Abstract
Essential amino acids (EAAs) are potent stimulators of mechanistic target of rapamycin complex 1 (mTORC1) signaling and muscle protein synthesis. However, regulators upstream of mTORC1 that are responsive to EAA availability are not well described, especially in human skeletal muscle. The purpose of this study was to determine changes in leucyl-tRNA synthetase (LARS/LARS) and Ras-related GTP binding B (RAGB/RAGB) mRNA and protein expression in healthy human skeletal muscle after acute EAA ingestion. Muscle biopsies sampled from the vastus lateralis were obtained from 13 young adults (7 males, 6 females; aged 22.9 ± 0.9 y; body mass index 21.7 ± 0.9 kg/m2) in the fasting state (baseline) and 1 and 3 h after EAA (13 g; 2.4 g of Leu) ingestion. Real-time quantitative polymerase chain reaction and Western blotting were used to determine changes in LARS/LARS and RAGB/RAGB mRNA and protein expression, respectively. Stable isotope tracers and gas chromatography mass spectrometry were used to determine Leu intracellular concentrations and muscle protein synthesis. EAA ingestion increased RAGB/RAGB mRNA (∼60%) and protein (∼100%) abundance in adult skeletal muscle (P ≤ 0.05). EAAs also increased muscle Leu concentrations (∼130%), mTOR phosphorylation (∼30%), and muscle protein synthesis (∼50%; P ≤ 0.05) but did not alter muscle LARS/LARS abundance (P > 0.05). We conclude that acute EAA ingestion is capable of increasing RAGB expression in human skeletal muscle. Future work is needed to determine whether this adaptive response is important to promote muscle protein anabolism in humans. This trial was registered at clinicaltrials.gov as NCT01669590.
Introduction
The mechanistic target of rapamycin complex 1 (mTORC1)7 is fundamental in stimulating human muscle protein synthesis (MPS) after essential amino acid (EAA) ingestion (1–4). However, less is known of the upstream molecular events that are responsible for mTORC1 activation in the presence of amino acid availability (5), especially in human skeletal muscle. An improved understanding of these regulators in human muscle will provide important information for future development of therapeutic approaches to regulate cell size in conditions of atrophy (e.g., sarcopenia, disuse).
Recently, leucyl-tRNA synthetase (LARS) was proposed to be critical for activating mTORC1 and protein synthesis (6, 7). In these studies, LARS, when bound to Leu, promoted the translocation of mTORC1 to the lysosome. Alternately, LARS knockdown cells prevented mTORC1 translocation to the lysosomal membrane (6, 7), whereas knockdown of isoleucyl-tRNA synthetase, methionyl-tRNA synthetase, or valyl-tRNA synthetase did not affect mTORC1 translocation. Together, these data suggest that LARS has a unique role compared with the other EAA amino acyl-tRNA synthetases in sensing Leu availability and regulating mTORC1 translocation.
The Ras-related GTP binding (RAG) GTPases (RAGA–RAGD) are also critical for amino acid–induced regulation of mTORC1 (8, 9) and were proposed to involve LARS (6, 7). The RAG proteins function as heterodimers such that RAGA or RAGB form a complex with RAGC or RAGD. In the presence of Leu, the Ragulator complex acts as a guanine–nucleotide exchange factor and facilitates RAGB into the GTP-bound state (10, 11), consequently activating the RAG heterodimeric complex. The Ragulator complex tethers the RAG proteins to the lysosomal membrane in which the RAGs physically interact with Raptor (10, 11). Interestingly, LARS bound with Leu interacted directly with RAGD and functioned as a GTPase activating protein to promote the transition from RAGD–GTP to RAGD–GDP, in turn activating mTORC1 (6, 7). Together, LARS and the RAG proteins (i.e., RAGB) are activated in the presence of increased amino acid availability and cooperate in the translocation and activation of mTORC1.
In our previous work in human skeletal muscle, we observed repeatedly an acute upregulation of the mRNA and protein of select amino acid transporters (important regulators of mTORC1 activation) after EAA ingestion alone, after a bout of resistance exercise, and after the combination of EAA or protein ingestion immediately after resistance exercise (12–15). Interestingly, these nutrient regulators had an overlapping time course response with changes in skeletal mTORC1 signaling and MPS and thus may play a role in promoting protein anabolism. Therefore, we hypothesized that, in response to acute EAA ingestion, skeletal muscle LARS/LARS and RAGB/RAGB (mRNA and protein) would similarly increase and would have an overlapping time course with mTORC1 signaling and MPS. As a follow-up to our findings, we also hypothesized that EAAs would increase the binding of LARS and RAGB with mTORC1 in human skeletal muscle.
Materials and Methods
Participants.
