Abstract
T cell infiltration into the metastatic melanoma microenvironment (MME) correlates with improved patient survival. However, diffuse infiltration into tumor occurs in only 8% of melanoma metastases. Little is known about mechanisms governing T cell infiltration into human melanoma metastases or about how those mechanisms may be altered therapeutically. We hypothesized that T cells in the MME would be enriched for chemokine receptors CCR4, CCR5, CXCR3, and homing receptors relevant to the tissue site. Viably cryopreserved single cell suspensions from nineteen melanoma metastases representing three metastatic sites (tumor-infiltrated lymph node (TIN), skin, and small bowel) were evaluated by multiparameter flow cytometry and compared to benign lymph nodes (NLN) and peripheral blood mononuclear cells (PBMC) from patients with Stage IIB-IV melanoma. T cells in the melanoma metastases contained large effector memory populations, high proportions of activated, moderately differentiated cells and few regulatory T cells. Site specific homing was suggested in bowel, with high expression of CCR9. We did not encounter the anticipated enrichment of integrin α4β7 in bowel, cutaneous leukocyte antigen (CLA) in skin, nor integrin α4β1 or receptor CXCR3 in metastatic sites. Retention integrins αEβ7, α1β1 and α2β1 were significantly elevated in metastases. These data suggest limited tissue site-specific homing to human melanoma metastases, but a significant role for retention integrins in maintaining intratumoral T cells. Our findings also raise the possibility that T cell homing, infiltration, and retention in melanoma metastases may be increased by increasing expression of ligands for CLA, α4β1 and CXCR3 on intratumoral endothelium.
Keywords: Metastatic melanoma, integrin, chemokine receptor, cancer immunology, tumor microenvironment
INTRODUCTION
Immune cell infiltration into the metastatic melanoma microenvironment (MME) is associated with better patient survival.1–3 T cells are the predominant immune cells in the MME. Patients with high CD8+ T cell infiltration (not CD4) with the highest overall immune cell infiltration into tumor have the best clinical outcomes.2, 3 Unfortunately, diffuse intratumoral infiltration of effector T cells is observed in less than 10% of melanoma metastases.3 The mechanisms that enable or prevent T cell infiltration into the MME are unknown. Cell surface adhesion receptors on effector and memory T cells mediate their homing to various tissues.4 Roles of some of these receptors have been elucidated for T cells entering inflamed peripheral tissues,4–6 but translation of these findings to the cancer setting is in its infancy. Murine models of vaccination for melanoma have shown that integrin expression on T cells is strongly influenced by the lymphoid compartment in which activation occurs. In particular, T cells activated in mesenteric lymph nodes (LN) are high in α4β7, and those activated in skin-draining LN selectively express E-selectin ligand (murine equivalent of cutaneous leukocyte antigen, CLA); homing appears to be integrin-dependent.7, 8 In human tumors, chemokine immune signatures of the MME have correlated with CD8+ T cell infiltration.9 However, little is known about the mechanisms governing T cell infiltration into human melanoma metastases, or about how they may be altered therapeutically.
This study aims to examine the expression of homing receptors and retention integrins on T cells infiltrating human melanoma metastases. We have hypothesized that tumor-infiltrating T cells should differentially express homing receptors critical to their ability to infiltrate and/or to be retained in the MME, compared to circulating peripheral blood. Based on prior published findings in murine studies, these may include integrin α4β1, chemokine receptors CCR4, CCR5, and CXCR3.8, 10 We also have hypothesized that T cell homing may be governed in part by the tissue site harboring the metastasis. Accordingly, we might expect enrichment of T cells expressing CLA in skin metastases and CCR9 with α4β7 in bowel metastases.7, 8, 11 This study also explores whether retention integrins (α1β1, α2β1, αEβ7) are differentially expressed on tumor-infiltrating T cells compared to lymphocytes in peripheral blood and LN.
MATERIALS AND METHODS
Tissue Specimens
Nineteen specimens of human metastatic melanoma known to contain immune cell infiltrates were identified from previous work.3 Viably cryopreserved human melanoma metastases representing three metastatic sites: tumor-infiltrated lymph node (TIN, n=10), skin (n=3) and small bowel (GI, n=5) were obtained for comparison to cryopreserved cell suspensions from benign lymph nodes (NLN, n=5) obtained from melanoma patients undergoing node dissections, and cryopreserved peripheral blood mononuclear cells (PBMC) from patients with resected stage IIB through IV melanoma (n=15). The tumor samples were collected from patients with stage IIIB-IV melanoma, with most (13/19, 68%) rendered clinically free of disease at surgery. Of these, 3, 6, and 4 had stages IIIB, IIIC, and IV disease, respectively. The remaining 6 patients had stage IV disease that was resected but with residual sites of disease after surgery. PBMC were not routinely collected from patients whose tumors were collected. The PBMC for this study were collected from patients on the Mel48 clinical trial (NCT00705640),12 for which patients were eligible if they had stage IIB,IIC, III or IV melanoma. 80% of those patients (12/15) had stage IIIB, IIIC, or IV melanoma, and all had stage IIB-IV melanoma (4 each). There was substantial overlap in stage of disease and disease status between the two groups. NLN were obtained from patients whose TIN were also included in this study. Among 18 patients from whom tumors and/or lymph nodes were resected, 11 had not had prior systemic therapy, 1 had had prior high-dose IL-2, 4 had been on vaccine trials that included low-dose IL-2, and 2 had been on vaccine trials without IL-2. None were on active therapy at the time of surgery.
The tumor specimens were obtained from surgical specimens and were mechanically dissociated by mincing with a scalpel and forceps under a laminar flow hood; resulting cells were then cryopreserved in 10% dimethyl sulfoxide (DMSO) and 90% fetal calf serum (FCS; Life Technologies; Carlsbad, CA) in a controlled-rate freezer. Remaining tissue fragments after mechanical dissociation were then incubated in RPMI1640 media (Life Technologies) supplemented with 10% FCS, collagenase (195 units/mL ; Worthington Biochemical Corp.; Lakewood, NJ), DNAase (12.5 units/mL; Worthington Biochemical), and hyaluronidase (2.5 units/mL; Worthington Biochemical)at room temperature in a sterile 50 ml tube, with gentle mixing on a tube rotator wheel (Glas-Col; Terre Haute, IN) overnight. Resulting viable cells were cryopreserved in 10% DMSO and 90% FCS using a controlled-rate freezer. When available, non-digested specimens were studied (5/5 NLN, 7/10 TIN, 0/3 skin, 2/5 GI); specimens assessed after overnight enzymatic digestion included 0/5 NLN, 3/10 TIN, 3/3 skin, 3/5 GI).
Immunostaining and multi-parameter flow cytometry
Single-cell suspensions of cryopreserved tumor specimens from melanoma patients were thawed in warm RPMI-1640 medium (Life Technologies) containing 5% fetal bovine serum (FBS; Sigma-Aldrich: St. Louis, MO), and 100,000 units of deoxyribonuclease (Cat. # LS00219; Worthington Biochemical Corp.) per mL of media. After thawing, cells were washed free of serum and DMSO in PBS. Cells were labeled with the LIVE/DEAD® Fixable Aqua dead cell stain (Life Technologies) for viability according to manufacturer’s instructions. After washing, fluorescently-labeled antibody mixtures for integrins, homing receptors, chemokine receptors, and activation/maturation markers were added to the Aqua-labeled cell suspensions. Cells and antibodies were incubated for 20 minutes at 4°C before washing cells free of unbound antibodies. After two washes in FACS buffer (PBS + 2% FBS), cells were fixed in 2% paraformaldehyde (Sigma-Aldrich) in PBS for 10 minutes at room temperature. Cells were washed one time to remove excess paraformaldehyde, then held overnight at 4°C. Immunostained cells were acquired on a CyAn ADP LX 9 color flow cytometer (Beckman Coulter; Indianapolis, IN) equipped with 3 lasers to accommodate up to 11 parameters. Analysis of flow data was done using FlowJo version 8.8.6 (Tree Star; Ashland, OR). The list of reagents, including source, isotype and clone is available in Supplemental Data Table 1.
