taste) that is the substrate of the memory. Obviously, combinations of alternative fates of glycogen are very difficult to evaluate, quantify, and interpret.
Glucose-sparing by glycogen, glucose, and glutamate
In spite of the considerable experimental difficulties with in vivo glycogen assays, astrocytic glycogen levels in brain of normal, carefully-handled, resting, awake rodents can be as high as ~10-24 mol/g, i.e., twice the tissue glycogen level, assuming that astrocytes occupy about half of the brain mass (see the section ‘Discoveries arising from investigation of the basis for unexpected, discrepant results’, above). Thus, astrocytic glycogen content can be 5-10 times higher than that of unmetabolized glucose in brain tissue (2-3 mol/g). The conclusion that glycogen can serve as a significant energy source for astrocytes and a regulator of their utilization of blood glucose during brain activation is derived from the remarkably-insightful modeling studies of DiNuzzo and colleagues (Dinuzzo et al. 2012; DiNuzzo et al. 2011; DiNuzzo et al. 2010). They proposed that glycogenolysis may serve to maintain a high concentration of Glc-6-P in astrocytes, thereby sustaining or increasing feedback inhibition of hexokinase in astrocytes by Glc-6-P, while simultaneously providing substrate for the astrocytic glycolytic pathway. Thus, the novel, new concepts are that blockade of glucose phosphorylation in astrocytes by the product of glycogen mobilization frees up extracellular and blood-borne glucose for use by neurons, and glycogenolysis influences cellular basis of utilization of blood-borne glucose during brain activation.
In addition to glycogen, unmetabolized glucose in brain can serve as an energy buffer during activation. The total rat brain glucose concentration is about 20% that of arterial plasma (Holden et al. 1991; Dienel et al. 1991), and brain level would be 2 mol/g when plasma glucose is 10 mM. If CMRglc increased 100% at the onset of brain activation (from 1 to 2 mol/g/min), an additional 1 mol/g of glucose would be consumed during the first minute. Assuming no glucose delivery to brain from blood during this interval (glucose is, in fact, delivered at a rate exceeding its phosphorylation rate), the stimulus-evoked rise in CMRglc would reduce the brain glucose level to 1 mol/g which is ~20 times the Km of hexokinase for glucose (~0.05 mM, (Wilson 2003)). However, if brain glucose level falls below ~ 0.5 mM (10 times the Km) the rate will progressively fall as glucose concentration decreases and hexokinase becomes unsaturated, as predicted by Michaelis-Menten kinetics. In normal, activated brain, hexokinase remains saturated and able to operate at maximal velocity when dis-inhibited by consumption of Glc-6-P. Lowry and colleagues determined that hexokinase rate in normal rodent brain in vivo is only about 3% of the maximal value assayed in vitro, indicating the enzyme is inhibited by about 97% in vivo (i.e., the rate can rise more than 20-fold during activation), mainly by feedback inhibition by Glc-6-P and perhaps other unidentified factors (Lowry and Passonneau 1964; Lowry et al. 1964). Thus, reduced astrocytic utilization of endogenous brain glucose and blood-borne glucose at stimulus onset due to hexokinase inhibition by glycogen-derived Glc-6-P further increases the ‘safety range’ for glucose supply to satisfy immediate neuronal energy demands at stimulus onset. Furthermore, unmetabolized glucose within astrocytes can diffuse down its concentration gradient to nearby neurons (Gandhi et al. 2009a), thereby sharing un-needed substrate with nearby activated neurons.
Three other physiological responses also help maintain brain glucose availability for its metabolism and glycogen turnover during brain activation: blood flow is quickly upregulated, glucose delivery exceeds demand, and alternative substrates can be oxidized. Hemodynamic responses to brain activation are very fast, even in anesthetized subjects, and many studies have shown that blood oxygen-level dependent (BOLD) signals, cerebral blood flow, and cerebral blood volume increase within 350-500 milliseconds and reach peak within a few seconds or minutes (Masamoto and Kanno 2012). Cremer, Cunningham, and colleagues (Hargreaves et al. 1986; Cremer and Seville 1983) demonstrated that glucose delivery exceeds its phosphorylation rate by about 60% over a 5-fold range of CMRglc and wide range of glucose concentrations (See Fig. 4 in (Dienel 2013)). Glutamate is a readily-oxidizable fuel, and oxidation of some of the neurotransmitter glutamate taken up by astrocytes that contain mitochondria in their fine processes would also serve a glucose-sparing role during excitatory neurotransmission because glutamate is delivered to the site where and when energy demands are enhanced (Dienel 2013). Some endogenous lactate may also be oxidized, but even during strong electrical stimulation the amount of lactate consumed is calculated to be quite small (see Table 7 in (Dienel 2012a)).
To summarize, endogenous substrates (glycogen, intracellular and extracellular glucose, glutamate, and lactate), along with rapid responses in glucose delivery in amounts that exceed demand, ensure that brain has adequate supplies of fuel and can replenish glycogen after its consumption. However, under mild-to-moderate hypoglycemic conditions, e.g., diabetic subjects given too much insulin, blood glucose levels are too low to fulfill the brain’s demand for glucose, and glycogen is consumed at higher rates to match supply-demand deficits (Choi et al. 2003), and under more severe hypoglycemic states, endogenous substrates are oxidized in increased amounts.
Gap junctional trafficking of metabolites within the astrocytic syncytium
The proposed role of Glc-6-P in governing fuel supplies to astrocytes and neurons has an added dimension because this regulatory metabolite is highly restricted from passage through gap junctional pores (Gandhi et al. 2009b). Deoxyglucose-6-P and the fluorescent glucose analog, 2-NBDG-6, also have very low gap junctional permeability, confirming the concept that hexose-6-phosphates do not participate in gap junctional communication. [14C]Glc-6-P was reported to traverse gap junctions in cultured astrocytes (Tabernero et al. 2006; Tabernero et al. 1996), but only label transfer was measured, and apparent diffusion of [14C]Glc-6-P through gap junctions must have been due to its metabolism by enzymes released during the scrape-load procedure to produce labeled downstream gap junction-permeant molecules that include glyceraldehyde-6-P and lactate (Gandhi et al. 2009b).
Retention of Glc-6-P in the cell where it was formed means that each astrocyte controls its utilization of blood-borne glucose by regulating the hexokinase reaction. Downstream fluxes in the pentose phosphate shunt and glycolytic pathways would be governed at the glucose-6-phosphate dehydrogenase and phosphofructokinase steps, respectively. Coupled astrocytes not only regulate their own metabolic activity, they can also share glucose, downstream metabolites, and redox and signaling compounds with other coupled astrocytes. The large syncytium is comprised of as many as 10,000 cells, and it is linked to the vasculature via endfeet (Gandhi et al. 2009a; Gandhi et al. 2009b; Ball et al. 2007). Astrocytes are crossroads of metabolite trafficking to and from blood.
