Abstract
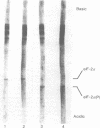
Partially purified Met-tRNAf binding factor, eIF-2, was phosphorylated by using heme-regulated inhibitor (HRI). Phosphorylated eIF-2 was freed from HRI by phosphocellulose column chromatography. Analysis by isoelectric focusing showed 100% phosphorylation of the 38,000-dalton subunit of eIF-2. Both eIF-2 and eIF-2(P) formed ternary complexes with Met-tRNAf and GTP with almost the same efficiency, and in both cases the ternary complex formation was drastically inhibited by prior addition of Mg2+. However, whereas the ternary complexes formed with eIF-2 could be stimulated by Co-eIF-2C at 1 mM Mg2+ and dissociated by Co-eIF-2B at 5 mM Mg2+, the ternary complexes formed with eIF-2(P) were unresponsive to both Co-eIF-2B and Co-e-IF-2C. Also, under conditions of eIF-2 phosphorylation, HRI drastically inhibited AUG-dependent Met-tRNAf binding to 40S ribosomes. However, HRI (in the presence of ATP) had no effect on the joining of preformed Met-tRNAf . 40S . AUG complex to the 60S ribosomal subunit to form Met-tRNAf-80S . AUG complex. These studies suggest that HRI inhibits protein synthesis initiation by phosphorylation of the 38,000-dalton subunit of eIF-2. HRI-phosphorylated eIF-2 does not interact with at least two other protein factors, Co-eIF-2B and Co-eIF-2C, and is thus inactive in protein synthesis initiation.
Full text
PDF

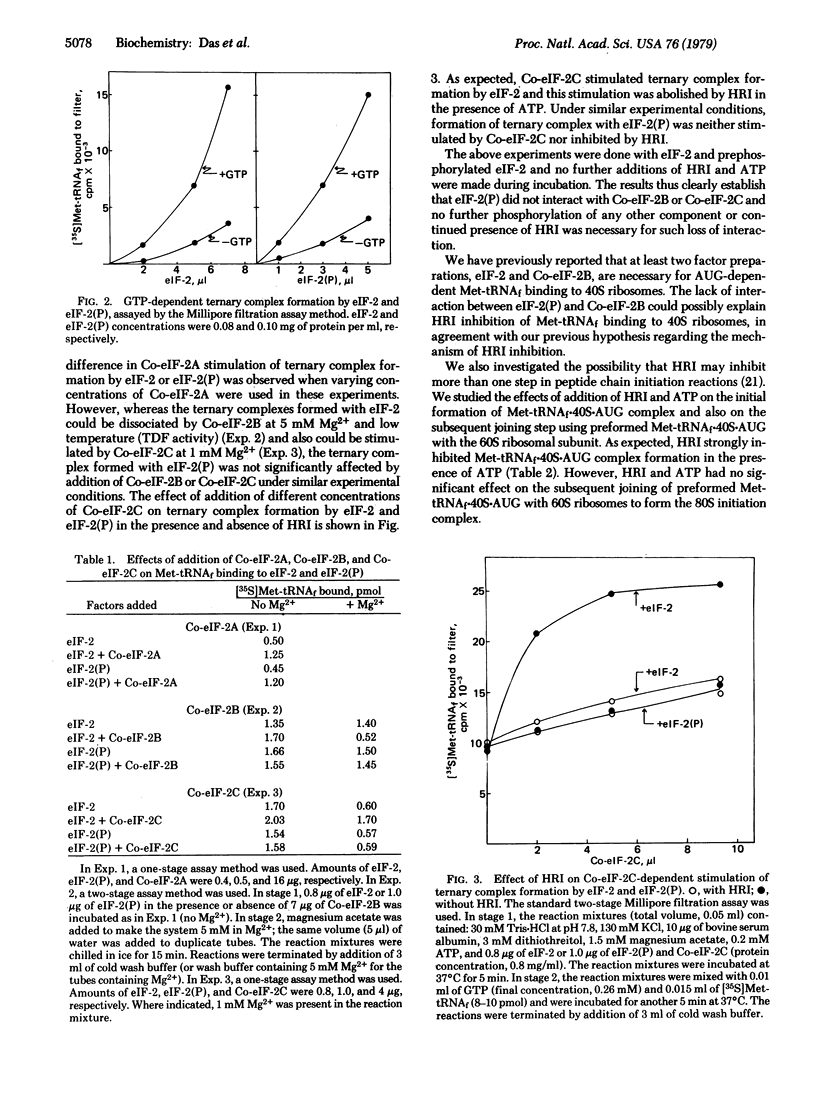

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Bosch L., Cohn W. E., Lodish H., Merrick W. C., Weissbach H., Wittmann H. G., Wool I. G. International symposium on protein synthesis. Summary of Fogarty Center-NIH Workshop held in Bethesda, Maryland on 18-20 October, 1976. FEBS Lett. 1977 Apr 1;76(1):1–10. doi: 10.1016/0014-5793(77)80109-9. [DOI] [PubMed] [Google Scholar]
- Benne R., Amesz H., Hershey J. W., Voorma H. O. The activity of eukaryotic initiation factor eIF-2 in ternary complex formation with GTP and Met-tRNA. J Biol Chem. 1979 May 10;254(9):3201–3205. [PubMed] [Google Scholar]
- Chatterjee B., Dasgupta A., Majumdar A., Palmieri S., Gupta N. K. Millipore filtration assay for AUG-directed met-tRNAf binding to 40 S and 80 S ribosomes. Methods Enzymol. 1979;60:256–265. doi: 10.1016/s0076-6879(79)60023-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Dasgupta A., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes. Characteristics of mRNA (AUG codon)-dependent binding of Met-tRNAfMet to 40 S and 80 S ribosomes. J Biol Chem. 1976 Oct 25;251(20):6379–6387. [PubMed] [Google Scholar]
- Das A., Gupta N. K. Protein synthesis in rabbit reticulocytes XX: a supernatant factor (TDI) inhibits ternary complex (Met-tRNAf-EIF-1-GTP) dissociation and Met-tRNAf binding to 40S ribosomes. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1433–1441. doi: 10.1016/0006-291x(77)91453-x. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Das A., Roy R., Ralston R., Majumdar A., Gupta N. K. Protein synthesis in rabbit reticulocytes XXI. Purification and properties of a protein factor (Co-EIF-1) which stimulates Met-tRNAf binding to EIF-1. J Biol Chem. 1978 Sep 10;253(17):6054–6059. [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Hunt T., Jackson R. J. Analysis of phosphorylation of protein synthesis initiation factor eIF-2 by two-dimensional gel electrophoresis. Eur J Biochem. 1978 Sep 1;89(2):517–521. doi: 10.1111/j.1432-1033.1978.tb12556.x. [DOI] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Evidence that the hemin-controlled translational repressor inhibits formation of 80 S initiation complexes from 48 S intermediate initiation complexes. J Biol Chem. 1979 Apr 10;254(7):2370–2377. [PubMed] [Google Scholar]
- Gross M., Mendelewski J. Additional evidence that the hemin-controlled translational repressor from rabbit reticulocytes is a protein kinase. Biochem Biophys Res Commun. 1977 Jan 24;74(2):559–569. doi: 10.1016/0006-291x(77)90340-0. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Adamson S. D., Herbert E. Studies on cessation of protein synthesis in a reticulocyte lysate cell-free system. Biochim Biophys Acta. 1970 Jul 16;213(1):237–240. doi: 10.1016/0005-2787(70)90028-6. [DOI] [PubMed] [Google Scholar]