Labeling of ACT 1 antibody
The antibody to the α4β7 integrin, ACT-1, was provided by Eugene C. Butcher at a concentration of 1 mg/mL of purified antibody. The fluorochrome allophycocyanin (APC) was added to the antibody using the Lightning-Link™ APC-XL conjugation kit (Innova Biosciences; Cambridge, UK) according to the manufacturer’s instructions.
Statistical comparisons of flow cytometry results among different sites were performed with the Mann-Whitney U test for independent samples using IBM SPSS version 20. Reported p values are 2-tailed asymptotic estimates for the comparison between each site and PBMC. In some comparisons, a particular category consisted of only two samples, precluding analysis by this method. Results for these groups are graphically depicted without statistical analysis.
RESULTS
Composition
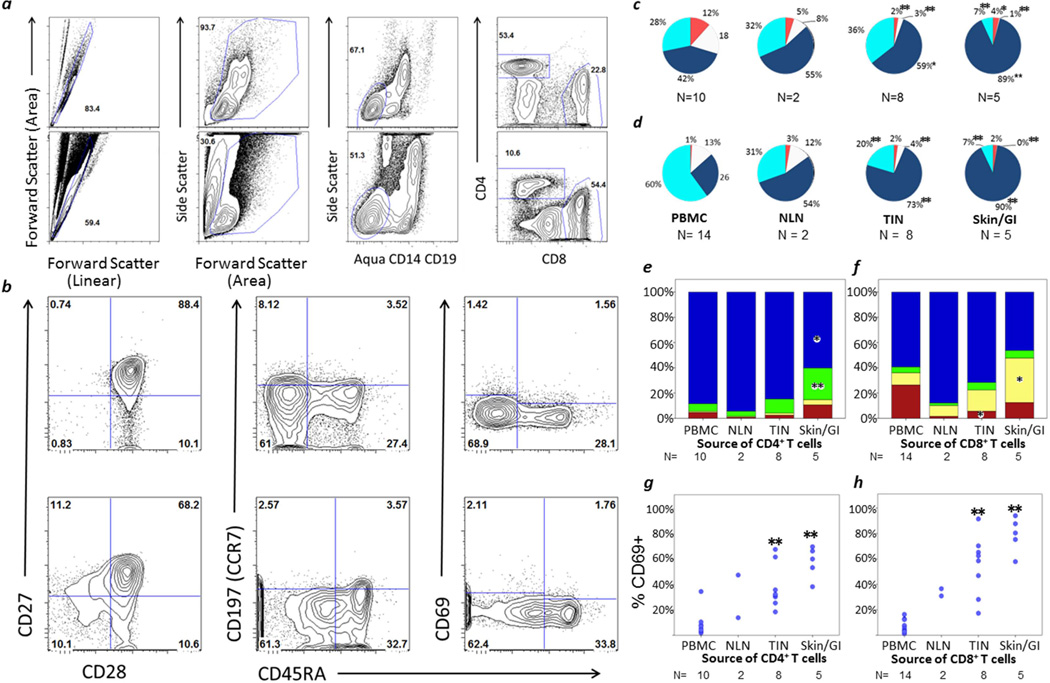
In melanoma metastases to the skin and bowel, there was a higher CD8+/CD4+ T cell ratio (mean 0.84) than in PBMC (mean 0.29, p=0.003). Regulatory T cells (Treg) were estimated as those that were CD4+ FoxP3+ CD127lo. Mean proportions of FoxP3+ CD127lo cells among CD4+ cells (TCD4) were higher in metastatic sites (9% in TIN, p=0.002; 20% in skin/GI metastases, p=0.027) than in PBMC (4%, data not shown). All metastatic sites together had greater than two-fold increase in Treg compared to PBMC and NLN combined (11% vs. 4%, p=0.001; Data not shown).
Maturation
T cell phenotypes were assessed based on CCR7 and CD45RA expression13 with gating strategies shown in Figure 1a,b. Maturation was substantially different for skin and GI metastases compared to PBMC (Figure 1c,d). Skin and GI metastases had three-fold higher proportions of effector memory (TEM, CCR7neg/CD45RAneg) CD8+ T cells (TCD8) and TCD4 compared to PBMC (90% vs. 26% of TCD8, p=0.002; 89% vs. 42% of TCD4, p<0.001). In contrast, naïve (CCR7+/CD45RA+), central memory (CCR7+/CD45RAneg) and RA+ effector memory (TEMRA+, CCR7neg/CD45RA+) T cells were substantially decreased in skin/GI metastases compared to PBMC. The proportion of naïve TCD4 in skin/GI metastases (mean 0.5%) was about one thirtieth of that in PBMC (mean 18%, p=0.002). For TCD8 cells, the difference was greater, with means of 0.3% and 13%, respectively (p=0.003).
FIGURE 1. Composition, Activation and Maturation of T cells in PBMC, NLN, TIN and Skin/GI Metastases.
Gating strategy: (a) Top row: PBMC; Bottom Row: GI metastatic melanoma. From left to right: Singlets; forward versus side scatter to identify mononuclear cells; dump gate excluding dead cells, CD14+ and CD19+ cells; CD4 and CD8 gates. (b) Gating of PBMC. Top row: CD4; Bottom Row: CD8. From left to right: CD28 vs. CD27; CD45RA vs. CCR7; CD45RA vs. CD69. Composition (mean values) of (c) TCD4 and (d) TCD8: Tnaive (CCR7+, CD45RA+) white; TEM (CCR7neg, CD45RAneg) navy; TEMRA (CCR7neg, CD45RA+) teal; TCM (CCR7+, CD45RAneg) orange. Maturation of (e) TCD4 and (f) TCD8: CD27+ CD28+ blue; CD27neg CD28+ green; CD27+ CD28neg yellow; CD27neg CD28neg red. Activation (CD69 expression) of TCD4 (g) TCD8 (h). PBMC = peripheral blood mononuclear cells. NLN = normal lymph node. TIN = tumor-infiltrated lymph node. Skin/GI = skin and gastrointestinal metastases. N = number of samples in each category. Comparisons are in reference to PBMC, with significant p values from Mann-Whitney U tests marked by asterisk: * p<0.05, ** p<0.005.
TCD4 and TCD8 infiltrating skin or GI metastases had pronounced loss of CD27 and CD28 expression, respectively (Figure 1e,f). Among TCD4, the proportion of CD27neg/CD28+ cells was five-fold higher in skin/GI metastatic sites than in PBMC (25% vs. 6%, p=0.002, Figure 1e). Among TCD8, the proportion of CD27+/CD28neg cells was more than three-fold higher in skin/GI metastases (35% vs. 10%, p=0.020, Figure 1f).
Activation
Metastatic sites had higher proportions of activated T cells than PBMC. CD69+ T cells were markedly enriched in the skin/GI metastatic microenvironment (79% vs. 7% for TCD8, p=0.001; 58% vs. 8% for TCD4, p=0.002, Figure 1g,h).
Homing receptors – Chemokine receptors
Chemokine receptors CCR4, CCR5, CCR9 and CXCR3 have been implicated in T cell homing to tumor. We found that CCR4 was enriched in metastases: among antigen-experienced (CD45RAneg) TCD4, 68% expressed CCR4 in bowel (compared to 26% in PBMC, p=0.034), and 77% expressed CCR4 in skin metastases (p=0.034 compared to PBMC, Figure 2a,c). Among TCD8, CCR4 was frequently expressed in PBMC, but there were no significant differences in CCR4 expression among different sites (Figure 2b,d).