Physiological roles for glycogen
Mechanistic and functional studies of glycogen in living brain are especially difficult, and progress has depended on studies in simpler systems, including brain slices and cell cultures. For example, it is well known that many, but not all, neurotransmitters mobilize glycogen via specific receptors (see discussion in (Cruz and Dienel 2002)), oxidative stress increases flux of glycogen-derived Glc-6-P through the astrocytic pentose phosphate shunt pathway to generate NADPH (Rahman et al. 2000), and graded increases in extracellular K+ concentration progressively increase glycogenolysis in brain slices (Hof et al. 1988). The complexity of the signaling and regulatory mechanisms involved in K+ uptake from extracellular fluid (Walz 2000; Hertz et al. 2007) to glycogenolysis into cultured astrocytes has only recently been elucidated (Xu et al. 2012). Extracellular glutamate does not stimulate glycogenolysis in brain slices (Magistretti et al. 1981), but glycogen metabolism is linked to glutamatergic neurotransmission in cultured cells (Sickmann et al. 2009), and in vivo studies in diabetic rat brain support roles for glycogen in glutamate and GABA homeostasis (Sickmann et al. 2012, 2010). Brain glycogen level rises during slow wave sleep to 70% above waking levels and falls within minutes after awakening (Karnovsky et al. 1983), whereas sleep deprivation for 12-24h causes a ~40% fall in glycogen level that is restored with sleep (Kong et al. 2002). Of considerable interest is the evidence from recent studies using different experimental systems that links glycogenolysis to learning and memory. Knockout of glycogen synthase reduces learning ability and diminishes hippocampal long term potentiation, strongly supporting the concept of key roles for glycogen in cognitive activities (Duran et al. 2013). A series of complex in vivo learning and biochemical experiments support the conclusion that glycogenolysis is required for memory consolidation in young chicks and that it is substrate for glutamate synthesis via the astrocytic TCA cycle (Gibbs and Hutchinson 2012; Hertz and Gibbs 2009). This deduction would be strengthened by direct demonstration of label transfer from glycogen to glutamate, which is especially difficult to carry out in vivo. Blockade of glycogenolysis impairs memory formation in rats, and glycogen-derived lactate is proposed to be shuttled from astrocytes to neurons during memory consolidation (Suzuki et al. 2011; Newman et al. 2011). Although intriguing, this notion of lactate shuttling demands much more rigorous proof than that provided by the authors, and these are also difficult in vivo studies. For example, no lactate shuttling was demonstrated, inhibition of glycogenolysis does not identify the role or fate of glycogen, memory rescue by lactate injections raises lactate levels in both neurons and astrocytes and causes redox shifts in them, and depletion of MCT levels by siRNA injection is slow (~50% at 13h, or a decrease by about 4%/h) and was not shown to be relevant within the timeframe of the actual learning event close to time zero (Dienel 2012b). To summarize, glycogen turnover not only plays an integral role in astrocytic energetics, it is also linked to glucose sparing, sleep-wake cycle, neurotransmitter actions on astrocytes, control of extracellular K+ level, management of oxidative stress, and memory consolidation. Astrocytic glycogen is necessary for complex brain functions.
Glycogenolysis is triggered by catecholamines released by locus coeruleus neurons that have a wide-spread forebrain innervation. It is, therefore, intriguing to consider the possibility that alerting stimuli involving responses of the locus coeruleus network may evoke widespread glucose-sparing responses in astrocytes by increasing their Glc-6-P levels and inhibiting hexokinase. According to the DiNuzzo hypothesis, catecholamine-evoked glycogen mobilization would shift metabolism of blood-borne glucose away from astrocytes to neurons in a large volume of forebrain. In other words, neurotransmitter signaling could act as a ‘priming’ mechanism for subsequent, larger shifts in function-activated metabolic activity that follow the alerting signals (as well as local functional activation and glycogen mobilization). The importance of locus coeruleus innervation on glycogen utilization is underscored by the striking observations of Harik and colleagues (1982). When the locus coeruleus was unilaterally ablated and the rats then subjected to ischemia or seizures, more glycogen remained in the ipsilateral hemisphere. Thus, neuronal signals are an important supplement to the metabolic regulatory mechanisms arising from energy failure to stimulate consumption of glycogen. Roles of various neurotransmitter systems and their receptor-mediated signaling pathways in the control of cellular glucose and glycogen utilization are research areas that need more experimental attention in future in vivo studies.
Rates of glycogenolysis
The relative importance of any reaction rate or pathway flux under different physiological or pathophysiological conditions can be evaluated by quantitative comparisons of rates of interest to that of a meaningful standard rate. Many in vivo studies carried out in different laboratories under various conditions have shown that CMRglc is directly related to functional activity. CMRglc is highest in gray matter structures with the greatest energy demand and lowest in white matter regions due to their rather modest energy requirements; CMRglc rises with increases in functional activity, it is highest in the neuropil compared with neuronal cell bodies, and it falls when neural activity is depressed (Sokoloff 2000). Based on these findings, it is reasonable to evaluate the importance of the energetic contributions of glycogen to specific functions in terms of glycogenolysis rates (expressed as mol glucosyl units/min/g) for direct comparison to rates of glucose utilization in the same structure under similar conditions.
Normal resting brain
Early studies of glycogen turnover in awake, non-stimulated rodents showed that the macromolecule turns over very slowly, with faster turnover of the outer tiers (0.010 mol/g/min) compared with the limit dextrin (0.004 mol/g/min) that comprises inner core of the glycogen polymer (Watanabe and Passonneau 1973). Thus, glycogenolysis rate in awake, unstimulated rodents is about 1% of the rate of utilization of blood-borne glucose in cerebral cortex (Sokoloff et al. 1977). Calculated rates of glycogen turnover determined in magnetic resonance studies with [13C]glucose in resting human (~0.003 mol/g/min (Öz et al. 2003)) and rat (~0.007-0.01 mol/g/min (van Heeswijk et al. 2010; Choi et al. 1999) brain are also extremely low. When these data are considered within the context of negligible effects of inhibition of glycogen phosphorylase on compensatory changes in CMRglc in resting animals (Dienel et al. 2007a), it is reasonable to conclude that basal turnover of glycogen has barely detectable contributions to overall brain energetics in non-stimulated subjects. However, the quantitative contribution of glycogen to resting astrocytic energetics would probably be much higher in assays at the cellular level.
Brain activation during sensory stimulation and pathophysiological conditions
Average glycogenolysis rates were calculated by dividing the net change in unlabeled glycogen concentration by the time interval, and the summary presented below is based on the data in Table 3 of (Dienel and Cruz 2004), Table 3 of (Dienel 2012b), and Fig. 3 of (Dienel 2013); interested readers are referred to these reviews for details. These rate estimates are not as accurate as desired because they do not reflect peak rates or short bursts of glycogen consumption, and they may be reduced by concomitant synthesis of glycogen in different regions of the same tissue sample. Nevertheless, the rates are useful for comparing the magnitude of glycogenolysis with CMRglc.
Average glycogenolysis rates in our rat sensory stimulation studies (Dienel et al. 2007a; Dienel et al. 2002; Cruz and Dienel 2002) were about 0.5 and 0.2 mol/g/min during stimulation and recovery, respectively, corresponding to 65 and 25% of resting CMRglc. These values are within the range of the highest net compensatory rates of CMRglc during activation in the presence of a glycogen phosphorylase inhibitor (i.e., 0.2-0.4 mol/g/min) (discussed above), suggesting that the compensatory responses may, in fact, reflect astrocytic CMRglc upregulation. Rates during whisker stimulation are calculated to be much lower, about 0.06 mol/g/min, equivalent to 6% of CMRglc. In this case rates were estimated from local percent glycogen changes in barrel cortex measured by quantitative autoradiography (Swanson et al. 1992) and cortical glycogen concentration taken from a separate, earlier study from the same lab, not from the same animals. The exact conditions of the handling of animals prior to experiments and the specific stimulation paradigms will influence the amount of glycogen present at the onset of stimulation and the rate of its degradation.