- Hunt T., Vanderhoff G., London I. M. Control of globin synthesis: the role of heme. J Mol Biol. 1972 May 28;66(3):471–481. doi: 10.1016/0022-2836(72)90427-5. [DOI] [PubMed] [Google Scholar]
- Kramer G., Cimadevilla J. M., Hardesty B. Specificity of the protein kinase activity associated with the hemin-controlled repressor of rabbit reticulocyte. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3078–3082. doi: 10.1073/pnas.73.9.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Henderson A. B., Pinphanichakarn P., Wallis M. H., Hardesty B. Partial reaction of peptide initiation inhibited by phosphorylation of either initiation factor eIF-2 or 40S ribosomal proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1445–1449. doi: 10.1073/pnas.74.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A., Dasgupta A., Chatterjee B., Das H. K., Gupta N. K. Purification and properties of rabbit reticulocyte protein synthesis initiation factors EIF-1, EIF-2, and EIF-3. Methods Enzymol. 1979;60:35–52. doi: 10.1016/s0076-6879(79)60006-x. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Reynolds S., Gupta N. K. Protein synthesis in rabbit reticulocytes. XIII. Lack of mRNA (poly r (A)) binding activity in highly purified EIF-1. Biochem Biophys Res Commun. 1975 Nov 17;67(2):689–695. doi: 10.1016/0006-291x(75)90867-0. [DOI] [PubMed] [Google Scholar]
- Majumdar A., Roy R., Das A., Dasgupta A., Gupta N. K. Protein synthesis in rabbit reticulocytes XIX: EIF-2 promotes dissociation of Met-tRNAf-EIF-1-GTP complex and Met-tRNAf binding to 40S ribosomes. Biochem Biophys Res Commun. 1977 Sep 9;78(1):161–169. doi: 10.1016/0006-291x(77)91235-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Rabinovitz M., Freedman M. L., Fisher J. M., Maxwell C. R. Translational control in hemoglobin syntheskis. Cold Spring Harb Symp Quant Biol. 1969;34:567–578. doi: 10.1101/sqb.1969.034.01.064. [DOI] [PubMed] [Google Scholar]
- Ralston R. O., Das A., Dasgupta A., Roy R., Palmieri S., Gupta N. K. Protein synthesis in rabbit reticulocytes: characteristics of a ribosomal factor that reverses inhibition of protein synthesis in heme-deficient lysates. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4858–4862. doi: 10.1073/pnas.75.10.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M., Das A., Dasgupta A., Majumdar A., Ralston R., Roy R., Gupta N. K. Regulation of protein synthesis in rabbit reticulocyte lysates by the heme-regulated protein kinase: inhibition of interaction of Met-tRNAfMet binding factor with another initiation factor in formation of Met-tRNAfMet.40S ribosomal subunit complexes. Proc Natl Acad Sci U S A. 1978 Feb;75(2):745–749. doi: 10.1073/pnas.75.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: additional initiation factor required for formation of ternary complex (eIF-2.GTP.Met-tRNAf) and demonstration of inhibitory effect of heme-regulated protein kinase. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1079–1083. doi: 10.1073/pnas.76.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranu R. S., London I. M. Regulation of protein synthesis in rabbit reticulocyte lysates: purification and initial characterization of the cyclic 3':5'-AMP independent protein kinase of the heme-regulated translational inhibitor. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4349–4353. doi: 10.1073/pnas.73.12.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Staehelin T. Binding and release of eukaryotic initiation factor eIF-2 and GTP during protein synthesis initiation. Proc Natl Acad Sci U S A. 1978 Jan;75(1):204–208. doi: 10.1073/pnas.75.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haro C., Datta A., Ochoa S. Mode of action of the hemin-controlled inhibitor of protein synthesis. Proc Natl Acad Sci U S A. 1978 Jan;75(1):243–247. doi: 10.1073/pnas.75.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]