Abstract
The unclear link between intake of polyunsaturated fatty acids (PUFAs) and risk of cardiovascular disease (CVD) could depend on genetic differences between individuals. Minor alleles of single-nucleotide polymorphisms (SNPs) in the ∆5 fatty acid desaturase (FADS) 1 gene were associated with lower blood concentrations of long-chain ω-3 (n–3) and ω-6 (n–6) PUFAs, indicating an associated loss of function effect. We examined whether the SNP rs174546 in FADS1 modifies the association between PUFA intakes and CVD risk. We included 24,032 participants (62% women, aged 44–74 y) from the Malmö Diet and Cancer cohort without prevalent CVD and diabetes. During a mean follow-up of 14 y, 2648 CVD cases were identified. Diet was assessed by a modified diet history method. A borderline interaction was observed between the α-linolenic acid (ALA) (18:3n–3)-to-linoleic acid (LA) (18:2n–6) intake ratio and FADS1 genotype on CVD incidence (P = 0.06). The ALA-to-LA intake ratio was inversely associated with CVD risk only among participants homozygous for the minor T-allele (HR for quintile 5 vs. quintile 1 = 0.72; 95% CI: 0.50, 1.04; P-trend = 0.049). When excluding participants reporting unstable food habits in the past (35%), the interaction between the ALA-to-LA intake ratio and FADS1 genotype on CVD incidence was strengthened and statistically significant (P = 0.04). Additionally, we observed a significant interaction between ALA and FADS1 genotype on ischemic stroke incidence (P = 0.03). ALA was inversely associated with ischemic stroke only among TT genotype carriers (HR for quintile 5 vs. quintile 1 = 0.50; 95% CI: 0.27, 0.94; P-trend = 0.02). In this large cohort, we found some weak, but not convincing, evidence of effect modification by genetic variation in FADS1 on the associations between PUFA intakes and CVD risk. For the 11% of the population homozygous for the minor T-allele of rs174546 that associates with lower ∆5 FADS activity, high ALA intake and ALA-to-LA intake ratio may be preferable in the prevention of CVD and ischemic stroke.
Introduction
For many years, there has been a major health research focus on the association between dietary intake of PUFAs and risk of cardiovascular disease (CVD)7 (1–5). However, the health effects of PUFAs may differ between individuals as a result of genetic variation. Long-chain PUFAs regulate the fluidity of the cell membrane, act as messengers in intracellular signaling pathways, and regulate transcription (4), and long-chain PUFAs, such as arachidonic acid (AA) (20:4n–6) and EPA (20:5n–3), are precursors of inflammatory molecules such as eicosanoids (6, 7). A review of prospective epidemiologic and intervention trials concluded that partial dietary replacement of SFAs with a balanced mixture of n–3 and n–6 PUFAs may lower the risk of CVD (8), and a high long-chain PUFA concentration in blood was associated with lower prevalence of metabolic syndrome and CVD (6, 9). However, several case-control and case-cohort studies found no association or even noticed a higher risk of myocardial infarction with a higher concentration of AA in adipose tissue, suggesting a more proatherosclerotic role of excess AA (10–12). Furthermore, a recent review of 48 randomized controlled trials also suggested that a small reduction in CVD risk may be achieved by decreasing the intake of SFAs and replacing them with unsaturated fats, whereas a reduction in total fat intake was not observed to lower the risk in longer trials (13).
The fatty acid desaturase (FADS) gene cluster includes the FADS1–FADS3 genes and encodes the key enzymes in the endogenous desaturation of α-linolenic acid (ALA) (18:3n–3) and linoleic acid (LA) (18:2n–6) into long-chain PUFAs, in which FADS1 is a ∆5 desaturase and FADS2 a ∆6 desaturase (6, 9). Minor alleles of single-nucleotide polymorphisms (SNPs) in FADS1 were associated with lower concentrations of long-chain PUFAs in blood (14–18), indicating an associated loss of function effect (19). Blood lipid and lipoprotein concentrations have an important role in the development of CVD, and previous studies suggested that the associated effects of SNPs in the FADS1 on lipid and lipoprotein concentrations may be modified by dietary PUFA intakes (20–22). In agreement with these findings, we recently observed a significant interaction between an SNP in FADS1 and long-chain n–3 PUFA intakes on LDLs among 4635 participants in the Malmö Diet and Cancer (MDC) study. The minor allele was observed to associate with lower LDLs only among participants in the lowest tertile of long-chain n–3 PUFA intakes in the MDC study (23). So far, the studies investigating the effect of desaturase activity on atherosclerotic risk are few, and the results are conflicting (9, 18, 24, 25). Therefore, we wanted to examine whether the genetic variation in FADS1 modifies the association between intakes of different PUFAs and the risk of CVD in the MDC cohort.
Participants and Methods
Study population.
The MDC cohort is an urban population-based prospective cohort including 30,447 individuals, with baseline data collection conducted from 1991 to 1996 (26). The study population includes participants born between 1923 and 1950 (27) and living in the southern part of Sweden, in the third largest city, Malmö. They were invited to participate in the study via personal letters and advertisements in the local newspaper and public places. The participation rate was ∼40% (28), and limited Swedish language skills and mental incapacity were the only exclusion criteria. In this study, we included 28,098 participants (11,063 men and 17,035 women) who completed the baseline examination regarding lifestyle factors, dietary intake, and anthropometrics. Of these, 25,528 participants were successfully genotyped for rs174546 in FADS1. After excluding participants with a history of coronary event or stroke (n = 758) or self-reported diabetes or medication (n = 778), 24,032 participants (62% women, aged 44–74 y) remained and create the study sample for this project. All participants provided a written informed consent, and the ethics committee of Lund University approved the MDC study protocols.
Case definition and follow-up.
In total, 2648 (1539 men and 1109 women) CVD cases [of whom 1559 had a coronary event (n = 576, 36.9% women) and 1089 had an ischemic stroke (n = 533, 48.9% women) as the first event] were identified during a mean follow-up time of 14 y (range of 0–18 y). Information about CVD (i.e., prevalent and incident coronary event and ischemic stroke) was taken from the national Swedish Hospital Discharge register, the cause-of-death register (29), and the local stroke register in Malmö (30). A coronary event was defined on the basis of codes 410–414 (fatal or nonfatal myocardial infarction or death due to ischemic heart disease) in the International Classification of Diseases, 9th Revision (ICD-9). Ischemic stroke was defined on the basis of ICD-9 code 434 and diagnosed when computed tomography, MRI, or autopsy could verify the infarction and/or exclude hemorrhage and nonvascular disease. If neither imaging nor autopsy was performed, the stroke was classified as unspecified. Hemorrhagic or nonspecific stroke cases (ICD-9 codes 430, 431, and 436) were excluded because these subtypes of stroke do not have the same underlying risk factors as ischemic stroke. The National Tax Board provided information on vital status and emigration. The participants contributed person-time from date of enrollment until the first cardiovascular event, death, emigration from Sweden, or end of follow-up on 30 June 2009.