During insulin-induced hypoglycemia, calculated rates from a number of studies have shown that glycogen mobilization is very modest, ranging from 0.01 to 0.07 mol/g/min (Choi et al. 2003; Ghajar et al. 1982; Suh et al. 2007; Herzog et al. 2008), or about 1-8% of CMRglc. Thus, mild-to-moderate hypoglycemia is a weak activator of glycogen utilization because glucose is still present in blood and delivered to brain by increased blood flow rates (Horinaka et al. 1997; Choi et al. 2001). However, hypoglycemia is carried out under aerobic conditions, and as it becomes more prolonged or more severe, brain cells also oxidize endogenous substrates, especially amino acids. For example, after insulin administration, CMRglc decreased faster than CMRO2 during the progression from lethargy to stupor to coma, whereas CMRglc increased more than CMRO2 during glucose infusion after hypoglycemic coma (Ghajar et al. 1982). These findings are consistent with oxidation of endogenous compounds when plasma glucose fell below the level needed to sustain brain energy demand, followed by greater use of glucose for de novo synthesis of these compounds to replenish their levels during recovery from severe hypoglycemia. Similar conclusions can be drawn from work by Siesjö and colleagues (reviewed in (Siesjö 1978)) who showed that glycolytic and TCA cycle intermediates and amino acids (e.g., glutamate, glutamine, GABA, and alanine) were consumed during hypoglycemia to a greater extent during the isoelectric state compared with the preceding convulsive phase. To sum up, compensatory responses to insulin-induced glucose deficiency in normoxic subjects include consumption of glycogen along with oxidation of endogenous metabolites, especially glucose-derived amino acids involved in neurotransmission.
Severe insults, such as electroshock, flurothyl-induced seizures, anoxia, and ischemia, all of which cause an energy crisis and drastically increase calculated cerebral cortical glycogen utilization rates to as high as 1.8-2.8 mol/g/min (Gross and Ferrendelli 1980; Lowry et al. 1964; Gatfield et al. 1966), or about 270-350% of the resting CMRglc. Deficiency or absence of oxygen increases dependence on the glycolytic pathway and greatly accelerates CMRglycogen. These enormous increases are, however, much smaller than the concomitant increases in CMRglc that rise as much as 6-fold high, to 6 mol/g/min (Lowry et al. 1964). Bicuculline-induced seizures had a smaller but substantial effect on glycogenolysis (0.24 mol/g/min (Folbergrova et al. 1981) or 30% of CMRglc). These findings demonstrate that severe energy failure rapidly accelerates glycogen mobilization, generally resulting in consumption of nearly all glycogen within a minute or so.
Under extreme conditions when glucose is absent or its levels are inadequate to maintain normal brain activity, glycogen has been shown to sustain various neuronal functions, and the higher the initial glycogen level the longer the functions were maintained. These types of studies were carried out in severely hypoglycemic subjects in vivo or under aerobic aglycemic in vitro conditions, some with intense stimulation. As an example, the estimated glycogenolysis rate during prolongation of the duration of EEG in parietal cortex of rats during severe hypoglycemia was about 0.03 mol/g/min (Suh et al. 2007) compared with resting CMRglc of 1.1 mol/g/min (Sokoloff et al. 1977). Glycogenolysis rates that extend the duration maximally-evoked compound action potentials produced by electrical stimulation of isolated optic or sciatic nerve in the absence of glucose (Brown et al. 2012; Brown and Ransom 2007; Brown et al. 2005) are also very low, within the range of about 0.01 to 0.06 mol/g/min. These rates are about <10-40% of the resting CMRglc in these white-matter structures (e.g., 0.13-0.16 mol/g/min in optic nerve and optic chiasm (Grunwald et al. 1988)) that have quite low metabolic rates compared with gray matter (Sokoloff et al. 1977; Grunwald et al. 1988). By contrast, aglycemic cultured astrocytes consume glycogen at a much higher rate of 0.5 mol/g/min (Dringen et al. 1993a). These rates in maximally-stimulated nerve are also much lower than CMRglc and CMRglycogen (0.32 and 0.17 mol/g/min, respectively) in ischemic sciatic nerve (Stewart et al. 1965). Because ischemia blocks oxidative metabolism due to lack of oxygen, it is likely that the lower rates in aerobic, aglycemic nerve is due to concomitant oxidation of amino acids or other endogenous substrates. Glycogen-derived lactate shuttling is proposed to fuel axonal activities, but the contribution of shuttled lactate compared with oxidative metabolism of endogenous metabolites is not known. Because lactate (0.12 mM) was detected outside of the axon (Brown et al. 2012), there must be lactate loss to the perfusion medium, and the rate and quantity of glycogen-derived lactate that helps support axonal function is overestimated by changes in glycogen content. Furthermore, it is not known how much glycogen-derived lactate contributes to axonal or brain function during normal physiological activity when normal amounts of glucose are available. In studies of synaptic function in hippocampal slices, the calculated rate of glycogen consumption in control slices (that were not preincubated with pyruvate to increase glycogen level) during preservation of evoked potentials in the absence of glucose (0.06 mol/g/min (Shetty et al. 2012)) is much less than that of CMRglc in hippocampal slices (0.35 mol/g/min (Newman et al. 1996) or hippocampus in vivo, i.e., 0.6-0.8 mol/g/min (Grunwald et al. 1988; Sokoloff et al. 1977). Finally, linkage of K+-evoked glycogenolysis to astrocyte-to-neuron lactate shuttling and preservation of synaptic function in the absence of glucose also involves very low rates of glycogen and glycogen-derived lactate consumption (~0.02 mol/g/min (Choi et al. 2012). To sum up, the minimal function-preserving rate of glycogenolysis during aerobic aglycemia (0.01 mol/g/min) is 180-280-fold lower than that during severe ischemic energy crises, and it is 6-50-fold lower than that evoked by sensory stimulation in normal subjects.
The low function-preserving rates of glycogenolysis illustrated above are very important for diabetic patients and they prolong various functions in the absence of glucose, but they do not constitute large or major fluxes compared with normal resting glucose utilization rates. These results raise the possibility that the processes assayed do not require much energy derived from glycogen. Normal subjects are never exposed to these types of extreme conditions, and even obese humans starved for 5-7 weeks have normal plasma glucose levels and normal glucose delivery to brain due to compensatory mechanisms (gluconeogenesis and ketone body production) that prevent cerebral hypoglycemia (Owen et al. 1967). However, when at the brink of energy failure, oxidative metabolism of endogenous substrates during aerobic aglycemic and severe hypoglycemic conditions probably generate most of the ATP required, and this amount is supplemented by glycogen to prolong the time until ultimate energy failure. Clearly, evaluation of the roles of glycogen in preserving various neural functions requires detailed analysis of all substrates consumed, particularly in the absence of glucose, and quantitative information regarding the fate of glycogen.
Summary and concluding comments
Glycogen is very difficult to study in living brain, and technical advances have enabled or improved quantitative and non-invasive assays of glycogen concentration, label incorporation into glycogen, label release from glycogen, and compensatory changes in CMRglc during blockade of glycogen mobilization. Glycogen is a ‘hidden’ substrate because the usual assays of glucose utilization (e.g., arteriovenous difference or metabolism of labeled deoxyglucose or glucose) cannot account for the utilization of this unlabeled endogenous substrate. Glycogen turnover can be of sufficiently-high magnitude that it can influence global ratios of oxygen to glucose utilization. Compartmentation of glycogen synthesis and degradation within single glycogen molecules, among different glycogen molecules in the same cell, and in different cells greatly complicates evaluation of these processes, as does compartmentation of downstream metabolism of glycogen and glucose. Without quantitative in vivo analysis, the physiological roles and contributions of glycogen to brain energy metabolism and control of astrocytic and neuronal glucose utilization cannot be appropriately established. The notion that glycogen- and glucose-derived lactate fuels neurons to a major extent is considered very unlikely because many studies provide strong evidence for substantial lactate release from brain and against high fluxes in this pathway; any lactate shuttle-oxidation model must accommodate these data. Similarly, a requirement for lactate shuttling in memory formation has not yet gone beyond the qualitative stage because knowledge of cellular source(s), rates, direction, and quantities of lactate transfer are lacking, and other possible roles for lactate (e.g., redox changes or receptor-mediated effects) have not been examined and ruled out. Roles for glycogen in brain function continue to emerge, revealing increasing complexity of the signaling and regulatory mechanisms that integrate glycogen mobilization with physiological activities. A novel function for glycogenolysis, besides providing substrate for astrocytes, is proposed to be regulation of astrocytic utilization of extracellular and blood-borne glucose during activation by inhibition of astrocytic hexokinase and diverting glucose for use by neurons. When combined with the locus coeruleus catecholaminergic-signaling system, local and global regulation of astrocytic glycogen utilization can have a high impact on cellular utilization of blood-borne glucose during various stages of a stimulatory event, i.e., alerting, onset, progression, and recovery. These fascinating aspects of glycogen biology need to be addressed in future studies.