A total of 13 healthy male and female young participants (aged 18–28 y) were recruited through poster advertisements on the University of Utah campus and in the surrounding Salt Lake City community. All participants gave their written informed consent before participating in the study. Participant characteristics are listed in Table 1. The participants were recreationally active but were not engaged in any regular exercise training program. All women were studied in the follicular phase of their menstrual cycle. Exclusion criteria included, but were not limited to, the following diseases: 1) heart; 2) lung; 3) blood; 4) vascular; 5) liver; 6) kidney; 7) infectious; 8) oncologic; and 9) neurologic. This study was approved by the University of Utah Institutional Review Board and conformed to the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects.
TABLE 1.
Participant characteristics of young healthy adults1
| Gender, M/F | 7/6 |
| Age, y | 22.9 ± 0.9 |
| Height, m | 1.7 ± 0.1 |
| Weight, kg | 65.9 ± 3.8 |
| BMI, kg/m2 | 21.7 ± 0.9 |
Values are means ± SEMs.
Experimental protocol.
The evening before the experiment, participants ingested a standardized evening meal (30% fat, 15% protein, 55% carbohydrate). The next morning, after an overnight fast and refrainment of intense physical activity over a 48-h period, participants arrived at 0600 by transportation to the Center for Clinical and Translational Sciences at the University of Utah. After arriving, an 18-gauge polyethylene catheter was inserted into the antecubital vein for the primed, constant infusion of l-[ring-13C6]Phe (Cambridge Isotopes Laboratories) to determine MPS and l-[1-13C]Leu for determination of intracellular Leu concentrations. The priming dose for the labeled Phe was 2 μmol/kg, and the infusion rate was 0.05 μmol ⋅ kg−1 ⋅ min−1. The priming dose for the labeled Leu was 4.8 μmol/kg, and the infusion rate was 0.08 μmol ⋅ kg−1 ⋅ min−1. Two and 4 h after the start of the infusion (0700), a muscle biopsy was taken from the vastus lateralis for determination of baseline MPS rates and usage for Western blotting and qPCR. Immediately after biopsy 2, participants ingested ∼13 g of crystalline EAAs (Glanbia Nutritionals) mixed in a 400-mL flavored low-calorie, noncaffeinated beverage. The EAA beverage contained 7.7% l-[ring-13C6]Phe and 5.2% l-[1-13C]Leu to minimize tracer dilution. Additional muscle biopsies were taken 1 and 3 h after EAA ingestion. The composition of EAA mixture was the following: l-His (1.6 g), l-Ile (1.0 g), l-Leu (2.4 g), l-Lys (3.1 g), l-Met (0.8 g), l-Phe (1.2 g), l-Thr (1.2 g), and l-Val (1.5 g). The dosage of EAAs used in this study, as determined by the quantity of Leu, is effective to maximally stimulate MPS in young adults (1, 4, 16).
Muscle biopsy.
Muscle biopsies were sampled from the vastus lateralis using aseptic technique, local anesthesia (1% lidocaine), and a 5-mm Bergström biopsy needle with manual suction (17). Muscle biopsies 1 and 2 were taken from a single incision. Biopsies 3 and 4 were taken from a separate incision, ∼7 cm proximal from the first incision. Biopsies taken from the same incision (biopsies 1 vs. 2, 3 vs. 4) were separated by ≥5 cm by angling the biopsy needle. All muscle tissue was immediately blotted and dissected of visible nonmuscle tissue, flash frozen in liquid nitrogen, and stored at −80°C for later analysis.
Western blotting.
We reported details of Western blotting previously (18). Membranes were incubated overnight in LARS (1:1000; Abcam; catalog #ab31534), RAGB (1:2000; Cell Signaling Technology; catalog #8150), phosphorylated mTOR (Ser2448; 1:1000; Cell Signaling Technology; catalog #2971), or phosphorylated mTOR (Ser2481; 1:1000; Cell Signaling Technology; catalog #2974). The next morning, secondary antibody was added to the membrane. Chemiluminescence reagent (ECL Plus; GE Healthcare) was applied to each blot and then assessed with a digital imager (ChemiDoc XRS+ Bio-Rad). Membranes were stripped of primary and secondary antibodies and then reprobed for total mTOR (1:1000; Cell Signaling Technology; catalog #2972) and GAPDH (Cell Signaling Technology; catalog #2118) on a separate day. Densitometric analysis was performed using Lab version 4.1 software (Bio-Rad). Whole-muscle homogenate data were normalized to the internal control (loaded in duplicate on each gel), and replicate samples were averaged and reported as fold change from baseline. Phosphorylated mTOR (Ser2448 and Ser2481) was reported as phosphorylated mTOR/total mTOR (fold change). GAPDH was used to verify equal sample loading.
Coimmunoprecipitation.