Genotyping.
The genotyping of the FADS1 rs174546 (C/T) was performed by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry on the Sequenom Mass-ARRAY platform. Genotyping was successful in 27,615 (96%) of the 28,768 participants with DNA available from the MDC cohort, and rs174546 was in Hardy-Weinberg equilibrium (P = 1.00).
Dietary information.
Dietary intake was measured by a modified diet history methodology combining a 168-item dietary questionnaire, a 7-d menu book, and a 1-h diet history interview specifically designed for the MDC study (31). The 168-item dietary questionnaire covered food items regularly consumed during the past year. The participants were asked to complete the frequency of food intake and estimate the usual portion sizes using a booklet with photographic aids. The 7-d menu book covered cooked lunch and dinner meals, cold beverages (including alcoholic beverages), medications, natural remedies, and dietary supplements used by the participants. The participants were interviewed over the course of 1 h about their food choices, food preparation practices, and portion sizes of the food reported in the menu book. The trained interviewers checked the menu book and questionnaire for very high reported intakes and overlapping information. We also noted the season of the dietary interview: winter (December through February), spring (March through May), summer (June through August), or autumn (September through November). In September 1994, the routines for coding dietary data were slightly altered to shorten the interview time. The diet assessment method version variable indicates whether the data were collected before or after 1 September 1994. This change did not reveal any major influence on the ranking of participants (32). Mean daily intake (grams per day) from food and supplements was calculated based on the information from the menu book, interview, and questionnaire and was converted into nutrient and energy intakes by using the MDC Food and Nutrient Database, developed from the PC KOST-93 food database of the Swedish National Food Administration (31).
The different PUFA intakes (diet plus supplements) were energy-adjusted by regressing the PUFA intakes on total energy intake (residual model), and the participants were divided into quintiles depending on their residual ranking. The PUFA intake variables included in this study were as follows: 1) ALA (18:3n–3); 2) long-chain n–3 PUFAs [EPA (20:5n–3), docosapentaenoic acid (DPA) (22:5n–3), and DHA (22:6n–3)]; 3) total n–3 PUFAs (ALA, EPA, DPA, and DHA); 4) LA (C18:2n–6); 5) total n–6 PUFAs [LA, γ-LA (18:3n–6), and AA (20:4n–6)]; 6) the ALA-to-LA intake ratio; and 7) the total n–3-to-total n–6 PUFA intake ratio. When expressed as energy percentage, the PUFA intake variables and the other diet variables (fat, protein, carbohydrates, MUFAs, PUFAs, and SFAs) were calculated by their contribution to non-alcohol energy intake. Fiber intake was expressed as grams per megajoules (total energy including alcohol).
The relative validity of the modified diet history method was examined in 105 women and 101 men. As the reference method, a total of 18 d of weighed food records was collected during 3 consecutive days, every second month during 1 y. The energy-adjusted correlation coefficients between the modified diet history method and the reference method in men were 0.22 for ALA, 0.23 for LA, 0.55 for AA, 0.24 for EPA, 0.37 for DPA, and 0.20 for DHA. In women, the coefficients were 0.58 for ALA, 0.68 for LA, 0.44 for AA, 0.38 for EPA, 0.40 for DPA, and 0.27 for DHA (33).
Information on dietary change in the past (yes/no) was based on the question “Have you substantially changed your eating habits because of illness or some other reasons?”. The participants’ reported dietary habits may only reflect a short period of their lives and may therefore have less influence on the development of chronic disease. Potential misreporters of energy intake were identified by comparing the individually estimated physical activity level, expressed as total energy expenditure divided by the basal metabolic rate (BMR), with energy intake divided by BMR, as explained in detail previously (34). When the ratio of the reported energy intake to BMR was outside the 95% CI limits of the calculated physical activity level (i.e., under-reporters and over-reporters), the participants were defined as misreporters.
Other variables.
BMI (kilograms per square meter) was calculated from direct measurement of weight and height, conducted by nurses. Participants wore light clothing and no shoes and used a balance-beam scale for weight and a fixed stadiometer for height (centimeters). A self-administered questionnaire was used to determine the following: 1) lifestyle factors, including cigarette smoking, alcohol intake, education, physical activity habits, and socioeconomic factors; 2) medication and diet supplement use; and 3) previous and current diseases. Three categories of smoking status were used: 1) current smokers (including irregular smoking); 2) former smokers; and 3) never smokers. Participants were divided into 5 categories based on their alcohol habits and education. Participants reporting no alcohol consumption during the past year in the questionnaire, who also were zero-reporters of alcohol in the 7-d menu book, were categorized as zero-consumers of alcohol. We divided the other study participants into categories (low, moderate, high, and very high) based on their alcohol consumption (grams per day) with different cutoffs according to sex. The cutoff levels for women were 5, 10, and 20 g/d alcohol, and the cutoff levels for men were 10, 20, and 40 g/d alcohol. Education categories were based on the type of education attained: 1) elementary; 2) primary and secondary; 3) upper secondary; 4) additional education without a degree; and 5) university degree. The leisure time physical activity level was calculated from a list of 17 different activities in the questionnaire. The time spent on each activity was multiplied with an intensity factor, creating a leisure time physical activity score. The leisure time physical activity score was then divided into quintiles, with the same cutoffs for both sexes. Separate categories for smoking, alcohol intake, education, and leisure time physical activity were constructed for the participants with missing data.
Statistical analyses.
SPSS (version 20.0; IBM) was used for all statistical analyses. Statistical significance was set at P < 0.05, and all P-values are 2 sided. The differences in baseline characteristics between the FADS1 rs174546 (C/T) genotype categories, cases and noncases, and between the lowest and highest PUFA intake quintiles were tested using χ2 analyses for categorical variables and with a general linear model for continuous variables. All continuous variables except age were logarithmically transformed to achieve normal distribution when testing differences of means between the lowest and highest PUFA intake quintiles, cases and noncases, and for trend across FADS1 genotype categories adjusted for age and sex, assuming an additive model. Before transformation, a very small amount (0.001 g) was added to n–3 PUFA intakes to handle 0 intakes. Spearman bivariate correlation coefficient was used to examine associations between PUFA and macronutrient intakes. Cox proportional hazard regression was used to examine the association between FADS rs174546 and incident CVD, as well as PUFA intakes and incident CVD, with the lowest quintile as reference. Years of follow-up was used as the underlying time variable. To examine the trends across quintiles, PUFA quintile variables were handled as continuous variables. The basic model was adjusted for age, sex, diet assessment method version, season, and total energy intake. Thereafter, we included smoking, alcohol, leisure time physical activity, education, and BMI in the multivariate analysis. These variables were selected from the literature for being known risk factors for CVD and for being associated with dietary PUFA intakes in this study. We also performed the multivariate model excluding BMI because it might be an intermediate between dietary habits and disease. In addition, we adjusted for intakes of fiber and SFAs because these dietary factors are suspected to be associated with eating pattern and with incidence of CVD (5). We also performed the analyses adjusting for use of hypertension medication. The association between PUFA intakes and incident coronary event and ischemic stroke was also analyzed separately. The interaction between PUFA intakes and FADS1 genotype on incidence of CVD were examined by introducing a multiplicative factor of genotypes and PUFA quintiles as continuous variables in addition to these main factors as separate variables in a multivariate-adjusted model. To further examine the interactions between PUFA intakes and FADS1 genotype categories on CVD risk, we performed the analyses in strata of PUFA intakes. All analyses were also examined separately in men and women because of sex differences in food selection and reporting, as well as biologic differences. Formal tests for interaction by sex were also performed.