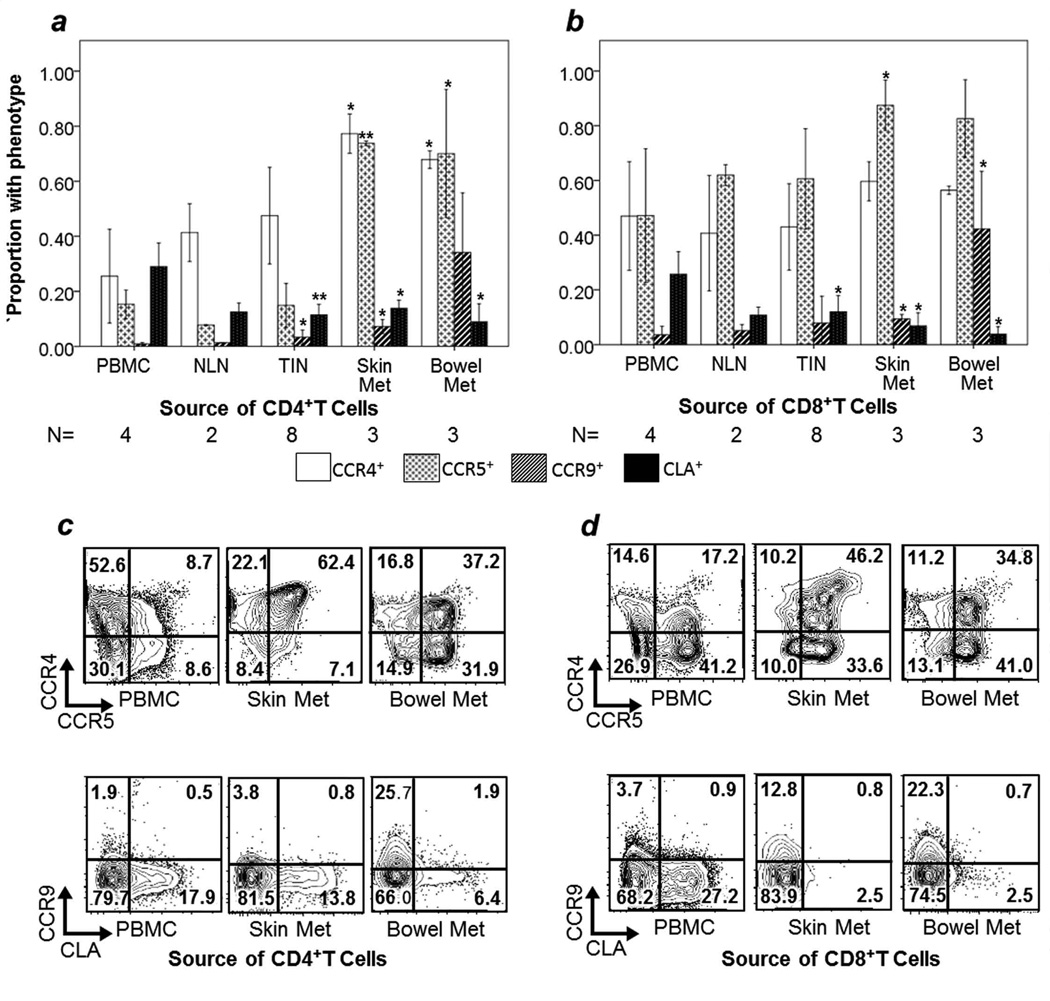
FIGURE 2. CCR4, CCR5, CCR9 and CLA expression by CD45RAneg T cells.
The mean proportion of CD45RAneg cells positive for each marker is reported in TCD4 (a) and in TCD8 (b), with error bars representing 1 standard deviation. Examples of individual sample multiparameter flow cytometry of CCR4/CCR5 and CCR9/CLA are shown for TCD4 (c) and TCD8 (d). Event counts (left to right): (c) Top- 33,584; 5,137; 8224. Bottom- 33,584; 5,137; 8,224. (d) Top- 14,743; 610; 13,608. Bottom- 14,743; 610; 13608. Comparisons are in reference to PBMC, with significant p values from Mann-Whitney U tests marked by asterisk: * p<0.05, ** p<0.005. N = number of samples evaluated for each data point. NLN = normal lymph node. TIN = tumor-infiltrated lymph node.
CD45RAneg T cells in GI and skin metastases were enriched for CCR5+ cells, compared to PBMC. In GI metastases vs. PBMC, means were 70% vs. 15% among TCD4 (p = 0.034), and 83% vs. 47% among TCD8 (p=0.077). In skin metastases, means were 74% (p=0.034) and 87% (p=0.034) for TCD4 and TCD8, respectively (Figure 2).
The highest levels of expression of CCR9 among antigen-experienced TCD8 were found in GI metastases, with low levels in other sites with statistical significance despite small sample size (mean for bowel metastases 42%, all other sites combined 6%, p=0.008). 34% of TCD4 in bowel metastases were CCR9+ compared to 4% in PBMC (p=0.008, Figure 2a,b).
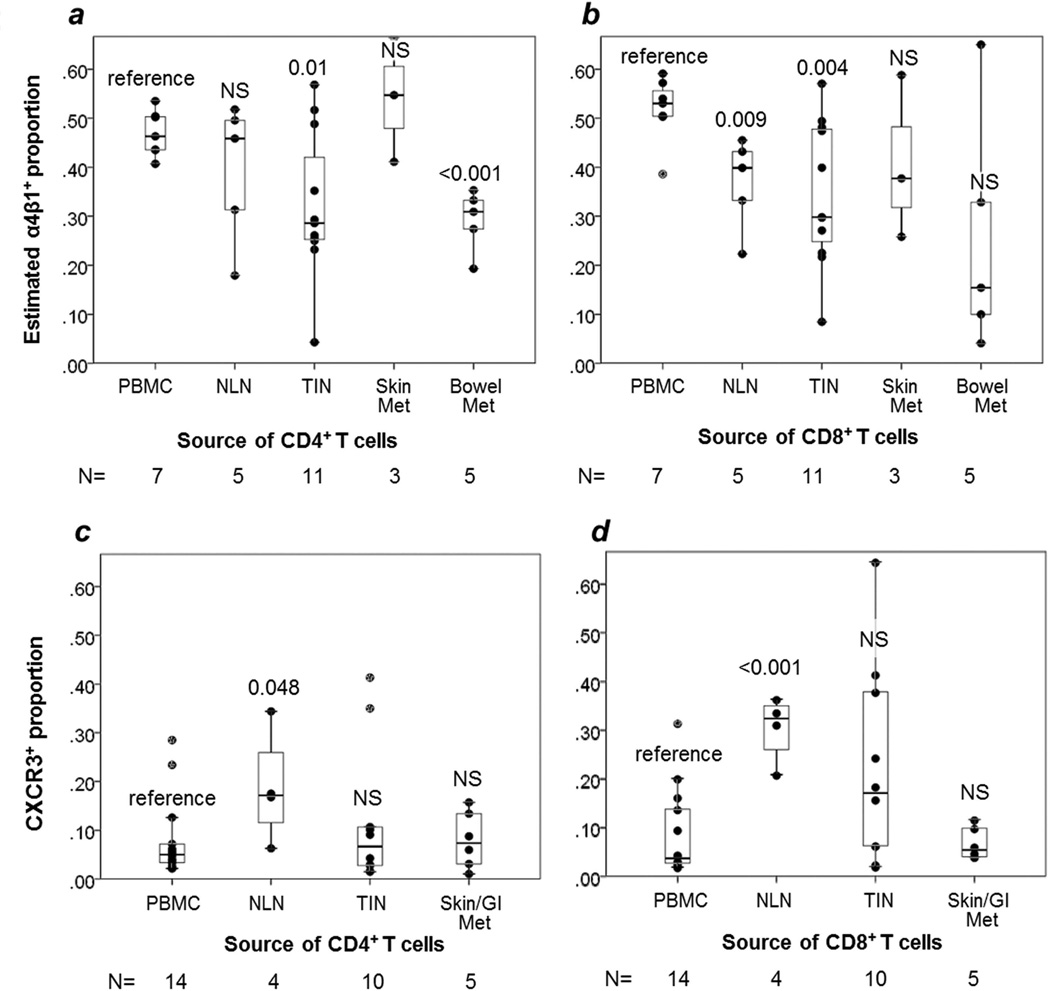
CXCR3 expression was low in metastases. Proportions of CXCR3+ TCD4 and TCD8 in skin and GI metastases were nearly equivalent to those in PBMC (7% vs. 8% for TCD8, p=0.53; 8% each for TCD4, p=0.80, Figure 3c,d).