To further describe whether the binding of LARS and RAGB to mTORC1 was altered after EAA ingestion, we evaluated a subset of muscle samples. We were restricted to n = 8 (4 males and 4 females; aged 22.8 ± 1.3 y; 22.8 ± 1.2 kg/m2) for this experiment because of limited available muscle tissue. Details of the coimmunoprecipitation for mTORC1 were reported by us and others previously (19, 20). Six microliters of mTOR antibody (Cell Signaling Technology; catalog #2972) was added to 700 μg of protein and then rotated overnight at 4°C. The mTOR protein–antibody complex was isolated by adding BioMag goat anti-rabbit IgG (Qiagen) beads, and then the immunoprecipitated sample (25 μL) was subjected to Western blotting as described above. Membranes were incubated in LARS or RAGB antibody overnight and then on another day reprobed for total mTOR. Coimmunoprecipitation data were normalized to the internal control, and replicate samples were averaged. Coimmunoprecipitation data were reported as target protein relative to total mTOR. No signal was present when using an IgG control antibody (1:1000; Cell Signaling Technology; catalog #2729) for precipitation.
RNA extraction, cDNA synthesis, and semiquantitative real-time PCR.
Total RNA, cDNA synthesis, and real-time qPCR were conducted as reported previously by our team (14). RNA integrity was performed using the Agilent 2100 Bioanalyzer (Agilent Technologies). The mean RNA integrity number was 9.5 ± 0.5 (1–10 scale, in which 1 is low and 10 is high), and the 28S:18S ratio was 1.53 ± 0.03, indicating high RNA integrity. Real-time qPCR was performed with a CFX Connect real-time PCR cycler (Bio-Rad). Primers [LARS, RAGB, and hydroxymethybilane synthase (HMBS)] were custom designed (Beacon Designer), purchased from Life Sciences, and carefully optimized (i.e., efficiency, DNA gel). The primer sequences and GenBank accession numbers were the following: 1) LARS (NM_020117): forward, ATGATTGACGCTGGAGAT and reverse, CAGAGCCACAACACATTC; 2) RAGB (NM_016656): forward, AAGGCGGAGGCAAGAATA and reverse, CGCAACAAGTCCTTTCCA; and 3) HMBS (NM_000190): forward, CACAGTTGGTAGGCATCA and reverse, AGTTAATGGGCATCGTTA. RAGD (NM_021244) primers were generated from PrimerBank (21) and also purchased from Life Sciences. The cycle threshold values for HMBS were similar across time points; therefore, HMBS was used to normalize the endpoints of interest. Fold change values were calculated using the 2−∆∆Ct method (22).
Intracellular Leu concentrations and MPS.
Bound muscle proteins and muscle intracellular free amino acids were extracted from biopsy samples (biopsies 1–4) as described previously (23). GC-MS (6890 Plus CG, 5973N MSD, 7683 autosampler; Agilent Technologies) was used to determine muscle intracellular free concentrations of Leu via the internal standard method as described previously (24), which included measurement of tracer enrichments for l-[1-13C]Leu and the internal standard, [5,5,5-2H3]Leu. However, because of technical issues with the intracellular Leu enrichments, only 12 of the 13 participants were included in the final analysis.
Mixed-muscle protein-bound Phe enrichment for l-[ring-13C6]Phe was analyzed by GC-MS after protein hydrolysis and amino acid extraction (23, 25), using the external standard curve approach (26). MPS was calculated by measuring the incorporation rate of the Phe tracer into the proteins and using the precursor-product model to calculate the synthesis rate. Data are expressed as percentage per hour.
Statistical methods.
A repeated-measures ANOVA followed by pairwise comparisons (Fisher’s least significant differences) were used to assess specific differences between baseline vs. 1-h EAA and baseline vs. 3-h EAA. Significance was set at P < 0.05. All values are presented as mean ± SEM. All analyses were performed with SigmaPlot (version 12.0).
Results
mRNA and protein abundance.