In sensitivity analyses, we excluded participants reporting a dietary change in the past because they may have unstable food habits. Furthermore, we excluded both participants reporting dietary change in the past and potential misreporters of energy intake. We also performed the analyses excluding cases that were diagnosed within 2 y after baseline examination. We did not correct for multiple testing.
Results
During a mean follow-up time of 14 y (330,774 person-years), we identified 2648 CVD cases.
Background information.
Lifestyle factors including the PUFA intakes did not differ according to FADS1 genotype categories (Table 1). As expected, men had a higher incidence of CVD, and the CVD cases had higher age and BMI compared with those who did not develop CVD (Supplemental Table 1). The distribution of FADS1 genotypes did not differ between incident CVD cases and noncases (P = 0.44).
TABLE 1.
Baseline characteristics among 24,032 participants in the Malmö Diet and Cancer cohort according to the FADS1 rs174546 genotype1
| Characteristics | Total | CC (n = 10,710) | CT (n = 10,661) | TT (n = 2661) | P-trend |
| n | |||||
| Women,2 % | 14,926 | 44.2 | 44.7 | 11.1 | 0.34 |
| Age, y | 24,032 | 57.9 (57.7, 58.0) | 57.9 (57.8, 58.1) | 57.9 (57.6, 58.2) | 0.86 |
| BMI, kg/m2 | 24,003 | 25.7 (25.6, 25.7) | 25.7 (25.6, 25.7) | 25.5 (25.3, 25.6) | 0.09 |
| Alcohol consumption, % | 23,881 | 0.40 | |||
| Zero-reporters | 1474 | 6.3 | 5.9 | 6.4 | |
| Low | 9712 | 40.9 | 40.0 | 40.3 | |
| Moderate | 5658 | 22.8 | 24.1 | 24.0 | |
| High | 5243 | 21.8 | 21.9 | 21.7 | |
| Very high | 1945 | 8.1 | 8.2 | 7.6 | |
| Cigarette smoking status, % | 24,022 | 0.85 | |||
| Current smoker | 6777 | 28.1 | 28.4 | 27.9 | |
| Former smoker | 7944 | 33.4 | 32.8 | 32.8 | |
| Never smoker | 9301 | 38.5 | 38.8 | 39.3 | |
| Education, % | 23,977 | 0.22 | |||
| Elementary | 9890 | 41.5 | 41.3 | 40.0 | |
| Primary and secondary | 6354 | 26.9 | 26.0 | 26.7 | |
| Upper secondary | 2142 | 9.0 | 8.8 | 9.4 | |
| Additional education without a degree | 2128 | 8.6 | 9.2 | 8.5 | |
| University degree | 3463 | 14.0 | 14.7 | 15.4 | |
| Leisure time physical activity, % | 23,881 | 0.95 | |||
| Very low | 2341 | 9.8 | 9.9 | 9.3 | |
| Low | 7916 | 33.1 | 33.0 | 33.8 | |
| Medium | 6849 | 28.8 | 28.6 | 28.7 | |
| High | 4638 | 19.2 | 19.6 | 19.6 | |
| Very high | 2137 | 9.1 | 8.9 | 8.5 | |
| Dietary intake | 24,032 | ||||
| Total energy intake, MJ/d | 9.53 (9.48, 9.57) | 9.53 (9.48, 9.57) | 9.53 (9.44, 9.62) | 0.98 | |
| Protein, E% | 15.7 (15.7, 15.8) | 15.8 (15.7, 15.8) | 15.7 (15.6, 15.8) | 0.47 | |
| Carbohydrates, E% | 45.2 (45.1, 45.4) | 45.2 (45.0, 45.3) | 45.1 (44.9, 45.3) | 0.44 | |
| Fiber, g/MJ | 2.16 (2.15, 2.17) | 2.16 (2.15, 2.17) | 2.15 (2.12, 2.17) | 0.53 | |
| Total fat, E% | 39.0 (38.9, 39.1) | 39.1 (39.0, 39.2) | 39.2 (39.0, 39.4) | 0.38 | |
| SFAs, E% | 16.8 (16.7, 16.9) | 16.8 (16.8, 16.9) | 17.0 (16.8, 17.1) | 0.08 | |
| MUFAs, E% | 13.6 (13.6, 13.6) | 13.6 (13.6, 13.6) | 13.6 (13.5, 13.7) | 0.69 | |
| PUFAs, E% | 6.16 (6.13, 6.19) | 6.18 (6.15, 6.21) | 6.12 (6.06, 6.18) | 0.57 | |
| Dietary PUFA intake | 24,032 | ||||
| ALA, E% | 0.74 (0.74, 0.75) | 0.75 (0.74, 0.75) | 0.74 (0.73, 0.75) | 0.53 | |
| Long-chain n–3 PUFAs, E% | 0.26 (0.25, 0.26) | 0.26 (0.25, 0.26) | 0.26 (0.25, 0.27) | 0.65 | |
| Total n–3 PUFAs, E% | 1.00 (0.99, 1.01) | 1.00 (1.00, 1.01) | 1.00 (0.99, 1.01) | 0.90 | |
| LA, E% | 4.91 (4.88, 4.94) | 4.93 (4.90, 5.96) | 4.87 (4.82, 4.93) | 0.49 | |
| Total n–6 PUFAs, E% | 4.98 (4.95, 5.00) | 5.00 (4.97, 5.02) | 4.94 (4.88, 4.99) | 0.48 | |
| ALA-to-LA intake ratio, E% | 0.16 (0.16, 0.16) | 0.16 (0.16, 0.16) | 0.16 (0.16, 0.16) | 0.44 | |
| Total n–3-to-total n–6 PUFA intake ratio | 0.21 (0.21, 0.21) | 0.21 (0.21, 0.21) | 0.21 (0.21, 0.22) | 0.45 |
Data are expressed as means (95% CIs) for continuous variables or as percentages for categorical variables. ALA, α-linolenic acid; E%, energy percentage; FADS, fatty acid desaturase; LA, linoleic acid.