FIGURE 3. T cell expression of homing integrin α4β1.
Box and whisker plots are shown for the estimated proportion of T cells expressing α4β1 integrin for TCD4 (a) and TCD8 (b), with individual data points represented by dots. Comparisons are in reference to PBMC, with p values from two-sided t-tests given for each site. NS= non-significant, p≥ 0.05. N = number of samples in each category. NLN = normal lymph node. TIN = tumor-infiltrated lymph node.
Homing receptors – E-selectin ligand (CLA)
Surprisingly, among antigen-experienced cells, CLA expression was lower among T cells in skin metastases than in PBMC for both TCD4 and TCD8 (mean 14% vs. 29% of TCD4, p=0.034; mean 7% vs. 26% of TCD8, p=0.034, Figure 2). There was no significant difference in CLA expression among skin, bowel, or LN metastases (One-way Kruskall Walllis Test, p=0.50 for TCD4, p=0.08 for TCD8).
Homing receptors - Integrins
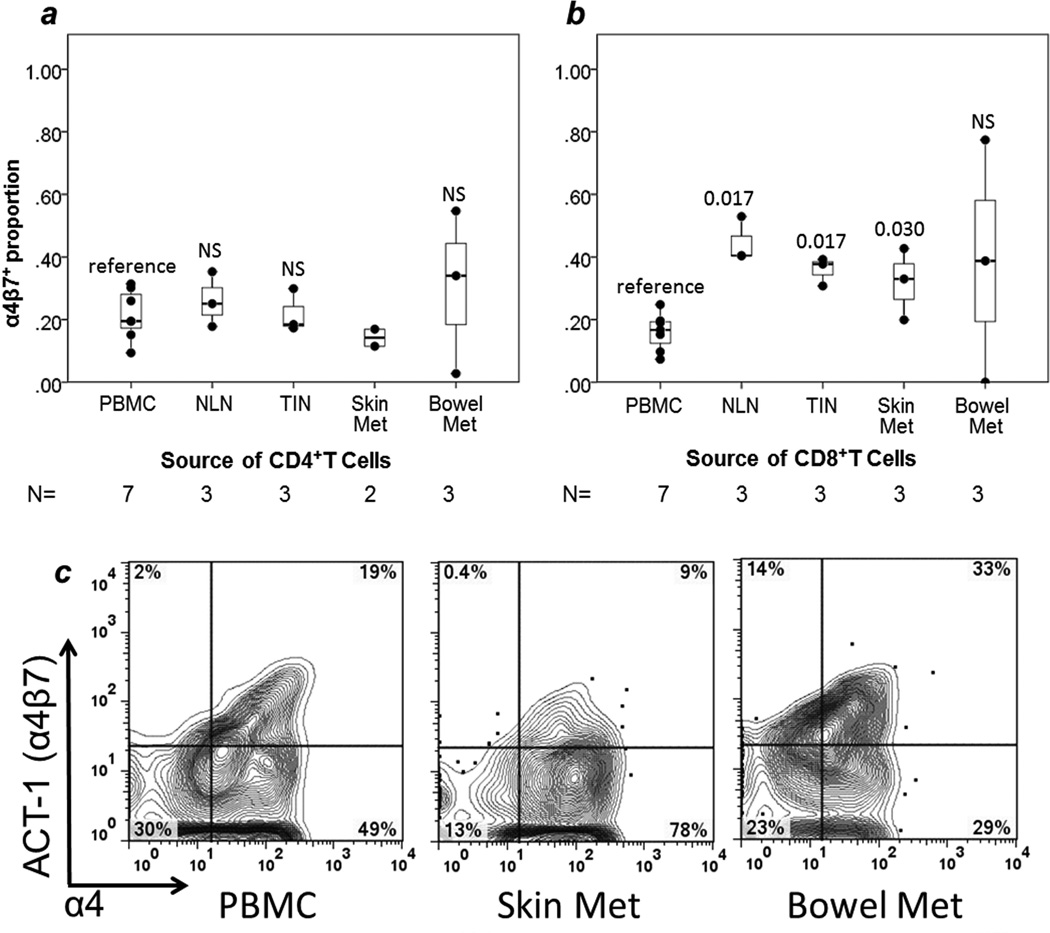
T cells were evaluated for expression of α4β7 using the heterodimer-specific antibody ACT-1.14 The frequency of these cells in bowel metastases was variable. Bowel metastases had no clear enrichment for α4β7-expressing cells in comparison to a PBMC alone or to all other sites combined (Figure 4).
FIGURE 4. Gut-specific homing integrin α4β7.
Box and whisker plots are provided for proportions of T cells expressing each integrin, with individual data points represented by dots. Comparisons are in reference to PBMC, with p values from Mann-Whitney U tests given for each site. NS= non-significant, p≥ 0.05. (a) TCD4 (b) TCD8. Examples of individual sample multiparameter flow cytometry for expression of α4b7 are shown for TCD4 (c) with event counts (left to right) 2,181; 2,448; 1,632. N = number of samples in each category. NLN = normal lymph node. TIN = tumor-infiltrated lymph node.
Quantitation of α4β1+ (VLA-4) expressing cells is limited by a lack of antibodies that stain the heterodimer specifically. α4 can bind β1 or β7, and β1 can bind multiple α subunits. We stained for α4 and α4β7, then estimated α4β1 expression by the fraction of cells that were α4+/ α4β7neg. This can underestimate the number of α4β1+ cells because cells that express both α4β1 and α4β7 are not included as α4β1+. Using this approach, the estimated proportion of α4β1+ cells in metastatic sites was less than in PBMC, for both TCD4 and TCD8 (mean 35% vs. 47% for TCD4, p=0.004; mean 33% vs. 52% for TCD8, p=0.088, Figure 3a,b). α4β1+ proportions of TCD4 and TCD8 varied greatly among individual samples, but did not vary significantly among NLN, TIN, skin metastases and bowel metastases (one-way Kruskall Wallis test, p=0.126 for TCD4, p=0.589 for TCD8).
