Abstract
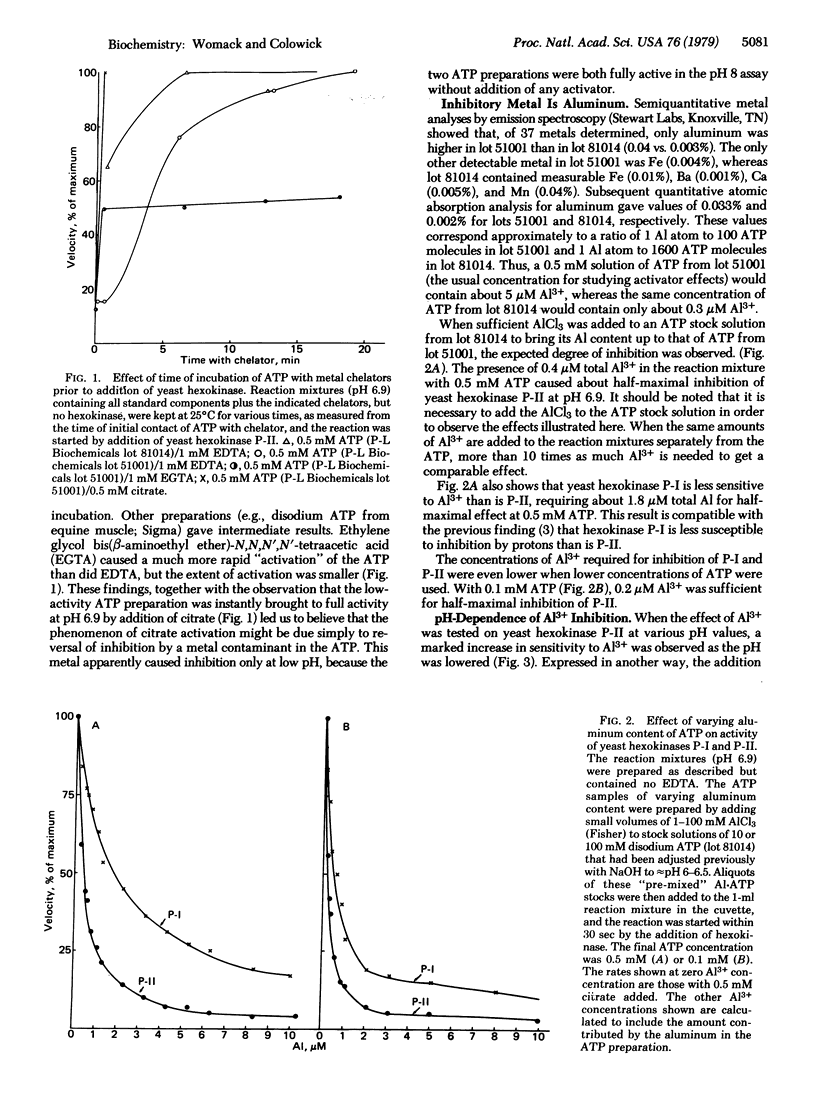
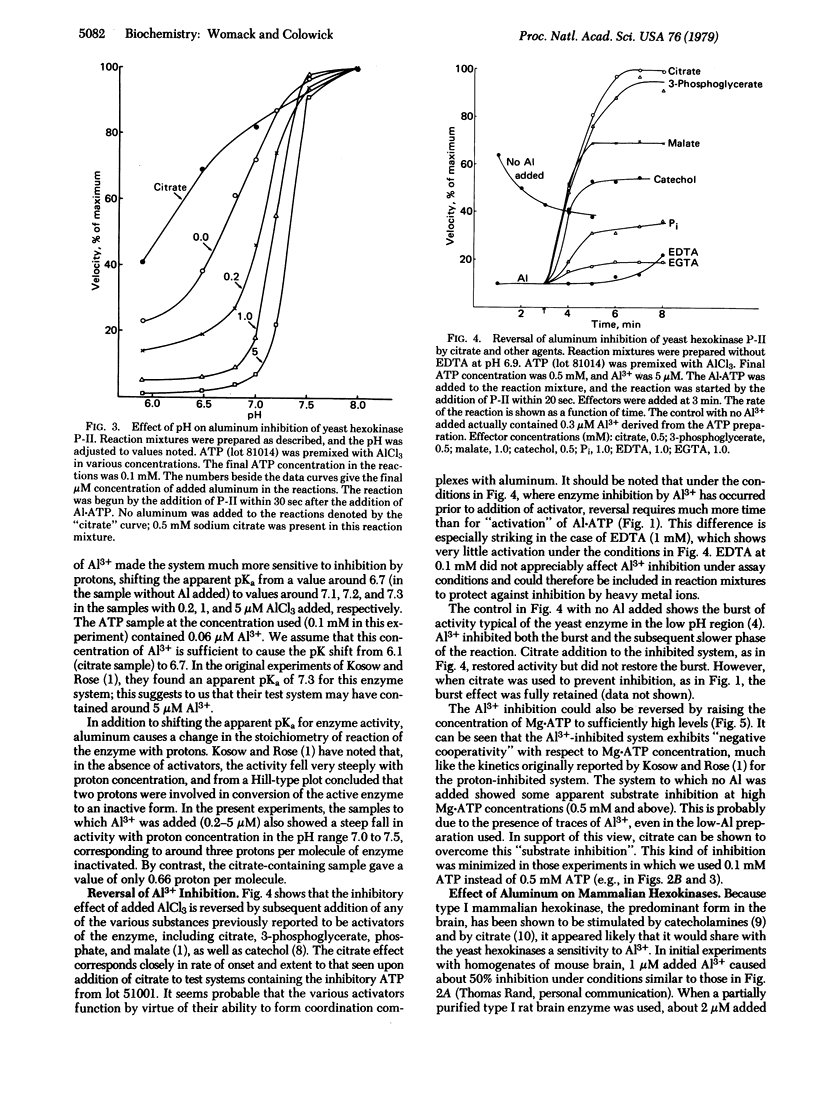
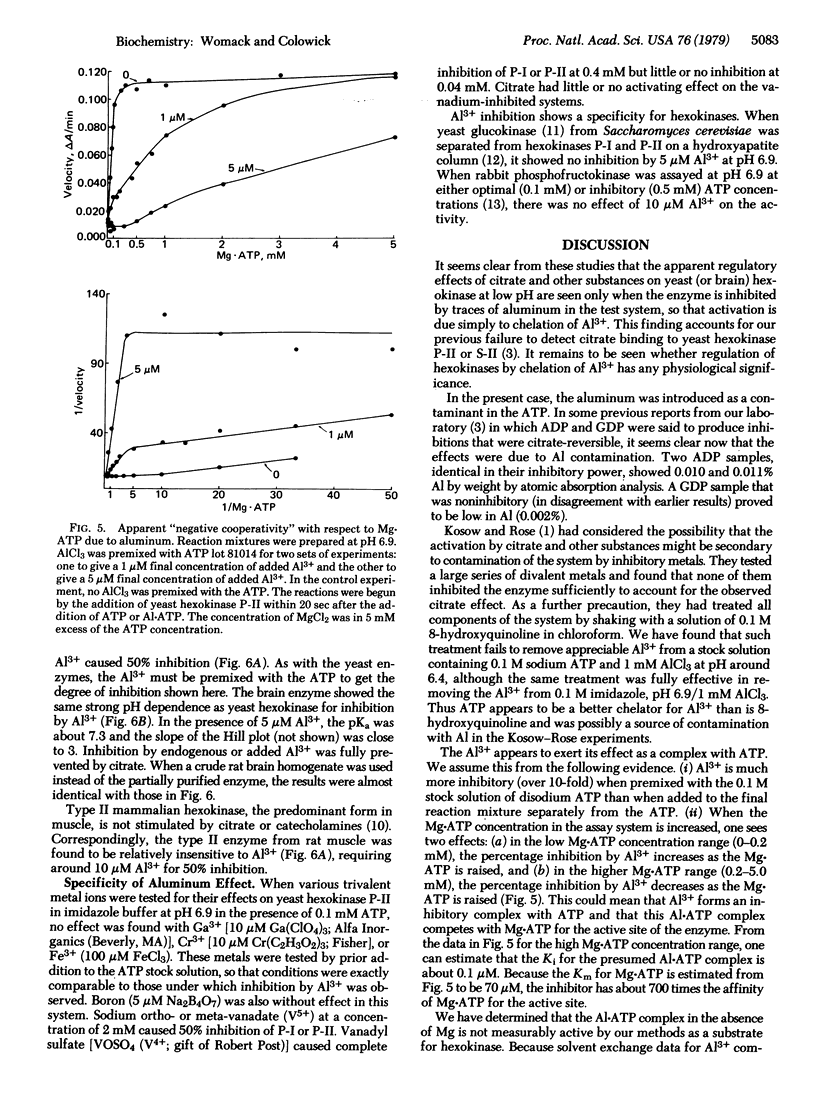
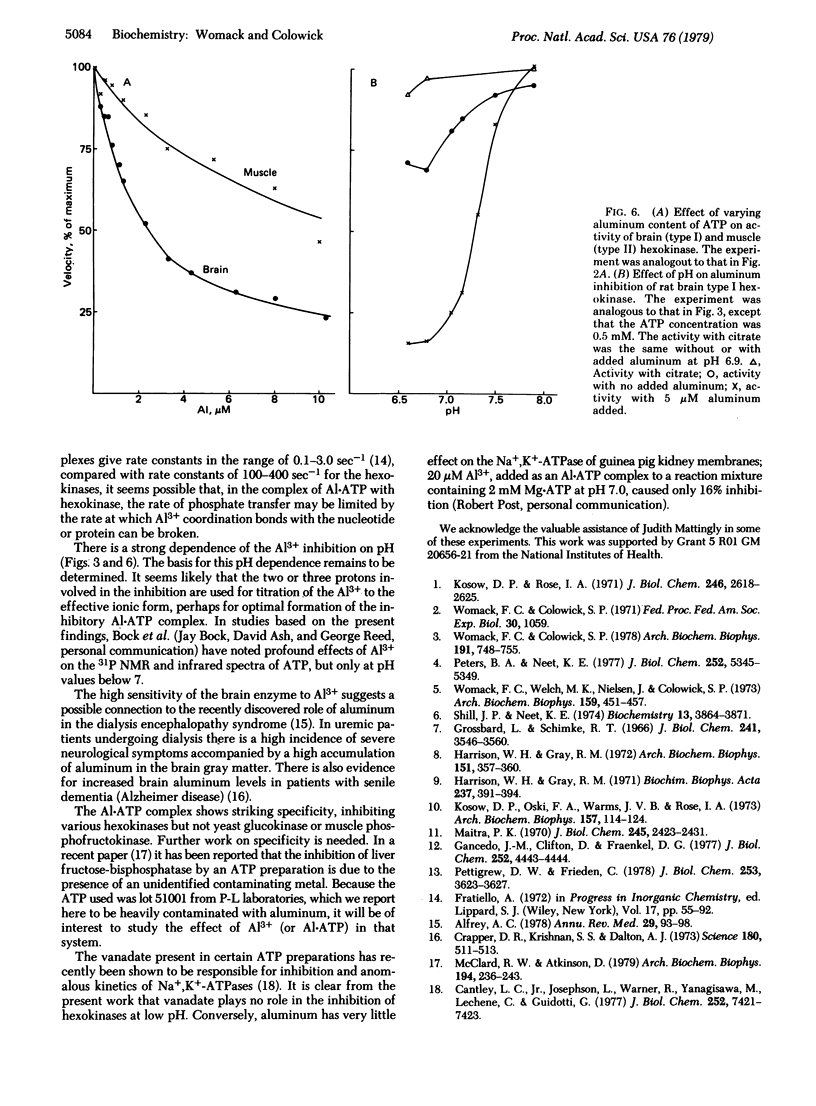
The aluminum present as a contaminant in ATP preparations can cause strong inhibition of yeast hexokinase P-II activity at pH 7.0 or below but has little or no inhibitory effect at a pH of 7.5 or greater. The inhibition is reversed by citrate, 3-phosphoglycerate, malate, phosphate, and catecholamines, all of which have previously been described as activators of hexokinase at low pH. We suggest that these agents activate the enzyme only by virtue of their ability to coordinate with aluminum present in the assay system. The presence of aluminum is also responsible for the "negative cooperativity" observed at low pH with respect to Mg . ATP concentration--i.e., the inhibition by aluminum is uncompetitive at low Mg . ATP concentrations but becomes competitive at high Mg . ATP concentrations. The inhibition is thought to be due to formation of a complex of Al . ATP with the enzyme, with a dissociation constant (Ki) of 0.1 microM. Yeast hexokinase P-I is somewhat less