The difference in baseline characteristics between FADS genotype categories was tested using χ2 analyses for categorical variables or with a general linear model for continuous variables, P < 0.05. Continuous variables were adjusted for age and sex and ln transformed when testing differences of means (except age).
A majority of the participant characteristics were unequally distributed when comparing the highest with the lowest quintile of dietary PUFA intakes (P < 0.05) (Supplemental Table 2). All of the PUFAs were positively correlated with fat intake and negatively correlated with carbohydrate intake (Supplemental Table 3). Furthermore, protein intake was negatively correlated with all PUFAs except long-chain n–3 PUFAs and the total n–3-to-total n–6 PUFA intake ratio.
PUFA intakes, FADS1 genotype, and CVD risk.
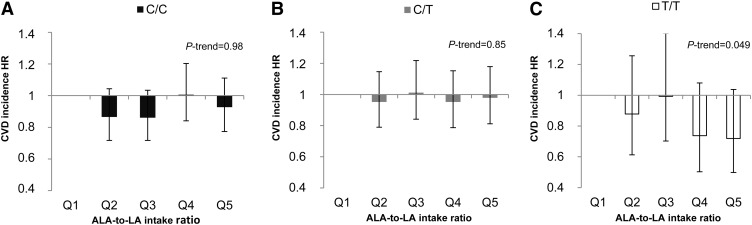
We did not observe any statistically significant association between any of the PUFA intakes and incident CVD in either the basic analysis model (P-trend ≥ 0.29) or the multivariate analysis (P-trend ≥ 0.28) (Table 2). Excluding BMI or including intakes of fiber and SFAs in the multivariate analyses did not change the results substantially. Furthermore, rs174546 was not associated with CVD risk (HR per allele = 0.99; 95% CI: 0.93, 1.04; P-trend = 0.61). A borderline interaction was observed between the ALA-to-LA intake ratio and FADS1 genotype on CVD incidence (P = 0.06) (Table 3). The ALA-to-LA intake ratio was only inversely associated with CVD risk among the TT genotype carriers of rs174546 (HR for quintile 5 vs. quintile 1 = 0.72; 95% CI: 0.50, 1.04; P-trend = 0.049) (Fig. 1). The above-reported results remained essentially the same when including intakes of fiber, SFAs, or use of antihypertensive medication in the multivariate model. To further examine the interaction between the ALA-to-LA intake ratio and FADS1 genotype on CVD risk, we performed the analyses in strata of the ALA-to-LA intake ratio. We only observed a significant association between the FADS1 genotypes and incidence of CVD in intake quintile 4 of the ALA-to-LA intake ratio (quintile 1: HR per allele = 1.11, 95% CI: 0.84, 1.47, and P-trend = 0.77; quintile 2: HR per allele = 1.11, 95% CI: 0.83, 1.48, and P-trend = 0.49; quintile 3: HR per allele = 1.26, 95% CI: 0.97, 1.64, and P-trend = 0.14; quintile 4: HR per allele = 0.77, 95% CI: 0.57, 1.05, and P-trend = 0.03; and quintile 5: HR per allele = 0.87, 95% CI: 0.66, 1.16, and P-trend = 0.29).
TABLE 2.
HRs of incident cardiovascular disease by PUFA intake quintiles and per 1 E% increase of PUFA intakes among 24,032 participants in the Malmö Diet and Cancer cohort1
| PUFA intake quintiles |
||||||
| Dietary PUFAs | 1 (n = 4806) | 2 (n = 4807) | 3 (n = 4806) | 4 (n = 4807) | 5 (n = 4806) | Per 1 E% increase of PUFA intakes2 |
| ALA | ||||||
| Median intake, E% | 0.52 | 0.63 | 0.72 | 0.82 | 0.99 | |
| HR (95% CI) | 1 | 0.93 (0.82, 1.06) | 1.04 (0.92, 1.17) | 0.97 (0.85, 1.09) | 0.98 (0.87, 1.11) | 1.07 (0.89, 1.29) |
| Long-chain n–3 PUFAs | ||||||
| Median intake, E% | 0.07 | 0.13 | 0.19 | 0.30 | 0.53 | |
| HR (95% CI) | 1 | 0.96 (0.85, 1.10) | 1.01 (0.89, 1.15) | 1.00 (0.88, 1.13) | 1.00 (0.88, 1.14) | 0.97 (0.82, 1.16) |
| Total n–3 PUFAs | ||||||
| Median intake, E% | 0.68 | 0.83 | 0.96 | 1.10 | 1.37 | |
| HR (95% CI) | 1 | 0.97 (0.85, 1.10) | 1.02 (0.90, 1-15) | 1.05 (0.93, 1.19) | 1.00 (0.88, 1.13) | 1.02 (0.90, 1.15) |
| LA | ||||||
| Median intake, E% | 3.26 | 4.05 | 4.73 | 5.49 | 6.80 | |
| HR (95% CI) | 1 | 1.15 (1.02, 1.30) | 1.08 (0.96, 1.22) | 0.99 (0.88, 1.13) | 1.15 (1.02, 1.30) | 1.01 (0.99, 1.04) |
| Total n–6 PUFAs | ||||||
| Median intake, E% | 3.32 | 4.11 | 4.79 | 5.55 | 6.86 | |
| HR (95% CI) | 1 | 1.15 (1.02, 1.30) | 1.09 (0.96, 1.23) | 0.99 (0.87, 1.12) | 1.16 (1.03, 1.31) | 1.01 (0.99, 1.04) |
| ALA-to-LA intake ratio | ||||||
| Median ratio | 0.12 | 0.14 | 0.15 | 0.17 | 0.21 | |
| HR (95% CI) | 1 | 0.90 (0.80, 1.02) | 0.94 (0.83, 1.06) | 0.96 (0.84, 1.08) | 0.92 (0.81, 1.04) | 0.96 (0.88, 1.05) |
| Total n–3-to-total n–6 PUFA intake ratio | ||||||
| Median ratio | 0.14 | 0.17 | 0.19 | 0.23 | 0.30 | |
| HR (95% CI) | 1 | 1.01 (0.89, 1.15) | 1.01 (0.89, 1.14) | 1.01 (0.89, 1.15) | 0.95 (0.83, 1.07) | 0.97 (0.92, 1.02) |
Cox proportional hazard regression was used to calculate the HR (95% CI) for each quintile of PUFA intakes with the lowest quintile as reference. Multivariate models were adjusted for age, sex, BMI, diet assessment method version, season, total energy intake, alcohol intake, leisure time physical activity, education, and smoking. ALA, α-linolenic acid; E%, energy percentage; LA, linoleic acid.