Retention integrins
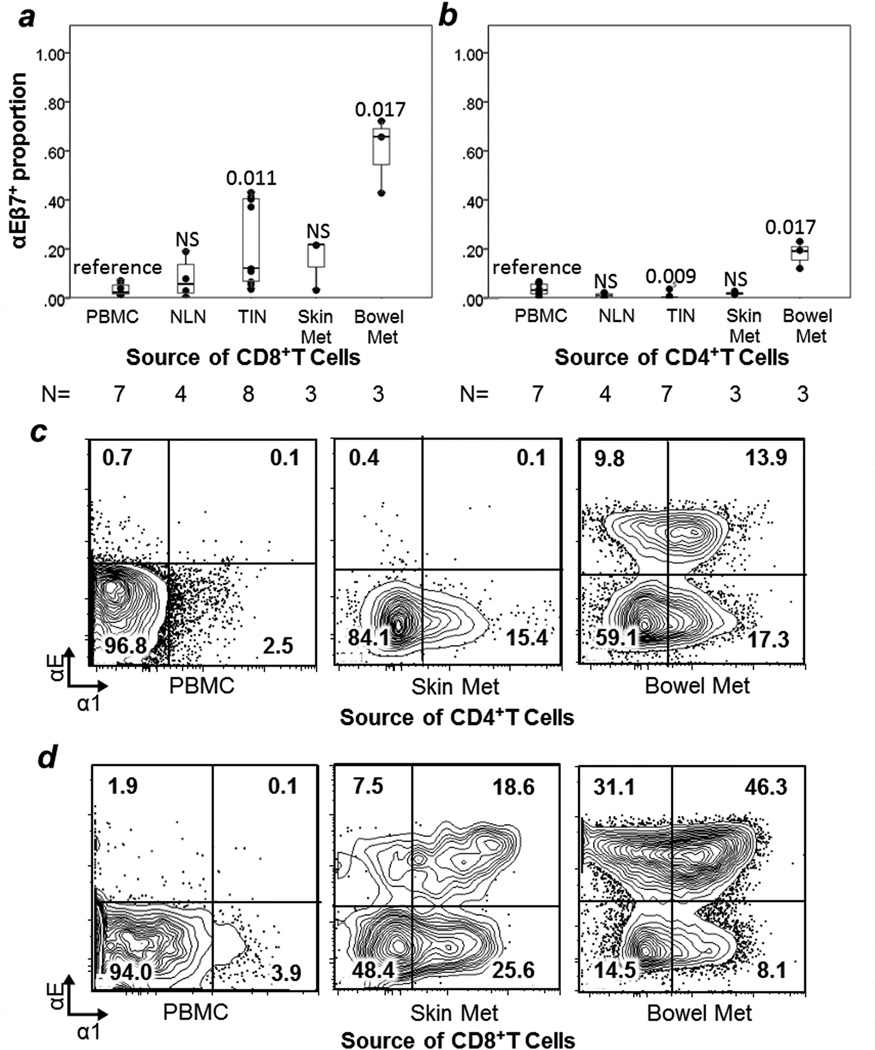
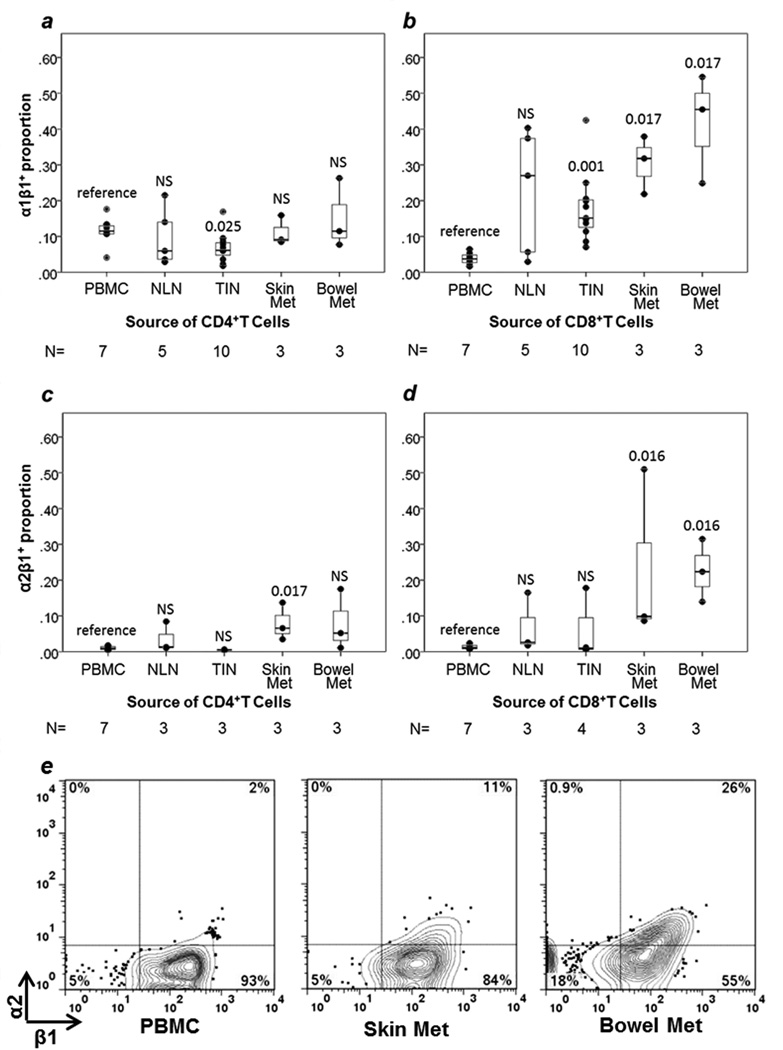
The retention integrins α1β1, α2β1, and αEβ7 were assessed by staining for α1, α2, and αE, respectively. αEβ7+ cells were enriched in bowel metastases (60% of TCD4 and 18% of TCD8) compared to other tissue sites (0.4% for TCD4 and 18% for TCD8, p=0.012, p=0.009, respectively) and compared to PBMC (3% for TCD4, p=0.017; 4% for TCD8, p=0.017, Figure 5). α1β1+ cells were enriched among TCD8 in bowel metastases (42%, p=0.017), skin metastases (31%, p=0.017) and TIN (18%, p=0.001) compared to PBMC (4%) (Figure 5d, Figure 6). TCD4 in bowel (15%) and skin (11%) metastases were not significantly enriched for α1β1+ cells compared to PBMC (11%, Figure 5C, Figure 6). TCD4 in TIN had slightly lower proportions of α1β1+ cells than PBMC (7% vs. 11%, p=0.025, Figure 6).
FIGURE 5. Epithelial retention integrin αEβ7 and collagen retention integrin α1β1 expression by T cells.
Box and whisker plots are shown for the mean proportion of T cells expressing integrin αEβ7 with individual data points represented by dots for TCD4 (a) and TCD8 (b). Comparisons are in reference to PBMC, with p values from Mann-Whitney U tests given for each site. NS= non-significant, p≥ 0.05. Examples of individual sample multiparameter flow cytometry of α1 and αE are shown for TCD4 (c) and TCD8 (d). Event counts (left to right): (c) 29,468; 4,981; 20,574. (d) 5,375; 1,396; 33,722. N = number of samples in each category. NLN = normal lymph node. TIN = tumor-infiltrated lymph node.
FIGURE 6. T cell expression of collagen retention integrins α1β1 and α2β1.
Box and whisker plots are shown for the proportion of T cells expressing each integrin with individual data points represented by dots. Comparisons are in reference to PBMC, with p values from Mann-Whitney U tests given for each site. NS= non-significant, p≥ 0.05. Retention integrin α1β1 expression in TCD4 (a) and TCD8 (b). Retention integrin α2β1 expression in TCD4 (c) and TCD8 (d). Representative examples of flow cytometric data for α1 are included in Figure 5(c, d). Representative examples of flow cytometric data for α2 by TCD8 cells are shown here (e) with event counts (left to right): 3,620; 1,163; 4,372. NLN = normal lymph node. TIN = tumor-infiltrated lymph node.