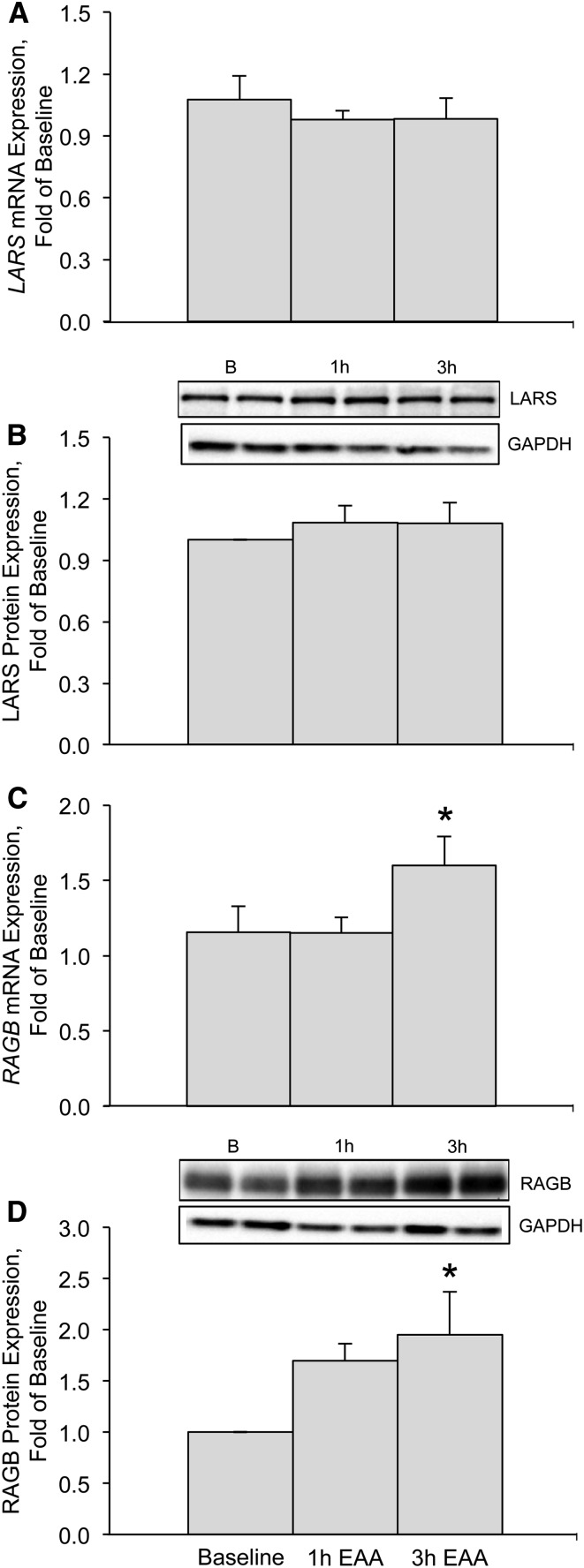
We found that LARS/LARS mRNA and protein abundance (Fig. 1A, B) were not different from baseline at either 1 h (mRNA, P = 0.45; protein, P = 0.32) or 3 h (mRNA, P = 0.46; protein, P = 0.34) after EAA ingestion. RAGB/RAGB mRNA and protein abundance (Fig. 1C, D) were not different from baseline at 1 h (mRNA, P = 0.98; protein, P = 0.095) after EAA ingestion but increased at 3 h at both the mRNA and protein levels (mRNA, P = 0.039; protein, P = 0.025) after EAA ingestion. RAGD mRNA abundance was not different from baseline at 1 h (0.97 ± 0.17-fold, P = 0.85) or 3 h (0.85 ± 0.22-fold, P = 0.76) after EAA ingestion (data not shown).
FIGURE 1.
LARS/LARS mRNA (A) and protein (B) expression and RAGB/RAGB mRNA (C) and protein (D) expression at baseline and 1 and 3 h after EAA ingestion in the skeletal muscle of young, healthy adults. Insets above panels B and D are Western blot images representing protein expression (in duplicate) for LARS, RAGB, and GAPDH at baseline and 1 and 3 h after EAA ingestion. Western blot images of GAPDH were used to verify equal protein across sample time points. Data are means ± SEMs (n = 13) and are reported as fold changes from baseline. *Different from baseline, P ≤ 0.05. B, baseline; EAA, essential amino acid; LARS/LARS, leucyl-tRNA synthetase; RAGB/RAGB, Ras-related GTP binding B.
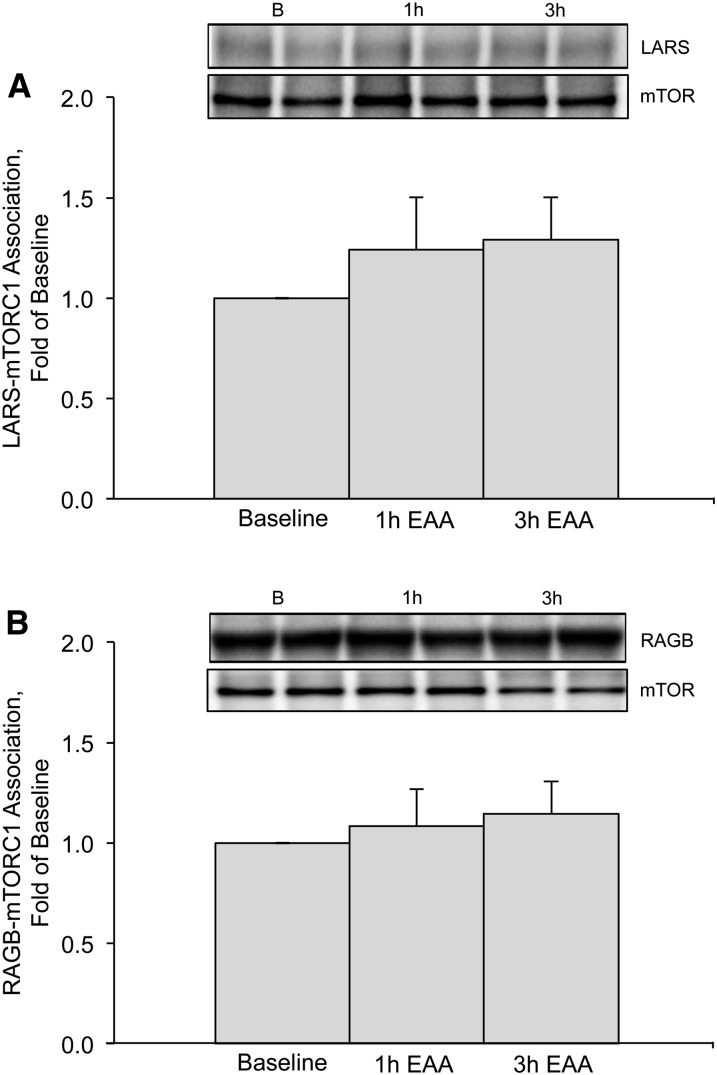
Coimmunoprecipitation.