Per-unit increase is 0.1 for ratios of PUFAs.
TABLE 3.
HRs for per 1 E% increase of PUFA intakes in strata of FADS1 rs174546 genotype on incidence of total CVD, coronary event, and ischemic stroke among 24,032 participants in the Malmö Diet and Cancer cohort1
| CC | CT | TT | P-interaction2 | |
| Total CVD | ||||
| ALA | 1.21 (0.92, 1.59) | 1.03 (0.77, 1.38) | 0.85 (0.47, 1.55) | 0.22 (0.07)3 |
| Long-chain n–3 PUFAs | 0.93 (0.72, 1.20) | 1.02 (0.78, 1.33) | 1.21 (0.69, 2.13) | 0.93 (0.73)3 |
| Total n–3 PUFAs | 1.04 (0.87, 1.26) | 1.02 (0.84, 1.24) | 1.02 (0.68, 1.53) | 0.64 (0.50)3 |
| LA | 1.03 (0.99, 1.07) | 1.00 (0.96, 1.04) | 1.04 (0.96, 1.13) | 0.74 (0.88)3 |
| Total n–6 PUFAs | 1.03 (0.99, 1.07) | 1.00 (0.96, 1.04) | 1.04 (0.96, 1.13) | 0.75 (0.90)3 |
| ALA-to-LA intake ratio | 0.97 (0.85, 1.11) | 1.01 (0.88, 1.16) | 0.76 (0.58, 1.00) | 0.06 (0.04)3 |
| Total n–3-to-total n–6 PUFAs intake ratio | 0.96 (0.89, 1.04) | 1.00 (0.92, 1.08) | 0.91 (0.77, 1.08) | 0.27 (0.34)3 |
| Coronary event | ||||
| ALA | 1.22 (0.85, 1.74) | 1.12 (0.78, 1.62) | 1.56 (0.78, 3.13) | 0.86 |
| Long-chain n–3 PUFAs | 1.02 (0.73, 1.41) | 0.95 (0.67, 1.36) | 0.74 (0.34, 1.61) | 0.48 |
| Total n–3 PUFAs | 1.10 (0.87, 1.39) | 1.02 (0.80, 1.32) | 1.09 (0.65, 1.82) | 0.64 |
| LA | 1.03 (0.98, 1.08) | 0.98 (0.93, 1.03) | 1.11 (1.00, 1.23) | 0.31 |
| Total n–6 PUFAs | 1.03 (0.98, 1.08) | 0.98 (0.93, 1.03) | 1.11 (1.00, 1.23) | 0.31 |
| ALA-to-LA intake ratio | 0.96 (0.81, 1.15) | 1.09 (0.92, 1.30) | 0.83 (0.59, 1.17) | 0.30 |
| Total n–3-to-total n–6 PUFAs intake ratio | 0.98 (0.89, 1.08) | 1.02 (0.92, 1.13) | 0.85 (0.68, 1.06) | 0.26 |
| Ischemic stroke | ||||
| ALA | 1.21 (0.79, 1.85) | 0.93 (0.59, 1.47) | 0.27 (0.09, 0.77) | 0.03 |
| Long-chain n–3 PUFAs | 0.81 (0.53, 1.22) | 1.11 (0.74, 1.67) | 2.21 (0.99, 4.96) | 0.47 |
| Total n–3 PUFAs | 0.97 (0.73, 1.30) | 1.02 (0.76, 1.38) | 0.91 (0.47, 1.75) | 0.88 |
| LA | 1.03 (0.97, 1.09) | 1.02 (0.96, 1.09) | 0.94 (0.81, 1.08) | 0.48 |
| Total n–6 PUFAs | 1.03 (0.97, 1.09) | 1.03 (0.96, 1.09) | 0.94 (0.82, 1.08) | 0.47 |
| ALA-to-LA intake ratio | 0.97 (0.80, 1.19) | 0.90 (0.72, 1.12) | 0.65 (0.41, 1.04) | 0.08 |
| Total n–3-to-total n–6 PUFAs intake ratio | 0.92 (0.82, 1.04) | 0.97 (0.85, 1.10) | 1.00 (0.77, 1.29) | 0.73 |
Values are HRs (95% CIs). Cox proportional hazard regression was used to calculate the HR for each quintile of PUFA intakes with the lowest quintile as reference and per-unit increase of PUFA intakes (1 E% for PUFA intakes and 0.1 for PUFA ratios) and P < 0.05. Multivariate models were adjusted for age, sex, BMI, diet assessment method version, season, total energy intake, alcohol intake, leisure time physical activity, education, and smoking. ALA, α-linolenic acid; CVD, cardiovascular disease; E%, energy percentage; FADS1, fatty acid desaturase 1; LA, linoleic acid.
Quintile of PUFA intakes × genotype.
Values in parentheses are P values for sensitivity analysis. Sensitivity analyses excluded those reporting a dietary change in the past and potential energy misreporters, n = 15,538.
FIGURE 1.
Association between the ALA-to-LA intake ratio and incidence of CVD according to FADS1 rs174546 genotype CC (A), CT (B), and TT (C) among 24,032 participants in the Malmö Diet and Cancer cohort. Cox proportional hazard regression was used to calculate HR for each quintile of PUFA intake, with the lowest quintile as reference. Multivariate models were adjusted for age, sex, BMI, diet assessment method version, season, total energy intake, alcohol intake, leisure time physical activity, education, and smoking. The median ALA-to-LA intake ratio and n per quintile: quintile 1 = 0.12, n = 4806; quintile 2 = 0.14, n = 4807; quintile 3 = 0.15, n = 4806; quintile 4 = 0.17, n = 4807; and quintile 5 = 0.21, n = 4806. ALA, α-linolenic acid; CVD, cardiovascular disease; FADS1, fatty acid desaturase 1; LA, linoleic acid; Q, quintile.
We also examined the associations separately for coronary events and ischemic stroke. FADS1 genotype was associated with neither coronary event nor ischemic stroke risk (HR per allele = 0.98, 95% CI: 0.91, 1.06, and P-trend = 0.66; and HR per allele = 0.99, 95% CI: 0.90, 1.08, and P-trend = 0.79, respectively). In line with previous results, we did not observe any significant association between any of the PUFA intakes and risk of coronary event or ischemic stroke (P-trend > 0.31). Furthermore, we did not observe any significant interaction between any of the PUFA intakes and FADS1 genotype on coronary event risk (lowest P-interaction was for the total n–3-to-total n–6 PUFA intake ratio, P = 0.26 to highest P = 0.86 for ALA) (Table 3). However, we observed a significant interaction between ALA and FADS1 genotype on risk of ischemic stroke incidence (P = 0.03) in which ALA was inversely associated with risk of ischemic stroke only among TT genotype carriers (HR for quintile 5 vs. quintile 1 = 0.50; 95% CI: 0.27, 0.94; P-trend = 0.02). Additionally, we observed a borderline interaction between the ALA-to-LA intake ratio and FADS1 genotype on ischemic stroke incidence (P = 0.08) (Supplemental Table 3). However, there was no significant association between the ALA-to-LA intake ratio and ischemic stroke incidence among the TT genotype carriers (P-trend = 0.17).