Acknowledgments
This work was supported, in part, by National Institutes of Health grants DK081936, NS038230, and NS36728.
Abbreviations
- CMRglc
rate of glucose utilization
- CMRO2
rate of oxygen utilization
- DG
2-deoxy-D-glucose
- DG-1-P
deoxyglucose-1-phosphate
- DG-6-P
deoxyglucose-6-phosphate
- Glc
glucose
- Glc-6-P
glucose-6-phosphate
- Lac
lactate
- Pyr
pyruvate
- TCA
tricarboxylic acid
Footnotes
The authors declare no conflict of interest.
References
- Adachi K, Cruz NF, Sokoloff L, Dienel GA. Labeling of metabolic pools by [6-14C]glucose during K(+)-induced stimulation of glucose utilization in rat brain. Journal of cerebral blood flow and metabolism. 1995;15(1):97–110. doi: 10.1038/jcbfm.1995.11. doi:10.1038/jcbfm.1995.11. [DOI] [PubMed] [Google Scholar]
- Ball KK, Cruz NF, Mrak RE, Dienel GA. Trafficking of glucose, lactate, and amyloid-beta from the inferior colliculus through perivascular routes. Journal of cerebral blood flow and metabolism. 2010;30(1):162–176. doi: 10.1038/jcbfm.2009.206. doi:10.1038/jcbfm.2009.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball KK, Gandhi GK, Thrash J, Cruz NF, Dienel GA. Astrocytic connexin distributions and rapid, extensive dye transfer via gap junctions in the inferior colliculus: implications for [(14)C]glucose metabolite trafficking. J Neurosci Res. 2007;85(15):3267–3283. doi: 10.1002/jnr.21376. doi:10.1002/jnr.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, Rothman DL. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(42):13983–13991. doi: 10.1523/JNEUROSCI.2040-10.2010. doi:10.1523/JNEUROSCI.2040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. The European journal of neuroscience. 2006;24(6):1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. doi:10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- Bouzier-Sore AK, Voisin P, Canioni P, Magistretti PJ, Pellerin L. Lactate is a preferential oxidative energy substrate over glucose for neurons in culture. Journal of cerebral blood flow and metabolism. 2003;23(11):1298–1306. doi: 10.1097/01.WCB.0000091761.61714.25. doi:10.1097/01.wcb.0000091761.61714.25. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. The Journal of physiology. 1983;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury MWB, Cserr HF. Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. In: Johnston MG, editor. Experimental biology of the lymphatic circulation. Research monographs in cell and tissue physiology. Vol. 9. Elsevier; New York: 1985. pp. 355–394. [Google Scholar]
- Brown AM. Brain glycogen re-awakened. J Neurochem. 2004;89(3):537–552. doi: 10.1111/j.1471-4159.2004.02421.x. doi:10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Brown AM, Evans RD, Black J, Ransom BR. Schwann cell glycogen selectively supports myelinated axon function. Ann Neurol. 2012;72(3):406–418. doi: 10.1002/ana.23607. doi:10.1002/ana.23607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Ransom BR. Astrocyte glycogen and brain energy metabolism. Glia. 2007;55(12):1263–1271. doi: 10.1002/glia.20557. doi:10.1002/glia.20557. [DOI] [PubMed] [Google Scholar]
- Brown AM, Sickmann HM, Fosgerau K, Lund TM, Schousboe A, Waagepetersen HS, Ransom BR. Astrocyte glycogen metabolism is required for neural activity during aglycemia or intense stimulation in mouse white matter. J Neurosci Res. 2005;79(1-2):74–80. doi: 10.1002/jnr.20335. doi:10.1002/jnr.20335. [DOI] [PubMed] [Google Scholar]
- Brunner EA, Passonneau JV, Molstad C. The effect of volatile anaesthetics on levels of metabolites and on metabolic rate in brain. J Neurochem. 1971;18(12):2301–2316. doi: 10.1111/j.1471-4159.1971.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34(2):131–144. doi: 10.1111/j.1365-2990.2007.00926.x. doi:10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Broadwell RD. Cytochemical identification of cerebral glycogen and glucose-6-phosphatase activity under normal and experimental conditions: I. Neurons and glia. Journal of Electron Microscopy Technique. 1986;3(4):413–437. doi: 10.1007/BF01611733. doi:10.1002/jemt.1060030406. [DOI] [PubMed] [Google Scholar]
- Cetin N, Ball K, Gokden M, Cruz NF, Dienel GA. Effect of reactive cell density on net [2-14C]acetate uptake into rat brain: labeling of clusters containing GFAP+- and lectin+-immunoreactive cells. Neurochem Int. 2003;42(5):359–374. doi: 10.1016/s0197-0186(02)00138-9. [DOI] [PubMed] [Google Scholar]
- Chih CP, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001;24(10):573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Chih CP, Roberts EL., Jr Energy substrates for neurons during neural activity: a critical review of the astrocyte-neuron lactate shuttle hypothesis. Journal of cerebral blood flow and metabolism. 2003;23(11):1263–1281. doi: 10.1097/01.WCB.0000081369.51727.6F. doi:10.1097/01.wcb.0000081369.51727.6f. [DOI] [PubMed] [Google Scholar]
- Choi HB, Gordon GR, Zhou N, Tai C, Rungta RL, Martinez J, Milner TA, Ryu JK, McLarnon JG, Tresguerres M, Levin LR, Buck J, Macvicar BA. Metabolic Communication between Astrocytes and Neurons via Bicarbonate-Responsive Soluble Adenylyl Cyclase. Neuron. 2012;75(6):1094–1104. doi: 10.1016/j.neuron.2012.08.032. doi:10.1016/j.neuron.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Gruetter R. In vivo 13C NMR assessment of brain glycogen concentration and turnover in the awake rat. Neurochem Int. 2003;43(4-5):317–322. doi: 10.1016/s0197-0186(03)00018-4. [DOI] [PubMed] [Google Scholar]
- Choi IY, Lee SP, Kim SG, Gruetter R. In vivo measurements of brain glucose transport using the reversible Michaelis-Menten model and simultaneous measurements of cerebral blood flow changes during hypoglycemia. Journal of cerebral blood flow and metabolism. 2001;21(6):653–663. doi: 10.1097/00004647-200106000-00003. doi:10.1097/00004647-200106000-00003. [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R. Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res. 2003;72(1):25–32. doi: 10.1002/jnr.10574. doi:10.1002/jnr.10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Tkac I, Ugurbil K, Gruetter R. Noninvasive measurements of [1-(13)C]glycogen concentrations and metabolism in rat brain in vivo. J Neurochem. 1999;73(3):1300–1308. doi: 10.1046/j.1471-4159.1999.0731300.x. [DOI] [PubMed] [Google Scholar]
- Cooper AJ. The Role of Glutamine Synthetase and Glutamate Dehydrogenase in Cerebral Ammonia Homeostasis. Neurochem Res. 2012;37(11):2439–2455. doi: 10.1007/s11064-012-0803-4. doi:10.1007/s11064-012-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremer JE, Seville MP. Regional brain blood flow, blood volume, and haematocrit values in the adult rat. Journal of cerebral blood flow and metabolism. 1983;3(2):254–256. doi: 10.1038/jcbfm.1983.35. doi:10.1038/jcbfm.1983.35. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Adachi K, Dienel GA. Rapid efflux of lactate from cerebral cortex during K+ - induced spreading cortical depression. Journal of cerebral blood flow and metabolism. 1999;19(4):380–392. doi: 10.1097/00004647-199904000-00004. doi:10.1097/00004647-199904000-00004. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Ball KK, Froehner SC, Adams ME, Dienel GA. Regional registration of [6-14C]glucose metabolism during brain activation of α-syntrophin knockout mice. J Neurochem. 2013;125(2):247–259. doi: 10.1111/jnc.12166. doi:10.1111/jnc.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz NF, Dienel GA. High glycogen levels in brains of rats with minimal environmental stimuli: implications for metabolic contributions of working astrocytes. Journal of cerebral blood flow and metabolism. 2002;22(12):1476–1489. doi: 10.1097/01.WCB.0000034362.37277.C0. doi:10.1097/00004647-200212000-00008. [DOI] [PubMed] [Google Scholar]