We found that the LARS–mTORC1 association was not different from baseline at 1 h (P = 0.28) or 3 h (P = 0.33) after EAA ingestion (Fig. 2A). Similarly, the RAGB–mTORC1 association was not different from baseline at 1 h (P = 0.87) or 3 h (P = 0.61) after EAA ingestion (Fig. 2B).
FIGURE 2.
LARS–mTORC1 (A) and RAGB–mTORC1 (B) associations (relative to total mTOR) at baseline and 1 and 3 h after EAA ingestion in the skeletal muscle of young, healthy adults. Insets above figures are Western blot images representing protein expression (in duplicate) for LARS, RAGB, and total mTOR at baseline and 1 and 3 h after EAA ingestion. Data are means ± SEMs (n = 8). LARS and RAGB data are reported as fold change from baseline. B, baseline; EAA, essential amino acid; LARS, leucyl-tRNA synthetase; mTOR, mechanistic target of rapamycin; mTORC1, mechanistic target of rapamycin complex 1; RAGB, Ras-related GTP binding B.
mTOR, Leu concentrations, and MPS.
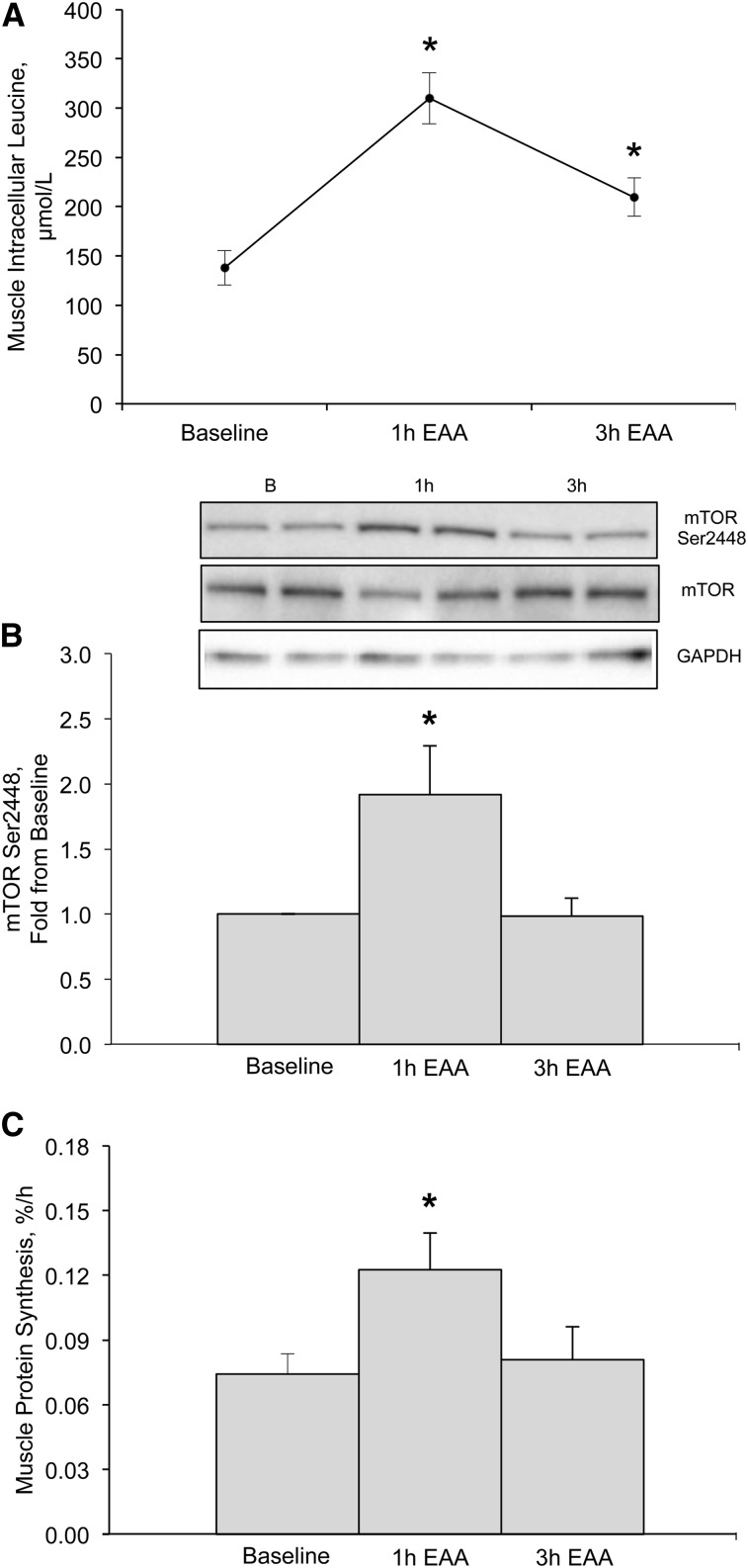
As expected, mTOR phosphorylation at Ser2448 was elevated above baseline at 1 h (P < 0.01) and returned to baseline at 3 h (P = 0.97) after EAA ingestion (Fig. 3A). Similarly, mTOR phosphorylation at Ser2481 increased at 1 h (1.29 ± 0.12-fold, P = 0.026) and returned to baseline 3 h (1.01 ± 0.12-fold, P = 0.95) after EAA ingestion (data not shown). Leu intracellular concentrations increased from baseline ∼2.3-fold at 1 h (P < 0.01) and ∼1.5-fold 3 h (P < 0.01) after EAA ingestion (Fig. 3B). Finally, mixed MPS increased from baseline ∼1.5-fold at 1 h (P = 0.036) but returned to baseline at 3 h (P = 0.76) after EAA ingestion (Fig. 3C).
FIGURE 3.
Intracellular Leu concentrations (μmol/L) (A), mTOR phosphorylation (Ser2448) relative to total mTOR (B), and mixed muscle protein synthesis rates (percentage per hour) (C) at baseline and 1 and 3 h after EAA ingestion in skeletal muscle of young, healthy adults. Inset above panel B is a Western blot image representing samples (in duplicate) at baseline and 1 and 3 h after EAA ingestion. Western blot images of GAPDH were used to verify equal protein across sample time points. Data are means ± SEMs (n = 13). Data for panel B are reported as fold change from baseline. Panel A only contains data from 12 participants. *Different from baseline, P < 0.05. B, baseline; EAA, essential amino acid; mTOR, mechanistic target of rapamycin.