We also examined all analyses separately for men and women. We did not observe any statistically significant association between any of the PUFA intakes and incidence of CVD in either men or women (P-trend > 0.24), and there was no heterogeneity of association between the sexes (P-interaction > 0.22). The previous inverse association between the ALA-to-LA intake ratio on CVD incidence among the TT genotype carriers was not significant in either men or women (HR for quintile 5 vs. quintile 1 = 0.69, 95% CI: 0.42, 1.15; and P-trend = 0.16; and HR for quintile 5 vs. quintile 1 = 0.67, 95% CI: 0.39, 1.15, and P-trend = 0.14, respectively), and there was no heterogeneity of association between the sexes (P-interaction = 0.53). There was no heterogeneity of association between the sexes (P-interaction = 0.80) for the previously observed inverse association between ALA intake and ischemic stroke incidence among TT genotype carriers. We did not observe any significant interactions in either men or women between the different PUFA intakes and FADS1 genotype on incidence of total CVD (lowest P-interaction was for the ALA-to-LA intake ratio, P = 0.08 in women), coronary event (LA, P = 0.11 in women), or ischemic stroke (ALA, P = 0.08 in men).
In sensitivity analyses, we excluded participants reporting a dietary change in the past and those suspected of being misreporters of energy (35% of the study sample). The findings after these exclusions were in line with previous results; however, the interaction between the ALA-to-LA intake ratio and FADS1 genotype on CVD incidence was slightly strengthened and statistically significant (P = 0.04) (Table 3). Similar results were observed when excluding only participants reporting a dietary change in the past. Finally, we excluded cases diagnosed within 2 y after baseline (2% of the study sample). However, this did not affect the previously observed results.
Discussion
In this large Swedish observational prospective study of middle-aged participants, we found some evidence of effect modification by genetic variation in FADS1 on the association between PUFA intakes and CVD risk. We observed a borderline interaction between the ALA-to-LA intake ratio and FADS1 genotype on CVD incidence. Interestingly, when excluding the participants with suspected unstable food habits and potential misreporters of energy, the interaction between the ALA-to-LA intake ratio and FADS1 genotype on CVD incidence was strengthened and significant. Additionally, we observed a significant interaction between ALA and FADS1 genotype on ischemic stroke incidence in which ALA intake was inversely associated with risk of ischemic stroke only among TT genotype carriers.
Both diet and genetic variation in the FADS1–FADS2 gene cluster are important factors that influence the blood and tissue concentrations of long-chain PUFAs and their essential precursors ALA and LA. In agreement with the results from Martinelli et al. (9), Lu et al. (18), and Baylin et al. (24), we did not observe any association between the FADS1 genotype and CVD risk when the analysis was not stratified for dietary intakes. However, our results suggest that individuals with genetically elevated ALA and LA concentrations together with a higher dietary ALA-to-LA ratio or ALA intake may reach higher tissue concentrations of ALA, which may have a protective effect against CVD. However, it is not clear whether ALA has an independent role in cardiovascular health or whether the potential beneficial effect is driven by the conversion to long-chain n–3 PUFAs (35, 36). In a meta-analysis of 27 studies, the included observational studies indicated that a higher exposure of ALA was associated with a moderately lower risk of CVD (36).
Our results suggest that individuals with higher ALA intake in combination with genetically higher ALA availability may have a protective effect against ischemic stroke. In fact, we only found a significant interaction on incidence of ischemic stroke and not on coronary events. In line with our results, previous studies indicated that insufficient intake and tissue levels of n–3 PUFAs increase the risk of stroke (37, 38). Although in particular EPA and DHA intake amounts were associated with neurovascular, cell membrane, and tissue functions (39, 40), ALA intake and the ALA-to-LA intake ratio were also recognized to be important (35, 36, 40, 41). For example, injection of ALA was observed to trigger vasodilatation in small cerebral arteries, leading to increased cerebral blood flow in mice (37, 40). Whether the interaction on stroke incidence and lack of interaction on myocardial infarction may reflect differences in risk factors and pathophysiology of these cardiovascular endpoints or whether it is simply a statistical power issue cannot be answered in the current study. Other studies need to replicate our findings to answer this question.
In agreement with our results noting a protective association with a high ALA-to-LA intake ratio among TT genotype carriers, Ghosh et al. (41) found that when pigs were fed diets differing in ALA and LA composition for 30 d, an increased dietary ALA intake was associated with decreased AA and increased EPA concentrations in heart membranes and with prevention of proinflammatory enzyme activation. The study suggests that the potential cardiovascular benefit of ALA may only be achieved when LA is simultaneously kept low and not when pigs are fed a high LA diet (41). However, in some studies, higher intakes of LA were associated with decreased risk of CVD (42, 43). Furthermore, because both a deficiency of LA and high intakes of LA may increase oxidative stress (44, 45), the roles of the absolute amounts of ALA and LA and the total n–3-to-total n–6 PUFA intake ratio in cardiovascular health need to be investigated further.
In our previous study, we observed a significant interaction between rs174547 in FADS1 and long-chain n–3 PUFA intakes on LDLs among 4635 participants in the MDC cohort. The minor allele was associated with lower LDLs only among participants in the lowest tertile of long-chain n–3 PUFA intakes (23). The present study did not show any significant interaction between the intake of long-chain n–3 PUFAs and FADS1 genotype on CVD incidence. An explanation for the inconsistency of the results may be that other PUFAs, such as ALA and LA (before the ∆5 FADS desaturation step), are more dependent on the FADS1 genotype to affect the long-term risk of CVD. For example, a benefit for ALA compared with long-chain n–3 PUFAs is that ALA can compete with LA in the first elongation–desaturation step, whereas the long-chain n–3 PUFAs only compete with the long-chain n–6 PUFAs in the late steps of eicosanoid metabolism. Thus, in contrast to long-chain n–3 PUFAs, ALA can prevent accumulation of long-chain n–6 PUFAs (46).
The large sample size and detailed information on dietary intakes, based on a 168-item dietary questionnaire, a 7-d menu book, and a 1-h interview, are the major strengths of our study. However, the estimated dietary intakes were based on self-reports, and the limitation of a short-term diet measurement to reflect “habitual” intake may have introduced misclassification of dietary intakes and attenuation of the associations. Although dietary data from the MDC cohort are in general of high relative validity (47), the validity coefficients of some PUFAs are rather low, especially long-chain n–3 PUFAs in men, which is a weakness of the current study. Finally, we performed multiple tests, and thus some of the observed significant associations and interactions could be due to chance. Therefore, it is important to replicate our results in well-powered studies with high-quality dietary data.