Among TCD8, α2β1+ cells were enriched in skin (mean 23%, p=0.016) and GI metastases (mean 23%, p=0.016), compared to PBMC (1%, Figure 6). Among TCD4, α2β1+ cells were enriched only in skin metastases (8%) compared to PBMC (1%, p=0.017, Figure 6).
DISCUSSION
There is ample evidence that infiltration of cancers with TCD8 is associated with better survival.1, 3, 15–17 T cell infiltration has been associated with immune signatures, which may identify patients most likely to respond to immune therapies and include many genes associated with T cell homing, including the chemokine ligands for CCR5 and CXCR3.16, 18, 19 However, little is known about the molecular mediators of T cell infiltration of cancer metastases, in particular the T cell homing receptors that govern transmigration of T cells through intratumoral endothelium and retention in the tumor. Here, we present data on homing receptor and retention integrin expression of human T cells that succeed at infiltrating the metastatic melanoma microenvironment. Our findings support site-specific homing to bowel metastases mediated by CCR9 and increased expression of retention integrins α1β1, α2β1, and αEβ7, particularly in skin and bowel metastases. A lack of T cells expressing several homing receptors and integrins with known endothelial ligands identifies potential therapeutic targets for increasing T cell infiltration into tumor for improved patient survival.
In bowel metastases, we encounter an expected enrichment of CCR9-expressing TCD8, but not the anticipated enrichment of α4β7+ cells compared to PBMC. The hypothesized increase in CCR9 and integrin α4β7 is based on previous implication of these molecules in T cell homing to the gut in mouse models of melanoma.8 This inconsistency may reflect post-transmigration down-regulation of α4β7 integrin expression in exchange for αEβ7 upregulation in the presence of TGF-β and cognate antigen.4, 20 This hypothesis is supported by our finding that αEβ7 is significantly elevated on TCD4 and TCD8 in GI metastases.
A similar lack of enrichment in tumor is observed for several other homing receptors. The low proportion of CLA+ cells in skin metastases is contrary to our hypothesis, based on the known association of CLA expression with T cell homing to skin.21 The low representation of CLA+ T cells in the MME likely reflects low E-selectin expression on intratumoral vasculature. Many cutaneous malignancies, including metastatic melanoma, have decreased endothelial expression of E-selectin ligand.11, 22, 23 Downregulation of E-selectin expression may limit tumor infiltration by a significant population of T cells, particularly in the case of lymphocytes induced by cutaneous vaccination, which express high levels of CLA.8 Similarly, integrin α4β1 does not appear to be elevated in metastases. Estimated α4β1 expression is high in PBMC, indicating that the supply of α4β1+ cells in circulation is not a significant factor in limiting T cell homing to tumor. Rather, it suggests that the expression of the endothelial ligand for α4β1, vascular cell adhesion molecule 1 (VCAM-1), may be low on tumor vascular endothelium. This is consistent with previous murine and human studies demonstrating low expression of VCAM-1 on the endothelium in melanoma metastases.24
The data suggest that CXCR3 also may not be enriched in metastases, an unexpected finding given that human melanoma metastases demonstrate elevation of CXCR3 ligands CXCL9-11 in tumors bearing the most T cells.9 Low levels of CXCR3 at the cell surface might be explained by internalization of the receptor upon binding its ligand.25, 26 Artefactual reductions in cell surface CXCR3 may also result from enzymatic digestion of some of the tumor samples in this study; however, low CXCR3 is observed in our TIN samples, 7 out of 10 of which were not exposed to tissue digestion enzymes. Regardless, we know that chemokines CXCL9-11 are not expressed at high levels in most melanoma metastases, though they can be induced by interferons, and low expression of these critical ligands is associated with low T cell infiltration.9
Lack of CLA+, α4β1+ and CXCR3+ T cells enrichment in the MME presents potential targets for therapeutic intervention. For human squamous cell carcinomas of the skin, the Toll-like receptor (TLR) 7 agonist imiquimod induces endothelial expression of the CLA ligand E-selectin, T cell infiltration into tumor, and tumor regression.23 For pre-malignant vulvar intraepithelial neoplasia, combined immunotherapy with imiquimod and vaccination increases T cell infiltration and induces many complete regressions.27 In human melanoma, decreased endothelial expression of VCAM-1 may be responsive to manipulation with TLR3 agonist Polyinosinic:polycytidylic acid (Poly I:C) and/or TLR4 agonist lipopolysaccharide, as experiments with human vascular endothelial cells yield upregulation of VCAM-1 after stimulation with these agents.28, 29 We have previously demonstrated that production of CXCL9-11, ligands for CXCR3, can be induced in human melanoma cells with IFN-α or IFN-γ.30 This provides a rationale for therapies employing direct delivery of TLR agonists or interferons to the MME.
In contrast to the molecules discussed above, we find that chemokine receptors CCR4 and CCR5 are enriched in the MME compared to PBMC. Gene expression profiling of human melanoma metastases has demonstrated elevation of chemokines CCL2, CCL3, CCL4, and CCL5 in samples containing T cells, with associated increase in the corresponding chemokine receptors CCR4 and CCR5.9 CCR4 also has been implicated in TCD4 homing to skin.8 Our findings are concordant with prior work suggesting roles of CCR4 and CCR5 in T cell homing to tumor.
The most striking finding differentiating tumor-infiltrating lymphocytes from those in peripheral blood is the high expression of retention integrins αEβ7, α1β1 and α2β1, with differential expression on TCD4 and TCD8. αEβ7 expression is highest in bowel metastases. Although a murine model of colon cancer has associated αE (CD103) with Treg,31 we find that in human GI metastases, αE expression among TCD4 far exceeds the proportion of Treg (means: 60% vs. 21%.), and that αE is also expressed by TCD8. Instead of marking an increase in Treg, αEβ7 expression may signal greater cytotoxic function. In a murine model of lung cancer, exposure to TGF-β with concomitant cognate antigen engagement of the T cell receptor induces αE expression, triggering increased cytotoxic function and contributing to T cell retention in place.20 The upregulation of αE by TGF-β stimulation has been described in non-malignant settings, as well,4 where CD103+ cytotoxic TCD8 have an activated memory phenotype and a role in tissue rejection.32
Integrins α1β1 and α2β1 have not been directly associated with cytotoxic function, but are known as collagen receptor retention integrins. α1β1 integrin binds collagen IV, and α2β1 integrin participates in T cell retention after binding collagen I.4 In our data, α1β1 is modestly elevated on TCD8 in TIN, skin and GI metastases, and α2β1 is moderately elevated on TCD8 in skin/GI metastases. Neither integrin is elevated on TCD4 in metastatic sites. Previous work examining skin infection has determined that CD4+ and CD8+ memory T cell subsets express different homing receptors, and that the effector memory TCD8 population remains localized to the initial site of antigen inoculation, while the TCD4 memory subset circulates freely, providing broad immunosurveillance.33 Possibly, the enrichment of retention integrins only on TCD8 in metastatic melanoma represents a similar phenomenon of retaining a tumor-resident TEM population while allowing broader circulation of memory T helper cells. Over 60% of metastatic melanomas have intratumoral lymphocytes confined to perivascular spaces3, and the increased proportion of α1β1 on TCD8 may reflect their intratumoral location in the collagen IV-rich perivascular space. α1β1 also has been found to be critical for development of memory TCD8 in peripheral tissues34 and can mediate autoimmune diseases including rheumatoid arthritis and psoriasis.35, 36 α2β1 is a critical mediator of experimental encephalitis.37 The retention integrins α1β1 and α2β1 we have found in melanoma metastases may have the potential to support antitumor immunity.