- Cruz NF, Lasater A, Zielke HR, Dienel GA. Activation of astrocytes in brain of conscious rats during acoustic stimulation: acetate utilization in working brain. J Neurochem. 2005;92(4):934–947. doi: 10.1111/j.1471-4159.2004.02935.x. doi:10.1111/j.1471-4159.2004.02935.x. [DOI] [PubMed] [Google Scholar]
- Dalsgaard MK, Madsen FF, Secher NH, Laursen H, Quistorff B. High glycogen levels in the hippocampus of patients with epilepsy. J Cereb Blood Flow Metab. 2007;27(6):1137–1141. doi: 10.1038/sj.jcbfm.9600426. doi:10.1038/sj.jcbfm.9600426. [DOI] [PubMed] [Google Scholar]
- de Graaf RA, Rothman DL, Behar KL. State of the art direct 13C and indirect 1H-[13C] NMR spectroscopy in vivo. A practical guide. NMR in biomedicine. 2011;24(8):958–972. doi: 10.1002/nbm.1761. doi:10.1002/nbm.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Lactate muscles its way into consciousness: fueling brain activation. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R519–521. doi: 10.1152/ajpregu.00377.2004. doi:10.1152/ajpregu.00377.2004. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012a;32(7):1107–1138. doi: 10.1038/jcbfm.2011.175. doi:10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Fueling and imaging brain activation. ASN Neuro. 2012b;4(5) doi: 10.1042/AN20120021. 4(5):art:e00093.doi:00010.01042/AN20120021 doi:10.1042/an20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA. Astrocytic energetics during excitatory neurotransmission: What are contributions of glutamate oxidation and glycolysis? Neurochem Int. 2013;63(4):244–258. doi: 10.1016/j.neuint.2013.06.015. doi: http://dx.doi.org/10.1016/j.neuint.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Ball KK, Cruz NF. A glycogen phosphorylase inhibitor selectively enhances local rates of glucose utilization in brain during sensory stimulation of conscious rats: implications for glycogen turnover. J Neurochem. 2007a;102(2):466–478. doi: 10.1111/j.1471-4159.2007.04595.x. doi:10.1111/j.1471-4159.2007.04595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Synthesis of deoxyglucose-1-phosphate, deoxyglucose-1,6-bisphosphate, and other metabolites of 2-deoxy-D-[14C]glucose in rat brain in vivo: influence of time and tissue glucose level. J Neurochem. 1993;60(6):2217–2231. doi: 10.1111/j.1471-4159.1993.tb03508.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Nutrition during brain activation: does cell-to-cell lactate shuttling contribute significantly to sweet and sour food for thought? Neurochem Int. 2004;45(2-3):321–351. doi: 10.1016/j.neuint.2003.10.011. doi:10.1016/j.neuint.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Imaging brain activation: simple pictures of complex biology. Ann N Y Acad Sci. 2008;1147:139–170. doi: 10.1196/annals.1427.011. doi:10.1196/annals.1427.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF. Exchange-mediated dilution of brain lactate specific activity: implications for the origin of glutamate dilution and the contributions of glutamine dilution and other pathways. J Neurochem. 2009;109(Suppl 1):30–37. doi: 10.1111/j.1471-4159.2009.05859.x. doi:10.1111/j.1471-4159.2009.05859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Ball K, Popp D, Gokden M, Baron S, Wright D, Wenger GR. Behavioral training increases local astrocytic metabolic activity but does not alter outcome of mild transient ischemia. Brain Res. 2003;961(2):201–212. doi: 10.1016/s0006-8993(02)03945-8. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Mori K, Holden JE, Sokoloff L. Direct measurement of the lambda of the lumped constant of the deoxyglucose method in rat brain: determination of lambda and lumped constant from tissue glucose concentration or equilibrium brain/plasma distribution ratio for methylglucose. Journal of cerebral blood flow and metabolism. 1991;11(1):25–34. doi: 10.1038/jcbfm.1991.3. doi:10.1038/jcbfm.1991.3. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Mori K, Sokoloff L. Acid lability of metabolites of 2-deoxyglucose in rat brain: implications for estimates of kinetic parameters of deoxyglucose phosphorylation and transport between blood and brain. J Neurochem. 1990;54(4):1440–1448. doi: 10.1111/j.1471-4159.1990.tb01981.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Cruz NF, Sokoloff L. Metabolites of 2-deoxy-[14C]glucose in plasma and brain: influence on rate of glucose utilization determined with deoxyglucose method in rat brain. Journal of cerebral blood flow and metabolism. 1993;13(2):315–327. doi: 10.1038/jcbfm.1993.40. doi:10.1038/jcbfm.1993.40. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Cruz NF. Local uptake of (14)C-labeled acetate and butyrate in rat brain in vivo during spreading cortical depression. J Neurosci Res. 2001a;66(5):812–820. doi: 10.1002/jnr.10063. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Liu K, Popp D, Cruz NF. Enhanced acetate and glucose utilization during graded photic stimulation. Neuronal-glial interactions in vivo. Ann N Y Acad Sci. 1999;893:279–281. doi: 10.1111/j.1749-6632.1999.tb07836.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Nelson T, Cruz NF, Jay T, Crane AM, Sokoloff L. Over-estimation of glucose-6-phosphatase activity in brain in vivo. Apparent difference in rates of [2-3H]glucose and [U-14C]glucose utilization is due to contamination of precursor pool with 14C-labeled products and incomplete recovery of 14C-labeled metabolites. J Biol Chem. 1988;263(36):19697–19708. [PubMed] [Google Scholar]
- Dienel GA, Popp D, Drew PD, Ball K, Krisht A, Cruz NF. Preferential labeling of glial and meningial brain tumors with [2-(14)C]acetate. J Nucl Med. 2001b;42(8):1243–1250. [PubMed] [Google Scholar]
- Dienel GA, Schmidt KC, Cruz NF. Astrocyte activation in vivo during graded photic stimulation. J Neurochem. 2007b;103(4):1506–1522. doi: 10.1111/j.1471-4159.2007.04859.x. doi:10.1111/j.1471-4159.2007.04859.x. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Wang RY, Cruz NF. Generalized sensory stimulation of conscious rats increases labeling of oxidative pathways of glucose metabolism when the brain glucose-oxygen uptake ratio rises. Journal of cerebral blood flow and metabolism. 2002;22(12):1490–1502. doi: 10.1097/01.WCB.0000034363.37277.89. doi:10.1097/00004647-200212000-00009. [DOI] [PubMed] [Google Scholar]