Discussion
The purpose of this study was to characterize the acute expression of 2 important molecular regulators of amino acid–mediated mTORC1 activation in human skeletal muscle. Our primary finding was that skeletal muscle RAGB/RAGB mRNA and protein expression (but not LARS/LARS) was acutely increased after EAA ingestion. However, despite an increase in mTOR phosphorylation, intracellular Leu concentrations, and MPS, we did not detect a change in LARS–mTORC1 and RAGB–mTORC1 associations from baseline at either 1 or 3 h after EAA ingestion in healthy human skeletal muscle. We conclude that an increase in amino acid availability is capable of acutely increasing RAGB/RAGB mRNA and protein abundance in human skeletal muscle. Future investigations are needed to determine whether this adaptive response is an important mechanism to improve sensitivity to a subsequent anabolic stimulus (e.g., meal, exercise).
The novel finding from our study was that RAGB/RAGB mRNA and protein abundance increased after EAA ingestion in human skeletal muscle. We observed previously acute increases in both protein and mRNA abundance of other nutrient regulators of mTORC1 after EAA ingestion in humans (18), suggesting that an adaptive upregulation of select amino acid–sensitive regulators of mTORC1 may be a common response in the presence of amino acid availability in humans. We speculate that an acute increase in RAGB/RAGB mRNA and protein may be an adaptive response important to amplify a subsequent anabolic stimulus. These findings, coupled with our previous findings (12–15), support conducting a future clinical trial to test whether a previous protein-enriched meal (i.e., breakfast) may serve as the basis for producing a magnified protein anabolic response to a second meal (i.e., lunch). Additionally, an acute upregulation of RAGB with EAA ingestion may be partly related to the additive anabolic response that is observed when EAAs are ingested in close proximity to a bout of resistance exercise (27–29).