The primary focus of the present study has been to determine the homing receptors and retention integrins expressed on T cells infiltrating melanoma metastases, with a focus on TCD8, and with assessment of the same molecules on TCD4. The predominant phenotype of intratumoral T cells in our study is an activated effector memory cell, with very few terminally differentiated effector cells. CD69, an early marker of T cell activation, is highly expressed, and there is a significant subset of TCD4 (25%) and TCD8 (35%) with an intermediate phenotype (CD27neg/CD28+ and CD27+/CD28neg, respectively), which has been shown to include cells producing IFN-γ as well as cells producing IL-4.38, 39 Future studies will explore other chemokine receptors that may extend the scope of this work to T cell subsets including Th17 cells (e.g. CCR640) and Treg (e.g. CCR5).41 Additional work will also assess the function of the T cells expressing selected receptors and integrins. A body of literature suggests that T cells infiltrating the MME may be dysfunctional, based on expression of negative costimulatory molecules PD-1, LAG-3 and others.42–45 Treatment with ipilimumab, PD-1 antibody, and vemurafenib have been associated with increases in T cell infiltration.46–49; CXCL10 may have a role with the PD-1 antibody.49 The mechanisms by which these alter T cell trafficking or retention, the T cells subsets affected, and their function in the tumor microenvironment all remain to be elucidated. Examination of tumors during treatment with these agents is now possible and may help to define further the critical mediators of T cell infiltration and function.
There are some limitations of the present work. Ideally, we would study lymphocytes infiltrating all sites of melanoma metastases. However, access to tumors is limited by the clinical settings in which surgery is performed. Metastases of the lung, liver, bone, and brain are commonly treated with systemic therapy rather than excision. Despite limited sample size, we have observed compelling differences between tumor infiltrating lymphocytes and those in PBMC. Some tumor specimens have required enzymatic digestion before cryopreservation, raising the possibility of higher in vivo expression of surface molecules than we have observed in this study. In a separate set of experiments, we have evaluated PBMC looking for changes in expression of the cell surface molecules reported for the present manuscript after incubation for 20hrs in the enzymatic mix used for tumor digestion (manuscript in preparation). With incubation, we observe a 20–30% reduction in the observed proportion of cells expressing CLA and an 80–90% reduction for CXCR3. In the present work, we cannot rule out an enzymatic contribution to observed low CLA expression. However, the reduction observed in the PBMC experiments is not large enough to suggest that CLA expression in our examined tumors could have been greater than that in PBMC, and CLA+ cells are also low in other cutaneous tumors.23 While the observed CXCR3 expression on intratumoral T cells reported here is likely an underestimate, it is difficult to assess the magnitude of diminution, as the some of the observed values are too high to possibly represent an 80–90% reduction from pre-digestion values. In the present work, data on CLA and CXCR3 expression should be interpreted with some caution. On the other hand, we have found that the chemokine receptors observed to be upregulated in tumor metastases are likely only modestly reduced on PBMC, and retention integrins appear wholly preserved on PBMC even after enzymatic digestion (data not shown). Although most patients in each group had resected stage IIIB-IV melanoma, PBMC samples were collected from different patients than those from whom tumors were collected, with some differences in the range of stages between those groups. There are too few patients to assess if melanoma stage has an effect on homing receptor expression. Another limitation of this study is that the use of single cell suspensions of tumor precludes knowledge of the location of T cells within the tumor architecture. Future studies will distinguish homing receptor expression and function of T cells in perivascular, peritumoral, and true intratumoral locations. Antigen specificity and function of infiltrating T cells are not assessed in this study. This is another important area for future investigation, especially as it relates to differences in recruitment, retention, and expansion of antigen-specific cells in the tumor microenvironment.
Given the clear association of improved survival with increased immune cell infiltrate into metastatic melanoma, the fact that less than 10% of patients have diffuse T cell infiltrate into tumor demands new approaches to enable infiltration by antitumor T cells. Our findings suggest several T cell homing receptors that may mediate T cell homing to the MME (CCR4, CCR5) and retention within metastases (integrins α1β1, α2β1, and αEβ7). We have also identified T cell homing receptors that are not enriched in the tumor microenvironment: CLA, α4β1 and likely CXCR3. Future goals of combination immunotherapies may aim to increase their ligands (endothelial E-selectin, VCAM-1, and CXCL9-11) in the MME as new approaches to increase infiltration of effector and effector-memory T cells. Toll-like receptor (TLR) agonists and interferons administered to tumor microenvironments may increase E-selectin and CXCL9-11, respectively;26, 34 they represent classes of therapeutic agents available for clinical intervention in humans, with some agents already FDA-approved for other indications. Future use of such agents in combination with other immune therapies may have a significant impact on the survival of patients with advanced melanoma.
Supplementary Material
ACKNOWLEDGEMENTS
Elise P. Salerno was supported by a research training grant from the National Institutes of Health Grant T32HL007849 (PI, Kron). Sofia Shea was supported by the Rebecca C Harris Memorial Fellowship. Craig L. Slingluff, Jr. was supported by the University of Virginia Cancer Center Core Grant NIH/NCI P30 CA44579 (PI, Weber), by NCI R01 CA57653, and by philanthropic gifts from Alice and Bill Goodwin and the Commonwealth Foundation for Cancer Research and George and Linda Suddock.
ABBREVIATIONS
- APC
allophycocyanin
- CLA
cutaneous leukocyte antigen
- LAG-3
lymphocyte activation gene-3
- LN
lymph node
- MAdCAM-1
mucosal addressin cell adhesion molecule-1
- MME
metastatic melanoma microenvironment
- NLN
benign lymph node
- PBMC
peripheral blood mononuclear cells
- PD-1
programmed cell death protein-1
- Poly I:C
Polyinosinic:polycytidylic acid
- TCD4
CD4+ T cells
- TCD8
CD8+ T cells
- TEM
effector memory T cells
- TEMRA
CD45RA+/CCR7neg effector memory T cells
- TGF-β
transforming growth factor beta
- TIN
tumor infiltrated lymph node
- TLR
toll-like receptor
- Treg
Regulatory T cells
- VCAM-1
vascular cell adhesion molecule 1
- VLA-4
α4β1 integrin, Very Late Antigen-4
REFERENCES
- 1.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 2.Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu YL, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, Lonardi S, Zavadil J, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A. 2009;106:20429–20434. doi: 10.1073/pnas.0905139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL., Jr Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeNucci CC, Mitchell JS, Shimizu Y. Integrin Function in T-Cell Homing to Lymphoid and Nonlymphoid Sites: Getting There and Staying There. Critical Reviews in Immunology. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinashi T. Overview of integrin signaling in the immune system. Methods in molecular biology. 2012;757:261–278. doi: 10.1007/978-1-61779-166-6_17. [DOI] [PubMed] [Google Scholar]
- 6.Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. 2007;218:126–134. doi: 10.1111/j.1600-065X.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 7.Sheasley-O'Neill SL, Brinkman CC, Ferguson AR, Dispenza MC, Engelhard VH. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. Journal of immunology. 2007;178:1512–1522. doi: 10.4049/jimmunol.178.3.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. Journal of immunology. 2010;184:4079–4086. doi: 10.4049/jimmunol.0901903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sasaki K, Zhu X, Vasquez C, Nishimura F, Dusak JE, Huang J, Fujita M, Wesa A, Potter DM, Walker PR, Storkus WJ, Okada H. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007;67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