- DiNuzzo M, Mangia S, Maraviglia B, Giove F. Glycogenolysis in astrocytes supports blood-borne glucose channeling not glycogen-derived lactate shuttling to neurons: evidence from mathematical modeling. Journal of cerebral blood flow and metabolism. 2010;30(12):1895–1904. doi: 10.1038/jcbfm.2010.151. doi:10.1038/jcbfm.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinuzzo M, Mangia S, Maraviglia B, Giove F. The Role of Astrocytic Glycogen in Supporting the Energetics of Neuronal Activity. Neurochem Res. 2012 doi: 10.1007/s11064-012-0802-5. doi:10.1007/s11064-012-0802-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Maraviglia B, Giove F. Why does the brain (not) have glycogen? Bioessays. 2011;33(5):319–326. doi: 10.1002/bies.201000151. doi:10.1002/bies.201000151. [DOI] [PubMed] [Google Scholar]
- Dringen R, Gebhardt R, Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993a;623(2):208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- Dringen R, Schmoll D, Cesar M, Hamprecht B. Incorporation of radioactivity from [14C]lactate into the glycogen of cultured mouse astroglial cells. Evidence for gluconeogenesis in brain cells. Biol Chem Hoppe Seyler. 1993b;374(5):343–347. doi: 10.1515/bchm3.1993.374.1-6.343. [DOI] [PubMed] [Google Scholar]
- Duran J, Saez I, Gruart A, Guinovart JJ, Delgado-Garcia JM. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J Cereb Blood Flow Metab. 2013;33(4):550–556. doi: 10.1038/jcbfm.2012.200. doi:10.1038/jcbfm.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran J, Tevy MF, Garcia-Rocha M, Calbo J, Milan M, Guinovart JJ. Deleterious effects of neuronal accumulation of glycogen in flies and mice. EMBO molecular medicine. 2012;4(8):719–729. doi: 10.1002/emmm.201200241. doi:10.1002/emmm.201200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folbergrova J, Ingvar M, Siesjo BK. Metabolic changes in cerebral cortex, hippocampus, and cerebellum during sustained bicuculline-induced seizures. J Neurochem. 1981;37(5):1228–1238. doi: 10.1111/j.1471-4159.1981.tb04673.x. [DOI] [PubMed] [Google Scholar]
- Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241(4864):462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Dienel GA. Astrocytes are poised for lactate trafficking and release from activated brain and for supply of glucose to neurons. J Neurochem. 2009a;111(2):522–536. doi: 10.1111/j.1471-4159.2009.06333.x. doi:10.1111/j.1471-4159.2009.06333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi GK, Cruz NF, Ball KK, Theus SA, Dienel GA. Selective astrocytic gap junctional trafficking of molecules involved in the glycolytic pathway: impact on cellular brain imaging. J Neurochem. 2009b;110(3):857–869. doi: 10.1111/j.1471-4159.2009.06173.x. doi:10.1111/j.1471-4159.2009.06173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield PD, Lowry OH, Schulz DW, Passonneau JV. Regional energy reserves in mouse brain and changes with ischaemia and anaesthesia. J Neurochem. 1966;13(3):185–195. doi: 10.1111/j.1471-4159.1966.tb07512.x. [DOI] [PubMed] [Google Scholar]
- Ghajar JB, Plum F, Duffy TE. Cerebral oxidative metabolism and blood flow during acute hypoglycemia and recovery in unanesthetized rats. J Neurochem. 1982;38(2):397–409. doi: 10.1111/j.1471-4159.1982.tb08643.x. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Anderson DG, Hertz L. Inhibition of glycogenolysis in astrocytes interrupts memory consolidation in young chickens. Glia. 2006;54(3):214–222. doi: 10.1002/glia.20377. doi:10.1002/glia.20377. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Hutchinson DS. Rapid Turnover of Glycogen in Memory Formation. Neurochem Res. 2012 doi: 10.1007/s11064-012-0805-2. doi:10.1007/s11064-012-0805-2. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Lloyd HG, Santa T, Hertz L. Glycogen is a preferred glutamate precursor during learning in 1-day-old chick: biochemical and behavioral evidence. J Neurosci Res. 2007;85(15):3326–3333. doi: 10.1002/jnr.21307. doi:10.1002/jnr.21307. [DOI] [PubMed] [Google Scholar]
- Gotoh J, Itoh Y, Kuang TY, Cook M, Law MJ, Sokoloff L. Negligible glucose-6-phosphatase activity in cultured astroglia. J Neurochem. 2000;74(4):1400–1408. doi: 10.1046/j.1471-4159.2000.0741400.x. [DOI] [PubMed] [Google Scholar]
- Gross RA, Ferrendelli JA. Mechanisms of cyclic AMP regulation in cerebral anoxia and their relationship to glycogenolysis. J Neurochem. 1980;34(5):1309–1318. doi: 10.1111/j.1471-4159.1980.tb09976.x. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Glycogen: the forgotten cerebral energy store. J Neurosci Res. 2003;74(2):179–183. doi: 10.1002/jnr.10785. doi:10.1002/jnr.10785. [DOI] [PubMed] [Google Scholar]
- Grunwald F, Schrock H, Theilen H, Biber A, Kuschinsky W. Local cerebral glucose utilization of the awake rat during chronic administration of nicotine. Brain Res. 1988;456(2):350–356. doi: 10.1016/0006-8993(88)90238-7. [DOI] [PubMed] [Google Scholar]
- Hargreaves RJ, Planas AM, Cremer JE, Cunningham VJ. Studies on the relationship between cerebral glucose transport and phosphorylation using 2-deoxyglucose. J Cereb Blood Flow Metab. 1986;6(6):708–716. doi: 10.1038/jcbfm.1986.127. doi:10.1038/jcbfm.1986.127. [DOI] [PubMed] [Google Scholar]
- Harik SI, Busto R, Martinez E. Norepinephrine regulation of cerebral glycogen utilization during seizures and ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1982;2(4):409–414. doi: 10.1523/JNEUROSCI.02-04-00409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RA, Miller AL. Loss of radioactive 2-deoxy-D-glucose-6-phosphate from brains of conscious rats: implications for quantitative autoradiographic determination of regional glucose utilization. Neuroscience. 1978;3(2):251–258. doi: 10.1016/0306-4522(78)90106-9. [DOI] [PubMed] [Google Scholar]
- Hawkins RA, Miller AL, Nielsen RC, Veech RL. The acute action of ammonia on rat brain metabolism in vivo. Biochem J. 1973;134(4):1001–1008. doi: 10.1042/bj1341001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Gibbs ME. What learning in day-old chickens can teach a neurochemist: focus on astrocyte metabolism. J Neurochem. 2009;109(Suppl 1):10–16. doi: 10.1111/j.1471-4159.2009.05939.x. doi:10.1111/j.1471-4159.2009.05939.x. [DOI] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27(2):219–249. doi: 10.1038/sj.jcbfm.9600343. doi:10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology. 2008;149(4):1499–1504. doi: 10.1210/en.2007-1252. doi:10.1210/en.2007-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Pascale E, Magistretti PJ. K+ at concentrations reached in the extracellular space during neuronal activity promotes a Ca2+-dependent glycogen hydrolysis in mouse cerebral cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8(6):1922–1928. doi: 10.1523/JNEUROSCI.08-06-01922.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JE, Mori K, Dienel GA, Cruz NF, Nelson T, Sokoloff L. Modeling the dependence of hexose distribution volumes in brain on plasma glucose concentration: implications for estimation of the local 2-deoxyglucose lumped constant. J Cereb Blood Flow Metab. 1991;11(2):171–182. doi: 10.1038/jcbfm.1991.50. doi:10.1038/jcbfm.1991.50. [DOI] [PubMed] [Google Scholar]