sensitive to A1 than is hexokinase P-II, and yeast glucokinase is not detectably affected. The hexokinase in rat brain (type I) shows a pH-dependent inhibition by Al similar to that observed with the yeast hexokinases, whereas the rat muscle (type II) enzyme is less sensitive, suggesting a possible relationship to aluminum encephalopathy in man.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfrey A. C. Dialysis encephalopathy syndrome. Annu Rev Med. 1978;29:93–98. doi: 10.1146/annurev.me.29.020178.000521. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Crapper D. R., Krishnan S. S., Dalton A. J. Brain aluminum distribution in Alzheimer's disease and experimental neurofibrillary degeneration. Science. 1973 May 4;180(4085):511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Clifton D., Fraenkel D. G. Yeast hexokinase mutants. J Biol Chem. 1977 Jul 10;252(13):4443–4444. [PubMed] [Google Scholar]
- Grossbard L., Schimke R. T. Multiple hexokinases of rat tissues. Purification and comparison of soluble forms. J Biol Chem. 1966 Aug 10;241(15):3546–3560. [PubMed] [Google Scholar]
- Harrison W. H., Gray R. M. Catecholamine stimulation of brain hexokinase. Biochim Biophys Acta. 1971 May 18;237(2):391–394. doi: 10.1016/0304-4165(71)90336-9. [DOI] [PubMed] [Google Scholar]
- Harrison W. H., Gray R. M. Stimulation of yeast hexokinase by catecholamines and related compounds. Arch Biochem Biophys. 1972 Aug;151(2):357–360. doi: 10.1016/0003-9861(72)90509-7. [DOI] [PubMed] [Google Scholar]
- Kosow D. P., Oski F. A., Warms J. V., Rose I. A. Regulation of mammalian hexokinase: regulatory differences between isoenzyme I and II. Arch Biochem Biophys. 1973 Jul;157(1):114–124. doi: 10.1016/0003-9861(73)90396-2. [DOI] [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Activators of yeast hexokinase. J Biol Chem. 1971 Apr 25;246(8):2618–2625. [PubMed] [Google Scholar]
- Maitra P. K. A glucokinase from Saccharomyces cerevisiae. J Biol Chem. 1970 May 10;245(9):2423–2431. [PubMed] [Google Scholar]
- McClard R. W., Atkinson D. E. Effects of fructose 6-phosphate and adenylates on the activities of rabbit liver fructose bisphosphatase and phosphofructokinase. Arch Biochem Biophys. 1979 Apr 15;194(1):236–243. doi: 10.1016/0003-9861(79)90614-3. [DOI] [PubMed] [Google Scholar]