In this large observational prospective study, we found some weak, but not convincing, evidence of effect modification by genetic variation in FADS1 on the association between PUFA intakes and CVD risk. For the 11% of the population homozygous for the minor T-allele of rs174546, we found some evidence that high ALA intake and ALA-to-LA intake ratio may be preferable in the prevention of CVD and ischemic stroke.
Supplementary Material
Acknowledgments
The authors thank Malin Svensson for excellent technical assistance. S.H., U.E., B.G., B.H., M.O.-M., and E.S. designed the research; S.H. analyzed the data; S.H., M.O.-M., and E.S. wrote the paper; U.E., B.G., and B.H. provided critical review; S.H. and E.S. had primary responsibility for the final content. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, arachidonic acid; ALA, α-linolenic acid; BMR, basal metabolic rate; CVD, cardiovascular disease; DPA, docosapentaenoic acid; FADS, fatty acid desaturase; ICD-9, International Classification of Diseases, 9th Revision; LA, linoleic acid; MDC, Malmö Diet and Cancer; SNP, single-nucleotide polymorphism.
References
- 1.Ramsden CE, Hibbeln JR, Majchrzak SF, Davis JM. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: a meta-analysis of randomised controlled trials. Br J Nutr 2010;104:1586–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 2012;308:1024–33. [DOI] [PubMed] [Google Scholar]
- 3.Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega-3 fatty acids. Lancet 2010;376:540–50. [DOI] [PubMed] [Google Scholar]
- 4.Jung UJ, Torrejon C, Tighe AP, Deckelbaum RJ. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr 2008;87:2003S–9S. [DOI] [PubMed] [Google Scholar]
- 5.Wallström P, Sonestedt E, Hlebowicz J, Ericson U, Drake I, Persson M, Gullberg B, Hedblad B, Wirfalt E. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmö Diet and Cancer Cohort: a prospective study. PLoS One 2012;7:e31637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lattka E, Illig T, Heinrich J, Koletzko B. Do FADS genotypes enhance our knowledge about fatty acid related phenotypes? Clin Nutr 2010;29:277–87. [DOI] [PubMed] [Google Scholar]
- 7.Mathias RA, Vergara C, Gao L, Rafaels N, Hand T, Campbell M, Bickel C, Ivester P, Sergeant S, Barnes KC, et al. FADS genetic variants and omega-6 polyunsaturated fatty acid metabolism in a homogeneous island population. J Lipid Res 2010;51:2766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders TA. Protective effects of dietary PUFA against chronic disease: evidence from epidemiological studies and intervention trials. Proc Nutr Soc 2014;73:73–9. [DOI] [PubMed] [Google Scholar]
- 9.Martinelli N, Girelli D, Malerba G, Guarini P, Illig T, Trabetti E, Sandri M, Friso S, Pizzolo F, Schaeffer L, et al. FADS genotypes and desaturase activity estimated by the ratio of arachidonic acid to linoleic acid are associated with inflammation and coronary artery disease. Am J Clin Nutr 2008;88:941–9. [DOI] [PubMed] [Google Scholar]
- 10.Baylin A, Campos H. Arachidonic acid in adipose tissue is associated with nonfatal acute myocardial infarction in the central valley of Costa Rica. J Nutr 2004;134:3095–9. [DOI] [PubMed] [Google Scholar]
- 11.Kark JD, Kaufmann NA, Binka F, Goldberger N, Berry EM. Adipose tissue n-6 fatty acids and acute myocardial infarction in a population consuming a diet high in polyunsaturated fatty acids. Am J Clin Nutr 2003;77:796–802. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen MS, Schmidt EB, Stegger J, Gorst-Rasmussen A, Tjonneland A, Overvad K. Adipose tissue arachidonic acid content is associated with the risk of myocardial infarction: a Danish case-cohort study. Atherosclerosis 2013;227:386–90. [DOI] [PubMed] [Google Scholar]
- 13.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, Moore HJ, Davey Smith G. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev 2012;5:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka T, Shen J, Abecasis GR, Kisialiou A, Ordovas JM, Guralnik JM, Singleton A, Bandinelli S, Cherubini A, Arnett D, et al. Genome-wide association study of plasma polyunsaturated fatty acids in the InCHIANTI Study. PLoS Genet 2009;5:e1000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gieger C, Geistlinger L, Altmaier E, Hrabe de Angelis M, Kronenberg F, Meitinger T, Mewes HW, Wichmann HE, Weinberger KM, Adamski J, et al. Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 2008;4:e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaeffer L, Gohlke H, Muller M, Heid IM, Palmer LJ, Kompauer I, Demmelmair H, Illig T, Koletzko B, Heinrich J. Common genetic variants of the FADS1 FADS2 gene cluster and their reconstructed haplotypes are associated with the fatty acid composition in phospholipids. Hum Mol Genet 2006;15:1745–56. [DOI] [PubMed] [Google Scholar]
- 17.Malerba G, Schaeffer L, Xumerle L, Klopp N, Trabetti E, Biscuola M, Cavallari U, Galavotti R, Martinelli N, Guarini P, et al. SNPs of the FADS gene cluster are associated with polyunsaturated fatty acids in a cohort of patients with cardiovascular disease. Lipids 2008;43:289–99. [DOI] [PubMed] [Google Scholar]
- 18.Lu Y, Vaarhorst A, Merry AH, Dolle ME, Hovenier R, Imholz S, Schouten LJ, Heijmans BT, Muller M, Slagboom PE, et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS One 2012;7:e41681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bokor S, Dumont J, Spinneker A, Gonzalez-Gross M, Nova E, Widhalm K, Moschonis G, Stehle P, Amouyel P, De Henauw S, et al. Single nucleotide polymorphisms in the FADS gene cluster are associated with delta-5 and delta-6 desaturase activities estimated by serum fatty acid ratios. J Lipid Res 2010;51:2325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Feskens EJ, Dolle ME, Imholz S, Verschuren WM, Muller M, Boer JM. Dietary n-3 and n-6 polyunsaturated fatty acid intake interacts with FADS1 genetic variation to affect total and HDL-cholesterol concentrations in the Doetinchem Cohort Study. Am J Clin Nutr 2010;92:258–65. [DOI] [PubMed] [Google Scholar]