- Horinaka N, Artz N, Jehle J, Takahashi S, Kennedy C, Sokoloff L. Examination of potential mechanisms in the enhancement of cerebral blood flow by hypoglycemia and pharmacological doses of deoxyglucose. J Cereb Blood Flow Metab. 1997;17(1):54–63. doi: 10.1097/00004647-199701000-00008. doi:10.1097/00004647-199701000-00008. [DOI] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, Nagelhus EA, Nedergaard M. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid beta. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. doi:10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(46):18190–18199. doi: 10.1523/JNEUROSCI.1592-13.2013. doi:10.1523/jneurosci.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky ML, Reich P, Anchors JM, Burrows BL. Changes in brain glycogen during slow-wave sleep in the rat. J Neurochem. 1983;41(5):1498–1501. doi: 10.1111/j.1471-4159.1983.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Koh L, Zakharov A, Johnston M. Integration of the subarachnoid space and lymphatics: is it time to embrace a new concept of cerebrospinal fluid absorption? Cerebrospinal Fluid Res. 2005;2:6. doi: 10.1186/1743-8454-2-6. doi:10.1186/1743-8454-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI, Geiger JD. Brain Glycogen Decreases with Increased Periods of Wakefulness: Implications for Homeostatic Drive to Sleep. The Journal of Neuroscience. 2002;22(13):5581–5587. doi: 10.1523/JNEUROSCI.22-13-05581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. The Relationships between Substrates and Enzymes of Glycolysis in Brain. J Biol Chem. 1964;239:31–42. [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV, Hasselberger FX, Schulz DW. Effect of ischemia on known substrates and cofactors of the glycolytic pathway in brain. J Biol Chem. 1964;239:18–30. [PubMed] [Google Scholar]
- Madsen PL, Cruz NF, Sokoloff L, Dienel GA. Cerebral oxygen/glucose ratio is low during sensory stimulation and rises above normal during recovery: excess glucose consumption during stimulation is not accounted for by lactate efflux from or accumulation in brain tissue. J Cereb Blood Flow Metab. 1999;19(4):393–400. doi: 10.1097/00004647-199904000-00005. doi:10.1097/00004647-199904000-00005. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, Wildschiodtz G, Paulson OB, Lassen NA. Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab. 1995;15(3):485–491. doi: 10.1038/jcbfm.1995.60. doi:10.1038/jcbfm.1995.60. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Morrison JH, Shoemaker WJ, Sapin V, Bloom FE. Vasoactive intestinal polypeptide induces glycogenolysis in mouse cortical slices: a possible regulatory mechanism for the local control of energy metabolism. Proc Natl Acad Sci U S A. 1981;78(10):6535–6539. doi: 10.1073/pnas.78.10.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masamoto K, Kanno I. Anesthesia and the quantitative evaluation of neurovascular coupling. J Cereb Blood Flow Metab. 2012;32(7):1233–1247. doi: 10.1038/jcbfm.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler FD, van Heeswijk RB, Xin L, Laus S, Frenkel H, Lei H, Gruetter R. Non invasive quantification of brain glycogen absolute concentration. J Neurochem. 2008;107(5):1414–1423. doi: 10.1111/j.1471-4159.2008.05717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Cruz N, Dienel G, Nelson T, Sokoloff L. Direct chemical measurement of the lambda of the lumped constant of the [14C]deoxyglucose method in rat brain: effects of arterial plasma glucose level on the distribution spaces of [14C]deoxyglucose and glucose and on lambda. J Cereb Blood Flow Metab. 1989;9(3):304–314. doi: 10.1038/jcbfm.1989.48. doi:10.1038/jcbfm.1989.48. [DOI] [PubMed] [Google Scholar]
- Nagra G, Koh L, Zakharov A, Armstrong D, Johnston M. Quantification of cerebrospinal fluid transport across the cribriform plate into lymphatics in rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(5):R1383–1389. doi: 10.1152/ajpregu.00235.2006. doi:10.1152/ajpregu.00235.2006. [DOI] [PubMed] [Google Scholar]
- Nelson SR, Schulz DW, Passonneau JV, Lowry OH. Control of glycogen levels in brain. J Neurochem. 1968;15(11):1271–1279. doi: 10.1111/j.1471-4159.1968.tb05904.x. [DOI] [PubMed] [Google Scholar]
- Nelson T, Lucignani G, Atlas S, Crane AM, Dienel GA, Sokoloff L. Reexamination of glucose-6-phosphatase activity in the brain in vivo: no evidence for a futile cycle. Science. 1985;229(4708):60–62. doi: 10.1126/science.2990038. [DOI] [PubMed] [Google Scholar]
- Newman GC, Hospod FE, Maghsoudlou B, Patlak CS. Simplified brain slice glucose utilization. J Cereb Blood Flow Metab. 1996;16(5):864–880. doi: 10.1097/00004647-199609000-00011. doi:10.1097/00004647-199609000-00011. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PloS one. 2011;6(12):e28427. doi: 10.1371/journal.pone.0028427. doi:10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel LF, Muller MS, Walls AB, Sickmann HM, Bak LK, Waagepetersen HS, Schousboe A. Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Frontiers in neuroenergetics. 2012;4:3. doi: 10.3389/fnene.2012.00003. doi:10.3389/fnene.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF. Brain Metabolism during Fasting*. The Journal of clinical investigation. 1967;46(10):1589–1595. doi: 10.1172/JCI105650. doi:10.1172/jci105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öz G, Henry PG, Seaquist ER, Gruetter R. Direct, noninvasive measurement of brain glycogen metabolism in humans. Neurochem Int. 2003;43(4-5):323–329. doi: 10.1016/s0197-0186(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Passonneau JV, Lowry OH. A Practical Guide. Humana Press; Totowa, N. J.: 1993. Enzymatic analysis. [Google Scholar]
- Rahman B, Kussmaul L, Hamprecht B, Dringen R. Glycogen is mobilized during the disposal of peroxides by cultured astroglial cells from rat brain. Neurosci Lett. 2000;290(3):169–172. doi: 10.1016/s0304-3940(00)01369-0. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Blaumanis OR, Grady PA. Rapid solute transport throughout the brain via paravascular fluid pathways. Advances in neurology. 1990;52:431–439. [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326(1):47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR in biomedicine. 2011;24(8):943–957. doi: 10.1002/nbm.1772. doi:10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Swanson RA. The regional distribution of glycogen in rat brain fixed by microwave irradiation. Brain Res. 1987;417(1):172–174. doi: 10.1016/0006-8993(87)90195-8. [DOI] [PubMed] [Google Scholar]
- Schmidt K, Lucignani G, Mori K, Jay T, Palombo E, Nelson T, Pettigrew K, Holden JE, Sokoloff L. Refinement of the kinetic model of the 2-[14C]deoxyglucose method to incorporate effects of intracellular compartmentation in brain. J Cereb Blood Flow Metab. 1989;9(3):290–303. doi: 10.1038/jcbfm.1989.47. doi:10.1038/jcbfm.1989.47. [DOI] [PubMed] [Google Scholar]
- Schmoll D, Fuhrmann E, Gebhardt R, Hamprecht B. Significant amounts of glycogen are synthesized from 3-carbon compounds in astroglial primary cultures from mice with participation of the mitochondrial phosphoenolpyruvate carboxykinase isoenzyme. European journal of biochemistry / FEBS. 1995;227(1-2):308–315. doi: 10.1111/j.1432-1033.1995.tb20390.x. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sickmann HM, Walls AB, Bak LK, Waagepetersen HS. Functional importance of the astrocytic glycogen-shunt and glycolysis for maintenance of an intact intra/extracellular glutamate gradient. Neurotox Res. 2010;18(1):94–99. doi: 10.1007/s12640-010-9171-5. doi:10.1007/s12640-010-9171-5. [DOI] [PubMed] [Google Scholar]