- Peters B. A., Neet K. E. Regulatory properties of yeast hexokinase PII. Metal specificity, nucleotide specificity, and buffer effects. J Biol Chem. 1977 Aug 10;252(15):5345–5349. [PubMed] [Google Scholar]
- Pettigrew D. W., Frieden C. Rabbit muscle phosphofructokinase. Modification of molecular and regulatory kinetic properties with the affinity label 5'-p-(fluorosulfonyl)benzoyl adenosine. J Biol Chem. 1978 May 25;253(10):3623–3627. [PubMed] [Google Scholar]
- Shill J. P., Peters B. A., Neet K. E. Monomer-dimer equilibria of yeast hexokinase during reacting enzyme sedimentation. Biochemistry. 1974 Sep 10;13(19):3864–3871. doi: 10.1021/bi00716a007. [DOI] [PubMed] [Google Scholar]
- Womack F. C., Colowick S. P. Purine nucleoside diphosphate regulation of yeast hexokinases. Arch Biochem Biophys. 1978 Dec;191(2):748–755. doi: 10.1016/0003-9861(78)90416-2. [DOI] [PubMed] [Google Scholar]
- Womack F. C., Welch M. K., Nielsen J., Colowick S. P. Purification and serological comparison of the yeast hexokinases P-I and P-II. Arch Biochem Biophys. 1973 Oct;158(2):451–457. doi: 10.1016/0003-9861(73)90536-5. [DOI] [PubMed] [Google Scholar]