- 21.Nakayama K, Bayasgalan T, Tazoe F, Yanagisawa Y, Gotoh T, Yamanaka K, Ogawa A, Munkhtulga L, Chimedregze U, Kagawa Y, et al. A single nucleotide polymorphism in the FADS1/FADS2 gene is associated with plasma lipid profiles in two genetically similar Asian ethnic groups with distinctive differences in lifestyle. Hum Genet 2010;127:685–90. [DOI] [PubMed] [Google Scholar]
- 22.Dumont J, Huybrechts I, Spinneker A, Gottrand F, Grammatikaki E, Bevilacqua N, Vyncke K, Widhalm K, Kafatos A, Molnar D, et al. FADS1 genetic variability interacts with dietary alpha-linolenic acid intake to affect serum non-HDL-cholesterol concentrations in European adolescents. J Nutr 2011;141:1247–53. [DOI] [PubMed] [Google Scholar]
- 23.Hellstrand S, Sonestedt E, Ericson U, Gullberg B, Wirfalt E, Hedblad B, Orho-Melander M. Intake levels of dietary long-chain polyunsaturated fatty acids modify the association between genetic variation in FADS and LDL cholesterol. J Lipid Res 2012;53:1183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baylin A, Ruiz-Narvaez E, Kraft P, Campos H. alpha-Linolenic acid, Delta6-desaturase gene polymorphism, and the risk of nonfatal myocardial infarction. Am J Clin Nutr 2007;85:554–60. [DOI] [PubMed] [Google Scholar]
- 25.Kwak JH, Paik JK, Kim OY, Jang Y, Lee SH, Ordovas JM, Lee JH. FADS gene polymorphisms in Koreans: association with n-6 polyunsaturated fatty acids in serum phospholipids, lipid peroxides, and coronary artery disease. Atherosclerosis 2011;214:94–100. [DOI] [PubMed] [Google Scholar]
- 26.Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmö Diet and Cancer Study. Design and feasibility. J Intern Med 1993;233:45–51. [DOI] [PubMed] [Google Scholar]
- 27.Manjer J, Elmstahl S, Janzon L, Berglund G. Invitation to a population-based cohort study: differences between subjects recruited using various strategies. Scand J Public Health 2002;30:103–12. [DOI] [PubMed] [Google Scholar]
- 28.Manjer J, Carlsson S, Elmstahl S, Gullberg B, Janzon L, Lindstrom M, Mattisson I, Berglund G. The Malmö Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non-participants. Eur J Cancer Prev 2001;10:489–99. [DOI] [PubMed] [Google Scholar]
- 29. National Board of Health and Welfare. V#x00E4rdering av diagnoskvaliteten f#x00F6r akut hj#x00E4rtinfarkt i patientregistret 1987 och 1995. [Evaluation of diagnosis quality of acute myocardial infarction in the National Patient Register 1987 and 1995.] Stockholm: National Board of Health and Welfare, 2000 (in Swedish).
- 30.Zia E, Pessah-Rasmussen H, Khan FA, Norrving B, Janzon L, Berglund G, Engstrom G. Risk factors for primary intracerebral hemorrhage: a population-based nested case-control study. Cerebrovasc Dis 2006;21:18–25. [DOI] [PubMed] [Google Scholar]
- 31.Callmer E, Riboli E, Saracci R, Akesson B, Lindgarde F. Dietary assessment methods evaluated in the Malmö food study. J Intern Med 1993;233:53–7. [DOI] [PubMed] [Google Scholar]
- 32.Wirfält E, Mattisson I, Johansson U, Gullberg B, Wallstrom P, Berglund G. A methodological report from the Malmö Diet and Cancer study: development and evaluation of altered routines in dietary data processing. Nutr J 2002;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riboli E, Elmstahl S, Saracci R, Gullberg B, Lindgarde F. The Malmö Food Study: validity of two dietary assessment methods for measuring nutrient intake. Int J Epidemiol 1997;26:S161–S73. [DOI] [PubMed] [Google Scholar]
- 34.Mattisson I, Wirfalt E, Aronsson CA, Wallstrom P, Sonestedt E, Gullberg B, Berglund G. Misreporting of energy: prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br J Nutr 2005;94:832–42. [DOI] [PubMed] [Google Scholar]
- 35.Goyens PL, Mensink RP. Effects of alpha-linolenic acid versus those of EPA/DHA on cardiovascular risk markers in healthy elderly subjects. Eur J Clin Nutr 2006;60:978–84. [DOI] [PubMed] [Google Scholar]
- 36.Pan A, Chen M, Chowdhury R, Wu JH, Sun Q, Campos H, Mozaffarian D, Hu FB. alpha-Linolenic acid and risk of cardiovascular disease: a systematic review and meta-analysis. Am J Clin Nutr 2012;96:1262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blondeau N, Petrault O, Manta S, Giordanengo V, Gounon P, Bordet R, Lazdunski M, Heurteaux C. Polyunsaturated fatty acids are cerebral vasodilators via the TREK-1 potassium channel. Circ Res 2007;101:176–84. [DOI] [PubMed] [Google Scholar]
- 38.Nguemeni C, Delplanque B, Rovere C, Simon-Rousseau N, Gandin C, Agnani G, Nahon JL, Heurteaux C, Blondeau N. Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke. Pharmacol Res 2010;61:226–33. [DOI] [PubMed] [Google Scholar]
- 39.Yates CM, Calder PC, Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 2014;141:272–82. [DOI] [PubMed] [Google Scholar]
- 40.Lauritzen I, Blondeau N, Heurteaux C, Widmann C, Romey G, Lazdunski M. Polyunsaturated fatty acids are potent neuroprotectors. EMBO J 2000;19:1784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ghosh S, Novak EM, Innis SM. Cardiac proinflammatory pathways are altered with different dietary n-6 linoleic to n-3 alpha-linolenic acid ratios in normal, fat-fed pigs. Am J Physiol Heart Circ Physiol 2007;293:H2919–27. [DOI] [PubMed] [Google Scholar]
- 42.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Balter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr 2009;89:1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr 2004;24:597–615. [DOI] [PubMed] [Google Scholar]
- 44.Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature 2005;435:502–6. [DOI] [PubMed] [Google Scholar]
- 45.Hennig B, Meerarani P, Ramadass P, Watkins BA, Toborek M. Fatty acid-mediated activation of vascular endothelial cells. Metabolism 2000;49:1006–13. [DOI] [PubMed] [Google Scholar]
- 46.Gibson RA, Neumann MA, Lien EL, Boyd KA, Tu WC. Docosahexaenoic acid synthesis from alpha-linolenic acid is inhibited by diets high in polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 2013;88:139–46. [DOI] [PubMed] [Google Scholar]
- 47.Elmstähl S, Riboli E, Lindgarde F, Gullberg B, Saracci R. The Malmö Food Study: the relative validity of a modified diet history method and an extensive food frequency questionnaire for measuring food intake. Eur J Clin Nutr 1996;50:143–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.