We are unsure of the mechanism responsible for the nearly 100% increase in skeletal muscle RAGB protein abundance that occurs within hours of EAA ingestion. However, it is not unreasonable to suspect that the upregulation of RAGB mRNA observed in this study may have contributed to the accumulation of RAGB protein. Moreover, it is possible that the transcriptional upregulation of RAGB mRNA may have been detected earlier if more sampling time points were feasible because RAGB protein tended to increase at 1 h after EAA ingestion (P = 0.095). However, we cannot rule out the possibility that post-translational mechanisms may contribute to RAGB protein accumulation. Finally, although out of the scope of our study, it is possible that endogenous insulin release could have had a role in the induction of RAGB. This is based on previous lines of evidence that circulatory insulin concentrations increase after a bolus of EAAs (i.e., 10 g) (14) and an understanding that insulin is capable of upregulating transcription factors (30) and participates in the assembly of an active mTORC1 complex (31).
To the best of our knowledge, a transcriptional regulator has not been identified for RAGB. We predict that activating transcription factor 4 (ATF4) may be a candidate regulator in the acute induction of RAGB mRNA in response to amino acid availability. Given the role of ATF4 as an important transcriptional mediator of many amino acid transporters and amino acyl-tRNA synthetases (30, 32, 33) coupled with our previous observation of increased ATF4 after EAA ingestion in human muscle (14), we suggest that this might be a possibility. Therefore, we examined known amino acid response element sites (32) within a proximal region of the transcriptional start site of the RAGB promotor (data not shown), and, as a result, we could not verify the existence of such a sequence. Although these known composite sites are not enriched in RAGB and given the complexity of ATF binding and enhancers, we cannot conclude that this is evidence against ATF4 regulating RAGB but simply that experiments need to be conducted to rule against or in favor of ATF4 as an activator of RAGB transcription.
Recent cell studies showed that a critical step in amino acid–induced mTORC1 activation is the recruitment of mTORC1 to the lysosome by LARS and RAG proteins (6, 9). Therefore, a follow-up experiment was to evaluate the mTORC1 association with LARS and RAGB. Contrary to our hypothesis, LARS–mTORC1 and RAGB–mTORC1 association was not altered after EAA ingestion. Certainly, for ethical considerations, we were limited to few sampling time points and number of participants (n = 8) to accurately detect differences. Nonetheless, we were surprised by our findings because EAA ingestion created a robust anabolic environment in skeletal muscle as evidenced by increased mTOR phosphorylation (Ser2448 and Ser2481), intracellular Leu concentrations, and MPS. The EAA dose used in the current study (13 g of EAAs; 2.4 g of Leu) was designed to elicit a maximal MPS response in young human skeletal muscle (4, 16). Furthermore, the time points we chose were in accordance with maximal amino acid–induced mTORC1 signaling and MPS in human muscle (2, 4, 14, 34, 35). Nonetheless, our findings are consistent with those of Suryawan and Davis (36), who found no change in RAGB–Raptor association after a 2-h amino acid infusion in skeletal muscle of neonatal pigs aged 6 and 28 d. Although we initially predicted that the LARS–mTORC1 and RAGB–mTORC1 associations might occur earlier than 2 h in human muscle (i.e., 1 h) and may be highly responsive to rapid increases in amino acid concentrations with a bolus (37), our chosen postprandial time points may have been too late to detect maximal LARS–mTORC1 or RAGB–mTORC1 associations. This notion is not unreasonable because an increase in plasma EAA/Leu concentrations and skeletal muscle ribosomal S6 kinase 1 and 4E-binding protein 1 phosphorylation (effectors of mTORC1) are detected in as little as 15–30 min after amino acid/protein ingestion in healthy young adults (4, 35, 38, 39). Additionally, changes in the LARS–mTORC1 and RAGB–mTORC1 associations may have been at a level undetectable by Western blotting methods yet still capable of maintaining an increase in mTORC1 signaling and MPS.
The lack of a change in the LARS–mTORC1 association after EAA ingestion is also difficult to explain beyond the limited sampling time points. A possible explanation is that other GTPase-activating proteins may exist in human muscle, such as folliculin tumor suppressor, to coordinate the nucleotide exchange of RAGD (40). Tsun et al. (40) noted that folliculin tumor suppressor and its binding partner, folliculin interacting protein 1, regulated RAG–mTORC1 binding by acting as a GTPase activating protein for RAGC/D, whereas LARS did not express GTPase activating activity. Therefore, the role of LARS in amino acid sensing should be investigated further in humans.
In conclusion, to our knowledge, this is the first time the integral nutrient signaling molecules LARS and RAGB, responsible for mTORC1 activation, were investigated in human skeletal muscle. The novel finding of this study was that skeletal muscle RAGB/RAGB mRNA and protein increased in response to amino acid availability in healthy humans. Additional work is needed to determine whether this adaptive response has a role in promoting protein anabolism.