- Shetty PK, Sadgrove MP, Galeffi F, Turner DA. Pyruvate incubation enhances glycogen stores and sustains neuronal function during subsequent glucose deprivation. Neurobiol Dis. 2012;45(1):177–187. doi: 10.1016/j.nbd.2011.08.002. doi:10.1016/j.nbd.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Cerebral energetics and the glycogen shunt: neurochemical basis of functional imaging. Proc Natl Acad Sci U S A. 2001;98(11):6417–6422. doi: 10.1073/pnas.101129298. doi:10.1073/pnas.101129298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann HM, Schousboe A, Fosgerau K, Waagepetersen HS. Compartmentation of lactate originating from glycogen and glucose in cultured astrocytes. Neurochem Res. 2005;30(10):1295–1304. doi: 10.1007/s11064-005-8801-4. doi:10.1007/s11064-005-8801-4. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Obesity and type 2 diabetes in rats are associated with altered brain glycogen and amino-acid homeostasis. J Cereb Blood Flow Metab. 2010;30(8):1527–1537. doi: 10.1038/jcbfm.2010.61. doi:10.1038/jcbfm.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sickmann HM, Waagepetersen HS, Schousboe A, Benie AJ, Bouman SD. Brain glycogen and its role in supporting glutamate and GABA homeostasis in a type 2 diabetes rat model. Neurochem Int. 2012;60(3):267–275. doi: 10.1016/j.neuint.2011.12.019. doi:10.1016/j.neuint.2011.12.019. [DOI] [PubMed] [Google Scholar]
- Sickmann HM, Walls AB, Schousboe A, Bouman SD, Waagepetersen HS. Functional significance of brain glycogen in sustaining glutamatergic neurotransmission. J Neurochem. 2009;109(Suppl 1):80–86. doi: 10.1111/j.1471-4159.2009.05915.x. doi:10.1111/j.1471-4159.2009.05915.x. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. John Wiley & Sons; Chichester: 1978. [Google Scholar]
- Sokoloff L. In vivo veritas: probing brain function through the use of quantitative in vivo biochemical techniques. Annual review of physiology. 2000;62:1–24. doi: 10.1146/annurev.physiol.62.1.1. doi:10.1146/annurev.physiol.62.1.1. [DOI] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Palay SL. The fine structure of the lateral vestibular nucleus in the rat. I. Neurons and neuroglial cells. The Journal of cell biology. 1968;36(1):151–179. [PubMed] [Google Scholar]
- Stewart MA, Passonneau JV, Lowry OH. Substrate changes in peripheral nerve during ischaemia and Wallerian degeneration. J Neurochem. 1965;12(8):719–727. doi: 10.1111/j.1471-4159.1965.tb06786.x. [DOI] [PubMed] [Google Scholar]
- Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K, Swanson RA. Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmethyl)pro pyl]-1H-indole-2-carboxamide) The Journal of pharmacology and experimental therapeutics. 2007;321(1):45–50. doi: 10.1124/jpet.106.115550. doi:10.1124/jpet.106.115550. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144(5):810–823. doi: 10.1016/j.cell.2011.02.018. doi:10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA. Physiologic coupling of glial glycogen metabolism to neuronal activity in brain. Can J Physiol Pharmacol. 1992;70(Suppl):S138–144. doi: 10.1139/y92-255. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MM, Sagar SM, Sharp FR. Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience. 1992;51(2):451–461. doi: 10.1016/0306-4522(92)90329-z. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Giaume C, Medina JM. Endothelin-1 regulates glucose utilization in cultured astrocytes by controlling intercellular communication through gap junctions. Glia. 1996;16(3):187–195. doi: 10.1002/(SICI)1098-1136(199603)16:3<187::AID-GLIA1>3.0.CO;2-#. doi:10.1002/(SICI)1098-1136(199603)16:3<187::AID-GLIA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Tabernero A, Medina JM, Giaume C. Glucose metabolism and proliferation in glia: role of astrocytic gap junctions. J Neurochem. 2006;99(4):1049–1061. doi: 10.1111/j.1471-4159.2006.04088.x. doi:10.1111/j.1471-4159.2006.04088.x. [DOI] [PubMed] [Google Scholar]
- Tesfaye N, Seaquist ER, Öz G. Noninvasive measurement of brain glycogen by nuclear magnetic resonance spectroscopy and its application to the study of brain metabolism. J Neurosci Res. 2011;89(12):1905–1912. doi: 10.1002/jnr.22703. doi:10.1002/jnr.22703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valles-Ortega J, Duran J, Garcia-Rocha M, Bosch C, Saez I, Pujadas L, Serafin A, Canas X, Soriano E, Delgado-Garcia JM, Gruart A, Guinovart JJ. Neurodegeneration and functional impairments associated with glycogen synthase accumulation in a mouse model of Lafora disease. EMBO molecular medicine. 2011;3(11):667–681. doi: 10.1002/emmm.201100174. doi:10.1002/emmm.201100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hall G, Stromstad M, Rasmussen P, Jans O, Zaar M, Gam C, Quistorff B, Secher NH, Nielsen HB. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29(6):1121–1129. doi: 10.1038/jcbfm.2009.35. doi:10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- van Heeswijk RB, Morgenthaler FD, Xin L, Gruetter R. Quantification of brain glycogen concentration and turnover through localized 13C NMR of both the C1 and C6 resonances. NMR in biomedicine. 2010;23(3):270–276. doi: 10.1002/nbm.1460. doi:10.1002/nbm.1460. [DOI] [PubMed] [Google Scholar]
- van Heeswijk RB, Pilloud Y, Morgenthaler FD, Gruetter R. A comparison of in vivo 13C MR brain glycogen quantification at 9.4 and 14.1 T. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2012;67(6):1523–1527. doi: 10.1002/mrm.23192. doi:10.1002/mrm.23192. [DOI] [PubMed] [Google Scholar]
- Vilchez D, Ros S, Cifuentes D, Pujadas L, Valles J, Garcia-Fojeda B, Criado-Garcia O, Fernandez-Sanchez E, Medrano-Fernandez I, Dominguez J, Garcia-Rocha M, Soriano E, Rodriguez de Cordoba S, Guinovart JJ. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nature neuroscience. 2007;10(11):1407–1413. doi: 10.1038/nn1998. doi:10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Walls AB, Heimbürger CM, Bouman SD, Schousboe A, Waagepetersen HS. Robust glycogen shunt activity in astrocytes: Effects of glutamatergic and adrenergic agents. Neuroscience. 2009;158(1):284–292. doi: 10.1016/j.neuroscience.2008.09.058. doi: http://dx.doi.org/10.1016/j.neuroscience.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem Int. 2000;36(4–5):291–300. doi: 10.1016/s0197-0186(99)00137-0. doi: http://dx.doi.org/10.1016/S0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Passonneau JV. Factors affecting the turnover of cerebral glycogen and limit dextrin in vivo. J Neurochem. 1973;20(6):1543–1554. doi: 10.1111/j.1471-4159.1973.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Weller RO, Preston SD, Subash M, Carare RO. Cerebral amyloid angiopathy in the aetiology and immunotherapy of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):6. doi: 10.1186/alzrt6. doi:10.1186/alzrt6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206(Pt 12):2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- Xu J, Song D, Xue Z, Gu L, Hertz L, Peng L. Requirement of Glycogenolysis for Uptake of Increased Extracellular K+ in Astrocytes: Potential Implications for K+ Homeostasis and Glycogen Usage in Brain. Neurochem Res. 2012:1–14. doi: 10.1007/s11064-012-0938-3. doi:10.1007/s11064-012-0938-3. [DOI] [PubMed] [Google Scholar]