- 11.Jokai H, Marschalko M, Csomor J, Szakonyi J, Kontar O, Barna G, Karpati S, Hollo P. Tissue-specific homing of immune cells in malignant skin tumors. Pathol Oncol Res. 2012;18:749–759. doi: 10.1007/s12253-012-9529-5. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer JT, Patterson JW, Deacon DH, Smolkin ME, Petroni GR, Jackson EM, Slingluff CL., Jr Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. J Transl Med. 2010;8:79. doi: 10.1186/1479-5876-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 14.Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. Journal of immunology. 1984;133:1857–1862. [PubMed] [Google Scholar]
- 15.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 16.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer journal. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 17.Hsu DS, Kim MK, Balakumaran BS, Acharya CR, Anders CK, Clay T, Lyerly HK, Drake CG, Morse MA, Febbo PG. Immune signatures predict prognosis in localized cancer. Cancer Invest. 2010;28:765–773. doi: 10.3109/07357900903095755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weiss GR, Grosh WW, Chianese-Bullock KA, Zhao Y, Liu H, Slingluff CL, Jr, Marincola FM, Wang E. Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17:7440–7450. doi: 10.1158/1078-0432.CCR-11-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, Pages F, Trajanoski Z, et al. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- 20.Franciszkiewicz K, Le Floc'h A, Jalil A, Vigant F, Robert T, Vergnon I, Mackiewicz A, Benihoud K, Validire P, Chouaib S, Combadiere C, Mami-Chouaib F. Intratumoral induction of CD103 triggers tumor-specific CTL function and CCR5-dependent T-cell retention. Cancer Res. 2009;69:6249–6255. doi: 10.1158/0008-5472.CAN-08-3571. [DOI] [PubMed] [Google Scholar]
- 21.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin Cancer Res. 2007;13:2549–2556. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- 23.Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, Lambert J, Kupper TS. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. 1995;181:811–816. doi: 10.1084/jem.181.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauty A, Colvin RA, Wagner L, Rochat S, Spertini F, Luster AD. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) Journal of immunology. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 26.Meiser A, Mueller A, Wise EL, McDonagh EM, Petit SJ, Saran N, Clark PC, Williams TJ, Pease JE. The chemokine receptor CXCR3 is degraded following internalization and is replenished at the cell surface by de novo synthesis of receptor. Journal of immunology. 2008;180:6713–6724. doi: 10.4049/jimmunol.180.10.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br J Cancer. 2010;102:1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Q, Cox LA, Glenn J, Tejero ME, Hondara V, VandeBerg JL, Wang XL. Molecular pathways mediating differential responses to lipopolysaccharide between human and baboon arterial endothelial cells. Clin Exp Pharmacol P. 2010;37:178–184. doi: 10.1111/j.1440-1681.2009.05260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee MT, Hooper LC, Kump L, Hayashi K, Nussenblatt R, Hooks JJ, Detrick B. Interferon-beta and adhesion molecules (E-selectin and s-intracellular adhesion motecule-1) are detected in sera from patients with retinal vasculitis and are induced in retinal vascular endothelial cells by Toll-like receptor 3 signalling. Clin Exp Immunol. 2007;147:71–80. doi: 10.1111/j.1365-2249.2006.03253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dengel LT, Norrod AG, Gregory BL, Clancy-Thompson E, Burdick MD, Strieter RM, Slingluff CL, Jr, Mullins DW. Interferons induce CXCR3-cognate chemokine production by human metastatic melanoma. Journal of immunotherapy. 2010;33:965–974. doi: 10.1097/CJI.0b013e3181fb045d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, Ellermeier J, Ellwart JW, Schnurr M, Bourquin C, Endres S. CD103 is a hallmark of tumor-infiltrating regulatory T cells. International journal of cancer Journal international du cancer. 2011;129:2417–2426. doi: 10.1002/ijc.25902. [DOI] [PubMed] [Google Scholar]
- 32.Hadley GA, Bartlett ST, Via CS, Rostapshova EA, Moainie S. The epithelial cell-specific integrin, CD103 (alpha E integrin), defines a novel subset of alloreactive CD8+ CTL. Journal of immunology. 1997;159:3748–3756. [PubMed] [Google Scholar]
- 33.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 34.Chapman TJ, Topham DJ. Identification of a unique population of tissue-memory CD4+ T cells in the airways after influenza infection that is dependent on the integrin VLA-1. Journal of immunology. 2010;184:3841–3849. doi: 10.4049/jimmunol.0902281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ben-Horin S, Goldstein I, Koltakov A, Langevitz P, Ehrenfeld M, Rosenthal E, Gur H, Bank I. The effect of blockade of tumor necrosis factor alpha on VLA-1+ T-cells in rheumatoid arthritis patients. J Clin Immunol. 2007;27:580–588. doi: 10.1007/s10875-007-9119-6. [DOI] [PubMed] [Google Scholar]
- 36.Conrad C, Boyman O, Tonel G, Tun-Kyi A, Laggner U, de Fougerolles A, Kotelianski V, Gardner H, Nestle FO. Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med. 2007;13:836–842. doi: 10.1038/nm1605. [DOI] [PubMed] [Google Scholar]
- 37.Tsunoda I, Terry EJ, Marble BJ, Lazarides E, Woods C, Fujinami RS. Modulation of experimental autoimmune encephalomyelitis by VLA-2 blockade. Brain pathology. 2007;17:45–55. doi: 10.1111/j.1750-3639.2006.00042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, Ogg GS, King A, Lechner F, Spina CA, Little S, Havlir DV, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 39.Ye SW, Wang Y, Valmori D, Ayyoub M, Han Y, Xu XL, Zhao AL, Qu L, Gnjatic S, Ritter G, Old LJ, Gu J. Ex-vivo analysis of CD8+ T cells infiltrating colorectal tumors identifies a major effector-memory subset with low perforin content. J Clin Immunol. 2006;26:447–456. doi: 10.1007/s10875-006-9040-4. [DOI] [PubMed] [Google Scholar]
- 40.Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, Taketani Y. Recruitment of CCR6-Expressing Th17 Cells by CCL 20 Secreted from IL-1 beta-, TNF-alpha-, and IL-17A-Stimulated Endometriotic Stromal Cells. Endocrinology. 2010;151:5468–5476. doi: 10.1210/en.2010-0398. [DOI] [PubMed] [Google Scholar]
- 41.Yurchenko E, Tritt M, Hay V, Shevach EM, Belkaid Y, Piccirillo CA. CCR5-dependent homing of naturally occurring CD4(+) regulatory T cells to sites of Leishmania major infection favors pathogen persistence. J Exp Med. 2006;203:2451–2460. doi: 10.1084/jem.20060956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baitsch L, Legat A, Barba L, Fuertes Marraco SA, Rivals JP, Baumgaertner P, Christiansen-Jucht C, Bouzourene H, Rimoldi D, Pircher H, Rufer N, Matter M, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS One. 2012;7:e30852. doi: 10.1371/journal.pone.0030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–896. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourcade J, Kudela P, Sun Z, Shen H, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, Kirkwood JM, Zarour HM. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. Journal of immunology. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fourcade J, Kudela P, Sun ZJ, Shen HM, Land SR, Lenzner D, Guillaume P, Luescher IF, Sander C, Ferrone S, Kirkwood JM, Zarour HM. PD-1 Is a Regulator of NY-ESO-1-Specific CD8(+) T Cell Expansion in Melanoma Patients. Journal of immunology. 2009;182:5240–5249. doi: 10.4049/jimmunol.0803245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, Tsao H, Wargo JA. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 47.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 48.Ribas A, Comin-Anduix B, Economou JS, Donahue TR, de la Rocha P, Morris LF, Jalil J, Dissette VB, Shintaku IP, Glaspy JA, Gomez-Navarro J, Cochran AJ. Intratumoral immune cell infiltrates, FoxP3, and indoleamine 2,3-dioxygenase in patients with melanoma undergoing CTLA4 blockade. Clin Cancer Res. 2009;15:390–399. doi: 10.1158/1078-0432.CCR-08-0783. [DOI] [PubMed] [Google Scholar]
- 49.Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizee G, Radvanyi L, Hwu P. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-gamma inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.