Acknowledgments
The authors thank Ming Zheng, Shelley Medina, and Susan Wilson at the University of Texas Medical Branch for their assistance with the GC-MS analysis and Glanbia Nutritionals for providing the premixed EAAs. M.J.D. developed the research proposal and design; M.J.D. and M.B.C. wrote the manuscript; M.J.D. and M.B.C. analyzed the data; M.J.D., M.B.C., R.E.T., and J.A. collected the data; and M.J.D., M.B.C., R.E.T., J.A., T.J., and D.A.M. reviewed the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: ATF4, activating transcription factor 4; EAA, essential amino acid; HMBS, hydroxymethybilane synthase; LARS, leucyl-tRNA synthetase; MPS, muscle protein synthesis; mTORC1, mechanistic target of rapamycin complex 1; RAG, Ras-related GTP binding.
References
- 1.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 2005;19:422–4. [DOI] [PubMed] [Google Scholar]
- 2.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient signalling in the regulation of human muscle protein synthesis. J Physiol 2007;582:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 2011;141:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glynn EL, Fry CS, Drummond MJ, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J Nutr 2010;140:1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014;24:400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–24. [DOI] [PubMed] [Google Scholar]
- 7.Bonfils G, Jaquenoud M, Bontron S, Ostrowicz C, Ungermann C, De Virgilio C. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol Cell 2012;46:105–10. [DOI] [PubMed] [Google Scholar]
- 8.Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 2008;10:935–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008;320:1496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 2012;150:1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson JM, Drummond MJ, Coben JR, Volpi E, Rasmussen BB. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr 2013;32:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond MJ, Fry CS, Glynn EL, Timmerman KL, Dickinson JM, Walker DK, Gundermann DM, Volpi E, Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol 2011;111:135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, Cope MB, Mukherjea R, Jennings K, Volpi E, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J Appl Physiol (1985) 2014;116:1353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, Prior T, Tarnopolsky MA, Phillips SM. Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 2009;89:161–8. [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 1975;35:609–16. [PubMed] [Google Scholar]
- 18.Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 2012;302:E1113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond MJ, Fry CS, Glynn EL, Dreyer HC, Dhanani S, Timmerman KL, Volpi E, Rasmussen BB. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol 2009;587:1535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson DL, Bolster DR, Kimball SR, Jefferson LS. Time course changes in signaling pathways and protein synthesis in C2C12 myotubes following AMPK activation by AICAR. Am J Physiol Endocrinol Metab 2006;291:E80–9. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Spandidos A, Wang H, Seed B. PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 2012;40:D1144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- 23.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E–BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol 2006;576:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. 2nd ed. Hoboken (NJ): Wiley-Liss; . 2005. [Google Scholar]
- 25.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr 2003;78:250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calder AG, Anderson SE, Grant I, McNurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0.09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Commun Mass Spectrom 1992;6:421–4. [DOI] [PubMed] [Google Scholar]
- 27.Moore DR, Atherton PJ, Rennie MJ, Tarnopolsky MA, Phillips SM. Resistance exercise enhances mTOR and MAPK signalling in human muscle over that seen at rest after bolus protein ingestion. Acta Physiol (Oxf) 2011;201:365–72. [DOI] [PubMed] [Google Scholar]
- 28.Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 2011;93:322–31. [DOI] [PubMed] [Google Scholar]
- 29.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab 2008;294:E392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams CM. Role of the transcription factor ATF4 in the anabolic actions of insulin and the anti-anabolic actions of glucocorticoids. J Biol Chem 2007;282:16744–53. [DOI] [PubMed] [Google Scholar]
- 31.Dennis MD, Baum JI, Kimball SR, Jefferson LS. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 2011;286:8287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 2009;20:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malmberg SE, Adams CM. Insulin signaling and the general amino acid control response. Two distinct pathways to amino acid synthesis and uptake. J Biol Chem 2008;283:19229–34. [DOI] [PubMed] [Google Scholar]
- 34.Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 2001;532:575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 2010;92:1080–8. [DOI] [PubMed] [Google Scholar]
- 36.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol 2010;109:1448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, Moore DR, Stellingwerff T, Phillips SM. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. Am J Clin Nutr 2011;94:795–803. [DOI] [PubMed] [Google Scholar]
- 38.Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 2009;107:987–92. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson DJ, Hossain T, Hill DS, Phillips BE, Crossland H, Williams J, Loughna P, Churchward-Venne TA, Breen L, Phillips SM, et al. Effects of leucine and its metabolite beta-hydroxy-beta-methylbutyrate on human skeletal muscle protein metabolism. J Physiol 2013;591:2911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsun ZY, Bar-Peled L, Chantranupong L, Zoncu R, Wang T, Kim C, Spooner E, Sabatini DM. The folliculin tumor suppressor is a GAP for the RagC/D GTPases that signal amino acid levels to mTORC1. Mol Cell 2013;52:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]